Abstract
Burial of seeds by sediment often happens in lakes. Laboratory experiments were conducted to investigate the effects of burial by river sand and fishpond sediment on seed germination and seedling establishment of Vallisneria natans at depths of 0, 1, 3, and 5 cm. Sediment type had significant effects only on dry weight and total leaf area of the seedlings and did not interact with burial depth. In contrast, a significant negative correlation was observed between burial depth and all of the variables, including seed germination rate, seedling emergence rate, shoot length, dry weight, leaf number, total leaf area, root number, and total root length. Moreover, no seedlings emerged above the sediment surface in the 5 cm depth treatment in either sediment, even though seed germination was observed. In addition, the results showed that fishpond sediment was more effective than river sand in accumulation of dry matter and increase of leaf area. Therefore, taking measures to accelerate seed germination and seedling emergence could promote V. natans restoration when sowing seeds in eutrophic lakes.
Introduction
Submerged macrophytes are important components of shallow lake ecosystems and have important functions (Zhao et al. Citation2012; Azzella et al. Citation2014). Therefore, restoration of submerged macrophytes has become one of the key components in eutrophic lake recovery (Dai et al. Citation2012; Rodrigo et al. Citation2013).
Vallisneria natans, a perennial submerged macrophyte, is widely distributed in freshwaters of many Asian-Pacific countries. With strong rhizomes and thick roots, this plant forms communities on a wide range of substrates from gravel bottoms to low-density organic sediments (Xie et al. Citation2005; Wang, Zhang et al. Citation2011). This plant stabilizes sediment and inhibits pollutant release (Chen et al. Citation2006; Wang, Wang et al. Citation2011). Because of its key ecological function and aesthetic values, it is widely used in restoration of eutrophic lakes (Zhao et al. Citation2011; Ge et al. Citation2014). Although the survival rate of transplants is high, manual transplantation of V. natans is time-consuming and laborious (Ke & Li Citation2006). In addition, heavy demand for the transplants could result in damage to the source populations. Fortunately, V. natans produces a large number of seeds that are easy to collect or purchase (Chen et al. Citation2006; Ke & Li Citation2006). However, experience has shown that it is difficult to establish V. natans by broadcasting seeds in eutrophic lakes.
Sediment in shallow lakes is easily resuspended and redistributed across multiple scales. It can be caused by natural or human-induced disturbances, such as waves, storm events, aquatic animal activities, dredging, and boating (Cabaço et al. Citation2008; Lövstedt & Bengtsson Citation2008; Schallenberg et al. Citation2012). Consequently, seeds and propagules of submerged macrophytes growing in these dynamic sedimentary environments can be subjected to burial (Ke & Li Citation2006; Xiao et al. Citation2010; van Zuidam et al. Citation2014). Research has shown that sediment burial has strong and species-specific effects on plants in terms of seed germination, seedling emergence, plant growth, and species composition (Cabaço et al. Citation2008; Valdemarsen et al. Citation2011; van Zuidam et al. Citation2014).
Although effects of other ecological factors on seed germination and seedling establishment of V. natans had been well documented (Li & Cui Citation2000; Li et al. Citation2005), there are few detailed quantitative reports that address sediment burial (Ke & Li Citation2006). We hypothesized that sediment burial would be detrimental to seeds and seedlings of V. natans, and that the extent of the influence might vary between different types of sediment. In this study, therefore, we examined the effects of burial by two types of sediment on V. natans seed germination and seedling establishment through laboratory experiments, in order to provide guidance in the restoration of V. natans populations by broadcasting seeds in eutrophic lakes.
Methods
Two types of sediment, fish pond sediment and river sand, were tested in the experiments. The pond sediment, collected from a fish pond near Ludong University (37° 31′ 32.00″ N, 121° 21′ 27.60″ E), Yantai, Shandong Province, had values of organic matter, total phosphorus and total nitrogen of 72.21 ± 6.44 mg/g, 0.32 ± 0.04 mg/g, and 1.58 ± 0.21 mg/g (mean ± standard deviation), respectively. The sand, collected from the Jiahe River near Ludong University, was sieved and that with diameters ranging 0.25–0.50 mm was rinsed three times with tap water before being used in the experiment.
Mature fruits of V. natans, collected from Gucheng Lake, Nanjing, Jiangsu Province, were air-dried naturally and stored in a dry, dark place in the laboratory. After the fruits were immersed in tap water for 24 hrs, the seeds were extruded out by hand and then were rinsed three times in tap water to remove the mucus before the fully developed seeds were tested.
The experiment design included four levels of burial depth: 0 (the control), 1, 3, and 5 cm. Each treatment consisted of three replicates. Plastic containers (10 cm diameter) of 6, 7, 9, and 11 cm in height were used for the treatments of 0, 1, 3, and 5 cm burial depths, respectively. Double-gauze pads were used to ensure that the very small, non-germinating seeds were all recovered at the end of this experiment. Three cm of sediment were added to a container and a double-gauze pad was spread on the sediment surface. Twenty seeds were scattered evenly on the pad and then were covered by another layer of double gauze. Sediment was then carefully added to 3 cm below the rim of the buckets. Tap water was used to fill the containers.
A plastic water tank (62 cm deep, 96 cm long, and 53 cm wide) was filled with the fish pond sediment up to 6 cm depth and then was filled with tap water to an appropriate height. The containers were randomly placed in the tank carefully, avoiding disturbance to the seeds inside. The tank was then filled with tap water up to 5 cm below the rim and was finally placed in the laboratory under natural light conditions. The water temperature was maintained at 20 °C by an aquarium heater with automatic temperature control (± 1.0 °C). Two days later, total phosphorus and total nitrogen of the water in the tank were 0.12 ± 0.03 and 1.62 ± 0.31 mg/L, respectively. Tap water was added to the water tank every 5 days to keep the normal water level.
This experiment started on 22 March 2014 and ended after 6 weeks. The gauze pads together with seeds inside were carefully taken out of each container and the seeds were examined carefully. The viable seeds were regarded as the non-germinating and the others were considered to have germinated (Chen & Maun Citation1999). Based on this, the germination rates were obtained.
Another set of seeds of V. natans were subjected to the identical treatments above except that they were not covered with or underlaid with gauze pads. All seedlings in each container were harvested 6 weeks later and were counted by hand to derive the seedling emergence rates. Leaf number and root number of each plant were counted. The area of each leaf and the length of each root were measured for each seedling to obtain the total leaf area and total root length of this plant, respectively. Plants were dried at 60 °C for 48 hrs in an oven to obtain the dry weight of each seedling.
Two-way ANOVA (analysis of variance) by SPSS software was used to examine the main effects of burial depth and sediment type and the interaction between the two factors. The effects of burial depth were examined separately on each type of sediment using one-way ANOVA with Duncan's test at α = 0.05. An independent samples t-test was used to examine the differences between the germination rate and the corresponding seedling emergence rate at α = 0.05.
Results
Sediment type had significant effects (p < 0.01) only on dry weight and total leaf area of V. natans seedlings and did not interact with burial depth (). In contrast, burial depth had significant effects (p < 0.01) on all the responses, including seed germination rate, seedling emergence rate, leaf number, root number, shoot length, dry weight, total leaf area, and total root length (). This indicated that burial depth rather than sediment type was the dominant factor controlling seed germination and seedling establishment of V. natans in the experiments.
Table 1. Results from two-way ANOVA on changes in seed germination rate, seedling emergence rate, shoot length, dry weight, leaf number, total leaf area, root number, and total root length in response to burial depth and sediment type. Underlined p values are those that are significant at p < 0.05.
Seeds at 3 and 5 cm burial depths exhibited significantly lower germination rates than those at 0 and 1 cm burial depths when seeds were buried by either sediment type [(A)]. Compared to the control, burial at 3 cm by fishpond sediment and river sand led to significant reductions of 51.2% (p < 0.05) and 42.8% (p < 0.05), respectively [(A)]. When seeds were buried by 5 cm of fishpond sediment and river sand, the percentage changes from the control were 62.1% (p < 0.05) and 42.8% (p < 0.05) [(A)], respectively.
Figure 1. (A) Seed germination rate and (B) seedling emergence rate of V. natans under burial by fishpond sediment and river sand (mean ± SE). Values with the same lowercase letter in treatments of the same sediment type are not significantly different according to results of one-way ANOVA with Duncan's test at p = 0.05.
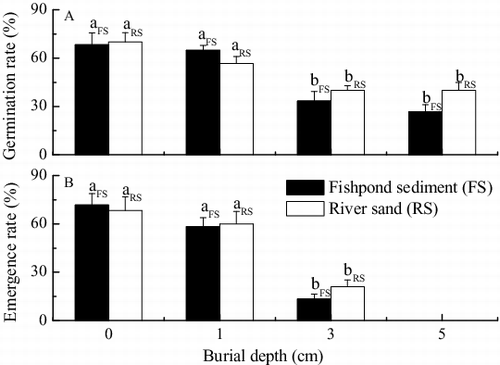
Compared to the control, burial at 1 cm by fishpond sediment and river sand resulted in decreases of 18.7% (p > 0.05) and 12.2% (p > 0.05) in seedling emergence rate, respectively [(B)]. When seeds were buried by 3 cm of fishpond sediment and river sand, the percentage changes from the control were 81.4% (p < 0.05) and 69.2% (p < 0.05), respectively. No seedling emergence was observed when seeds were buried at 5 cm by either type of sediment, although seed germination occurred [(A)]. The seedling emergence rates were significantly lower than the corresponding germination rates in treatments at 3 or 5 cm burial depth by either sediment type, yet not significantly in the two shallower treatments.
Burial depth produced strong negative effects on shoot length and dry weight of V. natans seedlings ( and ). When compared to the control, burial by 1 cm of fishpond sediment and river sand led to reductions of 15.0% (p > 0.05) and 17.8% (p > 0.05) in average shoot length [(A)], and reductions of 16.0% (p < 0.05) and 12.1% (p > 0.05) in average dry weight [(B)], respectively. When buried by 3 cm of fishpond sediment and river sand, significant reductions from the control of 46.2% (p < 0.05) and 50.4% (p < 0.05) occurred in average shoot length and 35.3% (p < 0.05) and 37.2% (p < 0.05) in average dry weight, respectively.
Figure 2. Effects of burial by fishpond sediment and river sand on (A) shoot length and (B) dry weight of V. natans seedlings after germination (mean ± SE). Values with the same lowercase letter in treatments of the same sediment type are not significantly different according to results of one-way ANOVA with Duncan's test at p = 0.05.
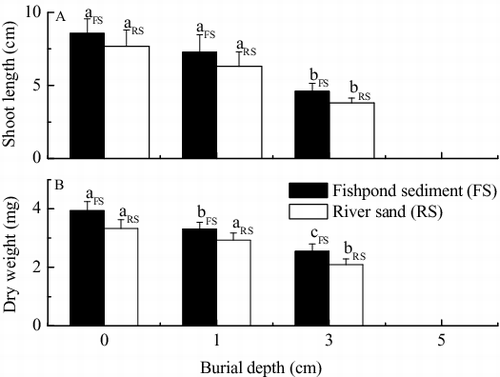
Dry weight was also significantly affected by sediment type () with weights in the fishpond sediment treatment higher than those in the river sand treatment [(B)], which indicated that fishpond sediment was more beneficial to V. natans seedlings in accumulation of dry matter than river sand.
Burial depth showed significant adverse effects on leaf number and total leaf area of V. natans seedlings after germination ( and ). There were no significant differences in average leaf number per plant between burial at 0 and 1 cm by either sediment type. Under burial at 3 cm by fishpond sediment and river sand, significant reductions of 28.0% (p < 0.05) and 15.5% (p < 0.05) from the control were observed, respectively.
Figure 3. Effects of burial by fishpond sediment and river sand on (A) leaf number per plant and (B) total leaf area per plant of seedlings of V. natans after germination (mean ± SE). Values with the same lowercase letter in treatments of the same sediment type are not significantly different according to results of one-way ANOVA with Duncan's test at p = 0.05.
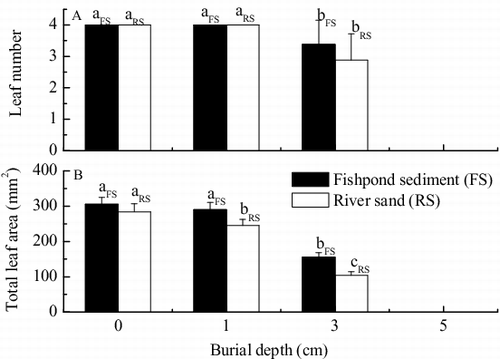
When compared to the control, burial by 1 cm of fishpond sediment and river sand resulted in reductions of 5.0% (p > 0.05) and 13.6% (p < 0.05) in average total leaf area per plant, respectively [(B)]. When buried by 3 cm of fishpond sediment and river sand, significant reductions of 49.0% (p < 0.05) and 63.3% (p < 0.05) were observed in average total leaf area per plant compared to the control, respectively.
Total leaf area was also significantly influenced by sediment type () and the values in fishpond sediment treatment were higher than those in river sand treatment [(B)], which indicated that fishpond sediment led to greater increases in leaf area than river sand.
There were no significant differences in average root number of V. natans seedlings among burial at 0, 1, and 3 cm by river sand [(A)]. However, when compared to the control, burial at 3 cm by fishpond sediment resulted in a significant reduction of 19.5% in root number [p < 0.05, (A)].
Figure 4. Effects of burial by fishpond sediment and river sand on (A) root number per plant and (B) total root length per plant of seedlings of V. natans after germination (mean ± SE). Values with the same lowercase in treatments of the same sediment type are not significantly different according to results of one-way ANOVA with Duncan's test at p = 0.05.
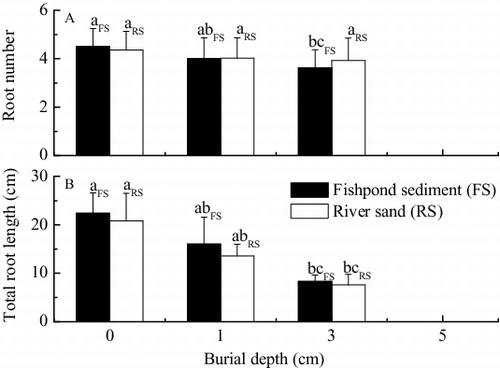
Burial depth had significant negative effects on total root length of V. natans seedlings [ and (B)]. Compared to the control, average total root length per plant decreased by 28.2% (p > 0.05) and 34.7% (p < 0.05) under burial at 1 cm by fishpond sediment and river sand, respectively. In the 3 cm treatments, burial by fishpond sediment and river sand resulted in values of average total root length per plant of 62.9% (p < 0.05) and 63.5% (p < 0.05) lower than in the control, respectively.
Discussion
It was reported that sediment type had significant effects on rate of seed germination, seed viability, biomass accumulation, and nutrient contents when seeds were subject to burial (Pan et al. Citation2012; Jarvis & Moore Citation2015). However, according to the results of our experiments (), burial depth rather than sediment type was mainly responsible for the negative effects on seed germination, which was similar to the results of Mills and Fonseca (Citation2003). Therefore, it is likely that the effect of sediment composition might have been masked by the more dominant factor, the burial depth. However, seedlings growing in fishpond sediment produced greater dry weight and larger total leaf area than those in river sand [(B) and (B)], which indicated that fertile sediment was promoted greater growth of V. natans seedlings than infertile sediment (Xie et al. Citation2007; Dong et al. Citation2011). The reason might be that combined absorption of nutrients by roots from sediment and by leaves from water could supply more nutrients to V. natans than leaves alone (Xu et al. Citation2012).
Seedling emergence of V. natans is affected by many ecological factors, including water salinity, microalgae, light intensity, water temperature, sediment type, dissolved oxygen, burial depth, and seed storage conditions (Li et al. Citation2005; Chen et al. Citation2006; Ke & Li Citation2006; Guan et al. Citation2011; Li et al. Citation2013). Our results showed that seed germination and seedling establishment of V. natans were very sensitive to burial depth ( and –). The seedling emergence rate of V. natans was above 15.0% at a depth of 3 cm by either sediment type, compared with the emergence rate of 3.0% at burial of 2 cm in the report of Ke and Li (Citation2006). Differences between the two results might be due to the differences in source of seeds, sediment types, and other experimental conditions.
Greater burial depth and per cent of organic matter could result in anoxic conditions and a decrease of redox potential in sediment (Cruz-Palacios & Tussenbroek Citation2005; Cabaço et al. Citation2008). Under such conditions, solubility of harmful metal ions would increase and sulphides could accumulate to high concentrations (van der Welle et al. Citation2006; Cabaço et al. Citation2008; van Zuidam et al. Citation2014) which could damage seeds and seedlings of V. natans.
Additionally, sediments above the buried seeds served as a physical barrier for the emergence of seedlings (Santamaria & Rodrigues-Girones Citation2002). Therefore, the amount of energy required for seedling emergence was determined not only by burial depth but also by the sediment density (Chen & Maun Citation1999; Cabaço et al. Citation2008; Ailstock et al. Citation2010). Greater burial depth or higher sediment density would cost more reserves of carbohydrate and other nutrients in the seed. Seeds of V. natans are very small and consequently are very susceptible to exhaustion of resources before emerging from sediment (Chen et al. Citation2006). In our point of view, the above are suggested as possible mechanisms through which sediment burial exerted adverse influences on seed germination and seedling establishment of V. natans.
Effects of sediment burial might be sediment-specific. Ailstock et al. (Citation2010) reported that coarse sediment had more negative effects on emergence of Ruppia maritima than fine sediment, which indicated that the adverse effects were mainly from physical obstacles in their experiments. It is known that sulphide itself is toxic to plants even at low concentrations and the toxicity could be reduced in the presence of reduced iron that can form metal sulphides. Therefore, effects of sediment burial could be moderated by the availability of free iron in sediment (van der Welle et al. Citation2006).
Tolerance of plants to sediment burial is believed to be species-specific and size-dependent. Maximum burial limits range from 2 to 5 cm for submerged macrophytes, including Zannichellia sp. (2 cm), Myriophyllum spicatum (3 cm), Potamogeton perfoliatus (3 cm), R. maritima (4 cm), and P. malaianus (5 cm, Bonis & Lepart Citation1994; Ailstock et al. Citation2010; Xiao et al. Citation2010). Low redox potentials ranging from −236 to −111 mV greatly reduced emergence of P. pusillus and Z. palustris but had no apparent effects on Chara cf. contraria (van Zuidam et al. Citation2014). Plants with larger leaves and thicker rhizomes were believed to be better able to survive burial stresses (Cabaço et al. Citation2008). Accordingly, adult V. natans might have strong capacity for coping with burial stresses. However, seedlings of V. natans just after germination were very slender (Chen et al. Citation2006). Therefore, seedling emergence of V. natans might be very susceptible to sediment burial, as shown in our experiments.
Additionally, seed germination of V. natans was generally unsynchronized and usually lasted a few weeks (Yuan & Jiang Citation2008), which could help seeds avoid being completely destroyed by sudden stresses. However, the postponed germination would increase the possibilities of being subjected to burial. Therefore, measures should be taken to accelerate seed germination and seedling emergence in restoration of V. natans when sowing seeds in eutrophic lakes.
Acknowledgements
The authors would like to thank Ming-Yang Kang and Rong-Jian Chen for their help during the experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ailstock MS, Shafer DJ, Magoun AD. 2010. Effects of planting depth, sediment grain size, and nutrients on Ruppia maritima and Potamogeton perfoliatus seedling emergence and growth. Restor Ecol. 18:574–583.
- Azzella MM, Rosati L, Iberite M, Bolpagni R, Blasi C. 2014. Changes in aquatic plants in the Italian volcanic-lake system detected using current data and historical records. Aqua Bot. 112:41–47.
- Bonis A, Lepart J. 1994. Vertical structure of seed banks and the impact of depth of burial on recruitment in two temporary marshes. Plant Ecol. 112:127–139.
- Cabaço S, Santos R, Duarte CM. 2008. The impact of sediment burial and erosion on seagrasses: a review. Estuarine Coast Shelf Sci. 79:354–366.
- Chen H, Maun MA. 1999. Effects of sand burial depth on seed germination and seedling emergence of Cirsium pitcheri. Plant Ecol. 140:53–60.
- Chen KN, Lan CJ, Shi LX, Chen WM, Xu H, Bao XM. 2006. Reproductive ecology of Vallisneria natans. J Plant Ecol. 30:487–495.
- Cruz-Palacios V, Tussenbroek VBI. 2005. Simulation of hurricane-like disturbances on a Caribbean seagrass bed. J Exp Mar Biol Ecol. 324:44–60.
- Dai Y, Jia C, Liang W, Hu S, Wu Z. 2012. Effects of the submerged macrophyte Ceratophyllum demersum L. on restoration of a eutrophic waterbody and its optimal coverage. Ecol Eng. 40:113–116.
- Dong B, Qin B, Gong Z, Wang Y. 2011. Effects of sediment amendment on Vallisneria natans growth. Chin J Ecol. 30:2726–2731.
- Ge XG, Wang GX, Chen CZ, Wang LZ. 2014. Effects on the transformation of phosphorus in sediment with growing of Vallisneria natans. Acta Ecol Sinica. 34:5802–5811.
- Guan WB, Lu F, Chen HH, He WH. 2011. Preliminary study on salt resistance ability of Vallisneria natans. Hunan Agri Sci. 13:51–53.
- Jarvis JC, Moore KA. 2015. Effects of seed source, sediment type, and burial depth on mixed-annual and perennial Zostera marina L. seed germination and seedling establishment. Estuaries Coasts 38:964–978.
- Ke X, Li W. 2006. Germination requirement of Vallisneria natans seeds: implications for restoration in Chinese lakes. Hydrobiologia. 559:357–362.
- Li Q, Xie YC, Li Q, Xie YC. 2013. Influence of low light on the growth and development of Vallisneria natans seedlings. Appl Mech Mater. 295–298:62–68.
- Li YD, Cui YQ. 2000. Germination experiment on seeds and stem tubers of Vallisneria natans in Lake Donghu of Wuhan. Acta Hydrobiol Sinica. 24:298–300.
- Li ZQ, Dan Y, Tu MH. 2005. Seed germination of three species of Vallisneria (Hydrocharitaceae), and the effects of freshwater microalgae. Hydrobiologia. 544:11–18.
- Lövstedt C, Bengtsson L. 2008. The role of non-prevailing wind direction on resuspension and redistribution of sediments in a shallow lake. Aqua Sci. 70:304–313.
- Mills KE, Fonseca MS, 2003. Mortality and productivity of eelgrass Zostera marina under conditions of experimental burial with two sediment types. Mar Ecol Prog Ser 255:127–134.
- Pan Y, Xie Y, Li F, Pan B. 2012. Morphological and physiological responses to burial depth and sediment type in the wetland macrophyte Miscanthus sacchariflorus. Fundament Appl Limnol. 180:271–277.
- Rodrigo MA, Rojo C, Alonso-Guillen JL, Vera P. 2013. Restoration of two small Mediterranean lagoons: the dynamics of submerged macrophytes and factors that affect the success of revegetation. Ecol Eng. 54:1–15.
- Santamaria L, Rodriguez-Girones MA. 2002. Hiding from swans: optimal burial depth of sago pondweed tubers foraged by Bewick's swans. J Ecol. 90:303–315.
- Schallenberg M, Goff J, Harper MA. 2012. Gradual, catastrophic and human induced environmental changes from a coastal lake, southern New Zealand. Sediment Geol. 273:48–57.
- Valdemarsen T, Wendelboe K, Egelund JT, Kristensen E, Flindt MR. 2011. Burial of seeds and seedlings by the lugworm Arenicola marina hampers eelgrass (Zostera marina) recovery. J Exp Mar Biol Ecol. 410:45–52.
- van der Welle MEW, Cuppens M, Lamers LPM, Roelofs JGM. 2006. Detoxifying toxicants: interactions between sulfide and iron toxicity in freshwater wetlands. Environ Toxicol Chem. 25:1592–1597.
- van Zuidam BG, Cazemier MM, Geest GJV, Roijackers RMM, Peeters ETHM. 2014. Relationship between redox potential and the emergence of three submerged macrophytes. Aqua Bot. 113:56–62.
- Wang C, Zhang S, Wang P, Hou J, Qian J, Ao Y, Lu J, Li L. 2011. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere. 84:136–142.
- Wang LZ, Wang GX, Yu ZF, Hou BB, Ge XG, Chen QM, Li ZG. 2011. Effects of Vallisneria natans on sediment phosphorus fractions and transfer during the growth period. J Lake Sci. 23:753–760.
- Xiao C, Wang X, Xia J, Liu G. 2010. The effect of temperature, water level and burial depth on seed germination of Myriophyllum spicatum and Potamogeton malaianus. Aqua Bot. 92:28–32.
- Xie Y, Li C, Liu Z, Chen G, Lei Z. 2007. Effects of sediments on the growth and morphology of Vallisneria natans. J Agro-Environ Sci. 26:1269–1272.
- Xie YH, An SQ, Yao X, Xiao KY, Zhang C. 2005. Short-time response in root morphology of Vallisneria natans to sediment type and water column nutrient. Aqua Bot. 81:85–86.
- Xu S, Li X, Zhong P, Liu Z. 2012. The uptake of nitrate and ammonium by the root of Vallisneria natans. Ecol Sci. 31:312–317.
- Yuan L, Jiang L. 2008. Effects of different concentration of NaCl solution on seed germination of Vallisneria spiralis, Vallisneria spinulosa and Ottelia alismoides. Anhui Agric Sci Bull. 14:77–80.
- Zhao FB, Xu HT, Wang LQ, Ji GH. 2011. Effects of environmental factors on Vallisneria natans restoration in Lake Dianshan. Ecol Environ Sci. 20:1097–1101.
- Zhao JG, He FF, Chen ZH, Li HJ, Hu JM, Lin FP. 2012. Effect of culture and extract solutions of macrophytes on the growth of three common algae. J Freshw Ecol. 27:367–379.
