Abstract
Brook trout (Salvelinus fontinalis) are generally restricted to headwaters in New York tributaries of Lake Ontario. In only a few streams are brook trout abundant in lower stream reaches that are accessible to adult Pacific salmonids migrating from the lake. Consequently, because of the rarity of native brook trout populations in these lower stream reaches it is important to understand how they use stream habitat in sympatry with juvenile Pacific salmonids which are now naturalized in several Lake Ontario tributaries. In this study, we examined the seasonal (spring, summer, and fall) habitat use of brook trout and juvenile steelhead (Oncorhynchus mykiss) in Hart Brook, a tributary of eastern Lake Ontario. We found interspecific, intraspecific, and seasonal variation in habitat use. Subyearling steelhead were associated with faster water velocities than subyearling brook trout and, overall, had the least habitat similarity to the other salmonid groups examined. Overyearling brook trout and yearling steelhead exhibited the greatest degree of habitat selection and habitat selection by all four salmonid groups was greatest in summer. The availability of pool habitat for overyearling salmonids may pose the largest impediment to these species in Hart Brook.
Introduction
The decline of brook trout (Salvelinus fontinalis) populations throughout much of their native range in eastern North America is well documented and has received considerable attention (Hudy et al. Citation2008). Among the many factors that are thought to have contributed to the decline of brook trout is interspecific interactions with introduced non-native salmonids, particularly brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss). Brown trout have been shown to adversely impact brook trout and intensive stocking of brown trout in some streams may eliminate wild brook trout in less than a decade (McKenna et al. Citation2013). Similarly, rainbow trout have been shown to have a depressant effect on brook trout in the southern part of their native range (Larsen & Moore Citation1985; Clark & Rose Citation1997). Because of concerns of impacts of naturalized rainbow trout on native brook trout populations interspecific studies in the northern part of the range of brook trout were carried out in Newfoundland (Cunjak & Green Citation1983).
Steelhead, a migratory form of rainbow trout, were first introduced into the Great Lakes in the 1870s and have become naturalized in each of the five lakes (MacCrimmon & Gots Citation1972). Steelhead currently spawn in several Great Lakes tributaries that historically supported brook trout. In most of these streams, brook trout are now abundant only in the headwaters and few are present in the lower stream reaches where juvenile steelhead are abundant (MacKay Citation1963). In tributaries of Lake Ontario, juvenile steelhead dominate the salmonid fish assemblage (Johnson et al. Citation2013). Perhaps, because of the longitudinal separation of occurrence between steelhead and brook trout in most Great Lakes tributaries, there is no information on their interspecific association in sympatry Rose (Citation1986). However, Rose (Citation1986) examined growth and habitat use of rainbow trout and brook trout in the Goulais River, a Lake Superior tributary, but it is unclear if the rainbow trout were steelhead. Although there is no evidence of different habitat use between rainbow trout and steelhead (Narum et al. Citation2008), interspecific habitat associations of steelhead with other species in lotic ecosystems only involve juvenile fish. Most studies of brook trout and rainbow trout interspecific associations have included adult rainbow trout. In the studies where the size of brook trout and rainbow trout has been reported, the average size of overyearling rainbow trout is larger than that of overyearling brook trout (Cunjak & Green Citation1983; Lohr & West Citation1992). Because only juvenile steelhead are present in Great Lakes tributaries, it is likely that the size of overyearlings may be less than is generally found in studies that examined interspecific habitat associations between rainbow trout and brook trout. Due to the absence of adult steelhead and because brook trout fry (fall spawners) emerge earlier than steelhead (spring spawners) in Great Lakes tributaries, brook trout should be larger than steelhead as both subyearlings and overyearlings. Because larger size often confers a competitive advantage for stream salmonids (Nakano Citation1995), this could aid brook trout in competitive interactions with juvenile steelhead when occurring sympatrically.
Brook trout are present in several New York tributaries of Lake Ontario but are not common in stream sections that are accessible to Pacific salmonids migrating from Lake Ontario. Although brook trout are not common in streams where Chinook salmon (Oncorhynchus tshawytscha) and coho salmon (O. kisutch) are present, they do occur sympatrically in a few streams with juvenile steelhead. We examined the habitat use of brook trout and juvenile steelhead during spring, summer, and fall in a tributary of Lake Ontario. We sought to determine if both intraspecific and interspecific differences in habitat use occurred and if these differences varied by season.
Methods
The habitat use of subyearling (0+) and overyearling (≥1+) brook trout and juvenile steelhead (0+ and 1+) was examined during spring, summer, and fall in Hart Brook (43° 49′ N, 76° 01′ W) in Jefferson County, NY. Spring habitat observations were made in late May prior to emergence of steelhead so only two seasons of observations, summer and fall, were recorded for subyearling steelhead. Hart Brook is a second-order stream that discharges into Sandy Creek, a tributary of eastern Lake Ontario. Brook trout and juvenile steelhead are the dominant fish species in the stream but white sucker (Catostomus commersoni) and cutlip minnow (Exoglossum maxillingua) are also present. Hart Brook is small (< 2.5 m average width) and has a gravel-cobble substrate. Stream gradient was 2.2% and was estimated using a clinometer. A combination of excellent riparian overstory and ground water input help maintain summer water temperatures below 17 °C. Low summer water temperatures support a viable population of wild brook trout that occurs sympatrically with juvenile steelhead. Small headwater streams such as Hart Brook are thought to be preferred spawning areas for brook trout because of the absence of predators and potential competitors (Petty et al. Citation2005). The small size of Hart Brook probably restricts use by Chinook salmon and coho salmon that also have access to the stream.
The representative study reach was selected after examining approximately 3 km (60%) of the stream. The 0.5 km study reach was selected based on proportional amounts of pools, riffles, and runs compared to the rest of the stream. Habitat assessments were done in late May (spring), late July (summer), and mid-October (fall). During each season both fish habitat use and available habitat were quantified. Fish habitat use was examined using the spot-electrofishing technique which is highly effective in small streams (Bovee Citation1986) that are too shallow for other methods of observations such as snorkeling (Heggenes et al. Citation1990). In an effort to minimize fish disturbance spot-electrofishing was carried out moving upstream in a slow methodical manner and sample sites were at least 3 m apart. A weighted numbered buoy was placed at the site of each fish collection and the species and age group were recorded. The habitat variables that were recorded at the site of each buoy included water depth, water velocity, substrate size, and the amount of cover. Water depth was measured with a calibrated wading rod and water velocity (taken at a depth of 60% from the water's surface) with a Marsh-McBirney model 201d digital flow meter. Substrate size was estimated visually using the modified Wentworth particle size scale with values ranging from 1 (detritus) to 8 (bedrock) (Orth Citation1983). Cover was estimated visually at increments of 5% within a radius of four fish lengths from the location of the buoy. Cover was classified as substrate, surface turbulence, and vegetative. For a more detailed description see Johnson & Nack (Citation2013). Using fish length as a determinant for the effective area of cover use by a fish allowed for a larger area to be considered for yearlings and overyearlings since they likely use cover over a larger area than subyearlings (Johnson et al. Citation1992). Available habitat was determined from 20 transects spaced 20 m apart within the study reach. Water depth, water velocity, substrate size, and the amount of cover were recorded at six stations spaced equidistant along each transect.
Variables for salmonid habitat use and available habitat were not normally distributed. Transformation of the data did not improve normality so a nonparametric analysis was performed. Differences in intraspecific and interspecific habitat use and between salmonid habitat and available habitat were assessed with a Kruskal–Wallis test using Statistix 8.2 software (Tidepool Scientific, Tallahassee, FL, USA). When differences were detected, Dunn's multiple comparison test was used to differentiate significant groups. Principal component analysis (PCA) ordination was used to determine the associations of salmonid habitats and available habitat (ter Braak & Smilauer Citation2002). We assessed habitat selection based on the distance between fish habitat centroids and available habitat centroids for each season. Neural networks (NN) typically produce more effective predictive models than many classical modeling techniques like linear regression or discriminant analysis, particularly when relationships are multivariate and nonlinear. We trained simple backpropagation NN models (with one hidden layer, NeuroShell 2.0 software, Wards Systems Group, Inc., Frederick, MD) to distinguish habitats supporting brook trout or rainbow trout from those representative of available habitat, for each of the sampled seasons. There was one neuron for each independent habitat variable (seven for spring and summer and six for fall), 15 (fall) or 16 (spring and summer) hidden layer neurons (see McKenna & Johnson, Citation2011), and one output neuron. Twenty percent of the data was held out as a validation data set, which provides the models with greater ability to extrapolate beyond the training data and prevents overfitting. Leaning (0.1) and inertia (0.1) rates were implemented to ensure global, rather than local, convergence during training. Weight (with sign) of each model input variable was determined by tracing the path and sequence of changes to each input value through the NN (McKenna Citation2005). A significance level of α = 0.05 was used for all comparisons.
Results
A total of 547 salmonid habitat observations were made, including 158 on subyearling brook trout, 157 on overyearling brook trout, 151 on subyearling steelhead, and 81 on yearling steelhead (). The mean size of subyearling brook trout varied the most among seasons, ranging from 43 mm in the spring to 74 mm in the fall (). Subyearling brook trout were, on average, 19 mm and 11 mm larger than subyearling steelhead during the summer and fall, respectively. The mean size of overyearling brook trout was slightly larger, about 18 mm, than that of yearling steelhead.
Table 1. Number of habitat use observations, mean total length, and length range of brook trout and juvenile steelhead as well as available habitat by season in Hart Brook, NY, USA.
Water depths during the spring were significantly greater than during the other two seasons, otherwise, there was little variation in available habitat in Hart Brook among seasons (). Overyearling brook trout and yearling steelhead used deeper areas than were commonly found within the stream reach (). Subyearling steelhead occupied significantly faster water velocities than were commonly found in the study reach during both the summer and fall. Compared to available substrate, no selection for substrate size was evident for any of the four groups of salmonids for any season. All four salmonid groups used areas with significantly more cover than was commonly found within the study reach (). Overyearling brook trout used deeper areas with more cover than underyearling brook trout and underyearling steelhead (). In only one comparison (cover with subyearling brook trout during spring) was the difference not significant. During summer and fall, subyearling steelhead occupied significantly faster areas than the other three groups of salmonids. Overyearling brook trout and yearling steelhead used deeper areas in the fall than during spring and summer (). Overyearling brook trout also were found at faster water velocities in spring compared to summer and fall. Subyearling brook trout occupied faster areas with more cover in spring than during summer or fall.
Table 2. Statistical analysis of mean seasonal habitat use (depth, velocity, substrate index, and percent cover) between seasons for steelhead and brook trout and available habitat (AH) for Hart Brook. Values followed by a * significantly differ (p < 0.05). BT0 = subyearling brook trout, BT1 = overyearling brook trout, ST0 = subyearling steelhead, and ST1 = yearling steelhead.
Table 3. Statistical analysis of mean habitat use (depth, velocity, substrate index, and percent cover) within each season for steelhead and brook trout and available habitat (AH) for Hart Brook. Values followed by a * significantly differ (p < 0.05). BT0 = subyearling brook trout, BT1 = overyearling brook trout, ST0 = subyearling steelhead, and ST1 = yearling steelhead.
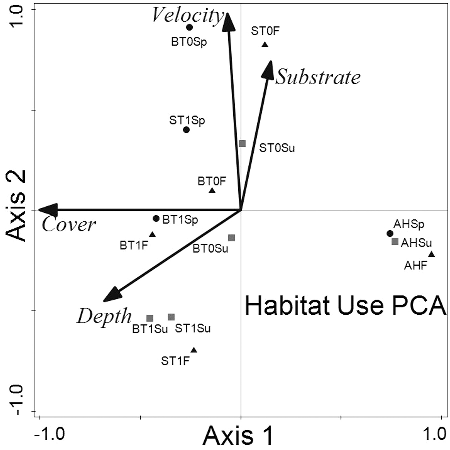
PCA axis 1 explained 97.3% of the variation in habitat variables and axis 2 explained 2.7% (). PCA showed that the habitat use of overyearling brook trout and yearling steelhead was most similar. Compared to subyearlings, the larger salmonids tended to be associated with deeper areas with more cover. The habitat used by subyearling steelhead was least similar to the other three salmonid groups and was most divergent from yearling steelhead (). Salmonid habitat use diverged most in the fall and was largely due to differences in habitat use between subyearling steelhead with yearling steelhead and overyearling brook trout. Overyearling brook trout exhibited the greatest amount of habitat selection followed by yearling steelhead. Habitat selection for subyearling brook trout and subyearling steelhead was similar. For the four salmonid groups (i.e. subyearling brook trout, overyearling brook trout, subyearling steelhead, yearling steelhead) habitat selection was greatest in summer ().
Figure 1. Ordinal representation of habitat data using principal component analysis. BT0 = subyearling brook trout, BT1 = overyearling brook trout, ST0 = subyearling steelhead, ST1 = yearling steelhead, AH = available habitat, Sp = spring, Su = summer, and F = fall.
The NN models performed well, explaining 76% to 82% of variation in each data set. Variables weightings showed great depths and high total cover but low substrate cover were most influential in spring; larger size substrate with low overhead and substrate cover were most influential in summer; and depth, overhead cover, and velocity were most influential in fall ().
Table 4. Variable influences and performance measures for neural networks predicting presence of salmonids or available habitat, based on a suite of habitat conditions. Mean weighting of each habitat variable is shown for each seasonal model. Adjusted coefficient of determination and mean squared error are provided after each season label.
Discussion
In small streams such as Hart Brook, the lack of habitat diversity can restrict the ability of sympatric species to segregate into habitats that are generally available in larger lotic systems (Matthews Citation1986; Peres-Neto Citation2004). However, Hart Brook had sufficient amounts of habitat variability whereby distinct differences in intraspecific and interspecific fish habitat use could be determined. Available habitat in Hart Brook varied little with season, although stream discharge was higher in the spring which resulted in greater water depths than during summer and fall. However, the availability of deeper areas in the spring did not appear to influence fish habitat selection as none of the four salmonid groups were associated with deeper areas in the spring when compared to other seasons.
Although rainbow trout are considered to have adversely affected brook trout populations in the southern portion of their range (Larson & Moore Citation1985), the presence of rainbow trout does not seem to influence the habitat use of brook trout (Cunjak & Green Citation1983). Both subyearling and overyearling brook trout tend to occupy lower velocity microhabitats both in allopatry (Johnson et al. Citation1992; Sotiropoulos et al. Citation2006) and in sympatry with rainbow trout (Rose Citation1986; Magoulick & Wilzbach Citation1998a). In Hart Brook, brook trout were associated with lower water velocities than yearling steelhead only during spring. However, both subyearling and overyearling brook trout occupied slower water velocities than subyearling steelhead in Hart Brook during summer and fall.
In Hart Brook, water depth and the amount of cover were the major habitat variables influencing habitat use by overyearling salmonids. Magoulick & Wilzbach (Citation1997) found that water depth was the most important variable governing habitat use of overyearling brook trout and rainbow trout in a small stream in Pennsylvania and that adult rainbow trout were found in deeper water than adult brook trout. However, Lohr & West (Citation1992) observed no difference in the depths used by adult brook trout and rainbow trout in a southern Appalachian stream. In Hart Brook, overyearling brook trout were found in deeper areas than yearling steelhead during each season but the difference was only significant in the spring. Although subyearling brook trout have been shown to occupy deeper areas than subyearling rainbow trout (Rose Citation1986; Lohr & West Citation1992), we observed no difference in the depths used by subyearlings in Hart Brook.
Ontogenetic differences in habitat use were greater than those between species in Hart Brook. Lohr & West (Citation1992) also found that the habitat use of adult brook trout and rainbow trout was more similar than between subyearling fish of each species. In Hart Brook the main habitat variables that differed between yearling and overyearling salmonid habitat use and subyearling habitat use were depth and cover. Similar to previous studies (Lewis Citation1969; Rose Citation1986) overyearling brook trout and yearling steelhead were associated with deeper areas with more cover than subyearlings. Although subyearling brook trout and subyearling steelhead occupied similar depth, cover, and substrates in Hart Brook, steelhead were associated with faster water velocities during all seasons. This contrasts with the findings of Rose (Citation1986) who found that there was no spatial segregation of brook trout and rainbow trout during summer. However, Rose (Citation1986) attributed the lack of segregation to the small size (i.e. <40 mm) of the subyearling rainbow trout at this time suggesting that they were not yet large enough to adapt to current velocities. The average size of subyearling steelhead in Hart Brook during summer was 51 mm and thereby large enough to deal with water currents as suggested by Rose (Citation1986).
There are several factors that govern interspecific associations of salmonids in streams including the relative temperature tolerance of each species, the type of habitat available, and fish size. Although introduced rainbow trout are widely considered to have caused declines in brook trout in eastern North America, Magoulick and Wilzbach (Citation1998a) have shown that brook trout are more aggressive and dominate rainbow trout at water temperatures of 13 °C and 18 °C. They speculated that higher temperatures could allow rainbow trout to dominate brook trout but emphasized that these higher temperatures would be at the upper tolerance for each species. In a study examining the behavior and dominance of brook trout and rainbow trout under laboratory conditions at 14 °C, rainbow trout were found to be more mobile, aggressive, and successful drift feeders than brook trout but, overall, in sympatry, neither specifically established social dominance over the other (Helfrich et al. Citation1982). Cunjak & Green (Citation1983) also found no evidence of social dominance by either brook trout or rainbow trout in sympatry but did find evidence suggesting that interactive segregation occurred. Studies examining habitat associations between these species have been carried out in lotic ecosystems ranging from small streams to small rivers that have different in-stream habitat conditions and this is thought to be related to the varied findings among the studies (Magoulick & Wilzbach, Citation1998b). Fish size may also affect interspecific habitat associations and could possibly explain differences between our observations with those of Magoulick and Wilzbach (Citation1997) regarding water depths used by adult rainbow trout while in sympatry with brook trout.
It is interesting that, given the generally held view that rainbow trout have had a negative impact on brook trout populations, no studies have been conducted to specifically examine predatory associations between these species in sympatry. However, McGrath and Lewis (Citation2007) examined predation as a possible mechanism for displacement of greenback cutthroat trout (Oncorhynchus clarkii stomias) by brook trout and concluded that it occurred at levels insufficient to account for population declines in Colorado streams. Although not a focus of our study, we examined the stomachs of 10 overyearling brook trout and 10 yearling steelhead from Hart Brook in June. No salmonid remains were found in the stomachs of yearling steelhead but one brook trout contained a subyearling brook trout and another contained a subyearling steelhead. These observations suggest that in small streams both habitat and predation may play an important role in interspecific interactions between brook trout and rainbow trout.
In Hart Brook, overyearling brook trout exhibited the highest degree of habitat selection, closely followed by yearling steelhead. Habitat selection was largely based on the use of pool habitat that provided ample cover for larger salmonids. Pool habitat, although present throughout the study reach, was a minor component of the available habitat, which is characteristic for small streams such as Hart Brook (Magoulick & Wilzbach Citation1997). The habitat use of subyearling steelhead diverged the most from the other salmonid groups and was largely due to their use of shallow and fast habitat which is typical for this age group (Everest & Chapman Citation1972). In small streams such as Hart Brook densities of small fishes are often highest in riffle habitats (Bratten & Berry Citation1997). Seasonally, the habitat use of the four groups of salmonids was most divergent in the fall, which aligns with the findings of Rose (Citation1986) who observed no spatial segregation between brook trout and rainbow trout prior to August in the Goulais River, Ontario.
Although overyearling brook trout and yearling steelhead used similar habitats in Hart Brook, differences in habitat use were observed between the subyearlings of each species. The greater rheophilic abilities of steelhead were only evident in habitat use of subyearling fish compared to habitat use of the other groups of salmonids examined. With the exception that yearling steelhead did not occupy deeper areas than overyearling brook trout, our observations on interspecific habitat associations between brook trout and juvenile steelhead were consistent with studies that examined habitat use of sympatric brook trout and rainbow trout. Based on our observations of salmonid habitat use in Hart Brook it is likely that the availability of pool habitat, the preferred habitat for both overyearling brook trout and yearling steelhead, may pose the largest limitation to these fish populations. Interspecific habitat overlap among yearling salmonids was highest during summer suggesting that competition may be most acute at this time. However, our data are insufficient to attempt to ascertain the effects of the presence of yearling steelhead on brook trout in pools.
Throughout much of their native range, small cold headwater streams provide refugia for brook trout populations. However, these streams face a variety of stressors from anthropogenic activities, including climate change (Hudy et al. Citation2008). In Hart Brook, removal of riparian canopy that could increase stream temperature or in-stream disturbance that reduces pool habitat could influence interspecific interactions between brook trout and juvenile steelhead.
Acknowledgements
This article is contribution 1996 of the USGS Great Lakes Science Center. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bovee KD. 1986. Development and evaluation of habitat suitability criteria for use in the instream flow incremental methodology. Washington (DC): U.S. Fish and Wildlife Service. (Instream Flow Information Paper No. 21. Biology Report, 86).
- Bratten PJ, Berry CR Jr. 1997. Fish associations with four habitat types in a South Dakota prairie stream. J Freshw Ecol. 12:477–489.
- Clark ME, Rose KK. 1997. Factors affecting competitive dominance of rainbow trout over brook trout in southern Appalachian streams: implications of an individual based model. Trans Am Fish Soc. 126:1–20.
- Cunjak RA, Green JM. 1983. Habitat utilization by brook char (Salvelinus fontinalis) and rainbow trout (Salmo gairdneri) in Newfoundland streams. Can J Zool. 61:1214–1219.
- Everest FH, Chapman DW. 1972. Habitat selection and spatial interaction by juvenile Chinook salmon and steelhead trout in two Idaho streams. J Fish Res Board Can. 29:91–100.
- Heggenes J, Brabrand A, Saltveit SJ. 1990. Comparison of three methods for studies of stream habitat use by young brown trout and Atlantic salmon. Trans Am Fish Soc. 119:101–111.
- Helfrich LA, Wolfe Jr. JR, Bromley PT. 1982. Agnostic behavior, social dominance, and food consumption of brook trout and rainbow trout in a laboratory stream. Proc Annu Conference Southeast Assoc Fish Wildl Agencies. 36:340–350.
- Hudy M, Thieting TM, Gillespie N, Smith EP. 2008. Distribution, status, and land use characteristics of subwatersheds within the native range of brook trout in the eastern United States. North American J Fish Manag. 28:1069–1085.
- Johnson JH, Dropkin DS, Shaffer PG. 1992. Habitat use by a headwater stream fish community in north central Pennsylvania. Rivers. 3:69–79.
- Johnson JH, McKenna Jr. JE, Douglas KA. 2013. Movement and feeding ecology of recently emerged steelhead in Lake Ontario tributaries. J Appl Ichthyology. 29:221–225.
- Johnson JH, Nack CC. 2013. Habitat use of American eel (Anguilla rostrata) in a tributary of the Hudson River, New York. J Appl Ichthyology. 29:1073–1079.
- Larson GL, Moore SE. 1985. Encroachment of exotic rainbow trout into stream populations of native brook trout in the southern Appalachian mountains. Trans Am Fish Soc. 114:195–203.
- Lewis SL. 1969. Physical factors influencing fish populations in pools of a trout stream. Trans Am Fish Soc. 98(1):14–19.
- Lohr SC, West JL. 1992. Microhabitat selection by brook and rainbow trout in a southern Appalachian stream. Trans Am Fish Soc. 121:729–736.
- MacCrimmon HR, Gots BL. 1972. Rainbow trout in the Great Lakes. Toronto: Ontario Ministry of Natural Resources.
- MacKay HH. 1963. Fishes of Ontario. Toronto: Ontario Ministry of Natural Resources.
- Magoulick DD, Wilzbach MA. 1997. Microhabitat selection by native brook trout and introduced rainbow trout in a small Pennsylvania stream. J Freshw Ecol. 12:607–614.
- Magoulick DD, Wilzbach MA. 1998a. Effect of temperature and macrohabitat on interspecific aggression, foraging success, and growth of brook trout and rainbow trout pairs in laboratory streams. Trans Am Fish Soc. 127:708–717.
- Magoulick DD, Wilzbach MA. 1998b. Are native brook charr and introduced rainbow trout differentially adapted to upstream and downstream reaches? Ecol Freshw Fish. 7:167–175.
- Matthews WJ. 1986. Fish faunal “breaks” and stream order in the eastern and central United States. Environ Biol Fish. 17:81–92.
- McGrath CC, Lewis Jr. WM. 2007. Competition and predation as mechanisms for displacement of greenback cutthroat trout by brook trout. Trans Am Fish Soc. 136:1381–1392.
- McKenna Jr. JE. 2005. Application of neural networks to prediction of fish diversity and salmonid production in the Lake Ontario basin. Trans Am Fish Soc. 134:28–43.
- McKenna Jr. JE, Johnson JH. 2011. Landscape models of brook trout abundance and distribution in lotic habitat with field validation. North American J Fish Manag. 31:742–756.
- McKenna Jr. JE, Slattery MT, Clifford KM. 2013. Broad-scale patterns of brook trout responses to introduced brown trout in New York. N Am J Fish Manage. 33:1221–1235.
- Nakano S. 1995. Individual differences in resource use, growth and emigration under the influence of a dominance hierarchy in fluvial red-spotted masu salmon in a natural habitat. J Anim Ecol. 64:75–84.
- Narum SR, Zendt JS, Graves D, Sharp WR. 2008. Influence of landscape on resident and anadromous life history types of Oncorhynchus mykiss. Can J Fisheries Aquatic Sci. 65:1013–1023.
- Orth DJ. 1983. Aquatic habitat measurements. In: Nielsen LA, Johnson DL, editors. Fisheries techniques. Bethesda (MD): American Fisheries Society; p. 61–84.
- Perez-Neto PR. 2004. Patterns in the occurrence of fish species in streams: the role of site suitability, morphology and phylogeny versus species interactions. Oecologia. 140:352–360.
- Petty JT, LaMothe PJ, Mazik PM. 2005. Spatial and seasonal dynamics of brook trout populations inhabiting a central Appalachian watershed. Trans Am Fish Soc. 134:572–587.
- Rose GA. 1986. Growth decline in subyearling brook trout (Salvelinus fontinalis) after emergence of rainbow trout (Salmo gairdneri). Can J Fisheries Aquatic Sci. 43:187–193.
- Sotiropoulos JC, Nislow KH, Ross MR. 2006. Brook trout, Salvelinus fontinalis, microhabitat selection and diet under low stream flows. Fisheries Manag Ecol. 13:149–155.
- ter Braak CJF, Smilauer P. 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Available from: Microcomputer Power, Ithaca, New York.
