Abstract
Sampling in springs has several technical problems due to their reduced dimensions and habitat heterogeneity. A standardized quantitative method for sampling crenic macroinvertebrates has never been proposed. The aim of this study was to compare different sampling methods and consider their environmental impacts. First, we present a review of sampling methods found in the literature and discuss their advantages and disadvantages with respect to selective collection of the target community and habitat disturbance. Altogether, 10 different methods have been reported, the use of nets being the most common protocol. Second, we report the results of macroinvertebrate samplings performed in three springs, each surveyed twice, using three different methods (multi-habitat proportional hand net, baited traps, and vegetation washing), in order to compare their effectiveness in collecting macroinvertebrates. Overall, 32 macroinvertebrate taxa, mostly identified at family level, were collected in the sampled springs. Significant differences in abundances were found using different methods, while results for community structure were comparable between the hand net sampling and the combined use of the other two methods, notwithstanding slight differences in the composition of Coleoptera and Diptera assemblages. The hand net, with a multi-habitat proportional approach, yielded more thorough results, making it suitable for biodiversity inventories but having some potentially negative effects on spring habitats. Traps and vegetation washing are also reliable methods with negligible impacts on spring ecosystems that can be conveniently used in ecological studies.
Introduction
In spite of their small size, the ‘mosaic’ ecotonal structure of springs results in a number of microhabitats that sustain high species richness (Cantonati et al. Citation2012). Several studies have highlighted the great biodiversity of macroinvertebrates in springs as well as the presence of rare and endemic species (e.g., Takhteev et al. Citation2010; Maiolini et al. Citation2011; Kubíková et al. Citation2012; Martin & Brunke Citation2012; Spitale Citation2012; Spitale et al. Citation2012). The uses and limitations of various methods for sampling benthic macroinvertebrates have been extensively discussed (e.g., Davies Citation2001). In contrast, despite great interest in spring biodiversity, a standardized, quantitative method for sampling crenic macroinvertebrate taxa has never been developed. Cantonati et al. (Citation2007) suggested effective methods for collecting spring invertebrates but a variety of methods have been adopted in crenic investigations. The technical difficulties of sampling in springs were well summarized by Gerecke et al. (Citation2007): ‘The main dilemma of limnological studies in springs probably derives from the generally reduced dimensions and extreme heterogeneity of the habitat.’ Furthermore, many authors (Gerecke et al. Citation1998; Zollhöfer Citation1999; Myers & Resh Citation2002; Staudacher & Füreder Citation2007; Tichá et al. Citation2012) noted that some survey methods, which implied samplings in all microhabitats, could be destructive to the environment and the biota of these fragile ecosystems.
Previous studies on macroinvertebrates in different aquatic ecosystems have shown that sampling methods affect the data precision and the selection of sampling technique is among the most important decisions for freshwater studies (Carter & Resh Citation2001). Standardizing the sampling procedure is thus necessary in order to obtain precise and comparable biological data for spring surveys and assessment. The aim of this study was to summarize the literature on sampling methods in springs and to compare the effectiveness of some semi-quantitative sampling methods, taking into account their potential impacts on spring habitat and biota.
Methods
Three rheocrenic, permanent springs located between 474 and 589 m above sea level in the Mount Prinzera protected area (44° 37' N; 10° 03' E), an ophiolitic outcrop in northern Italy near Parma, were selected for the study. Samplings were carried out in two seasons (May–June and August–September 2014). Macroinvertebrates were collected using three methods.
Multi-habitat proportional net – a hand net (frame dimensions: 10 × 10 cm; mesh size: 255 µm) was used to collect 10 replicates at each site. For each replicate, a substrate area equivalent to the net frame was sampled for 15 seconds. The number of replicates for each microhabitat was proportional to its percentage cover in the spring, up to a total number of 10 replicates. For example, given a substrate composition of 50% gravel, 30% mosses, and 20% silt, five samples were collected in gravel, three in mosses, and two in silt. All 10 replicates were composited into a single sample.
Vegetation washing – about 250 mL volume of submerged vegetation was collected and washed in the laboratory through a 255-µm sieve.
Traps – traps were constructed following Bottazzi et al. (Citation2011). They were obtained from polyvinyl chloride (PVC) centrifuge tubes (length 100 mm; diameter 28 mm), by cutting the conical end, drilling an opening (0.5 cm of diameter) in its apex, and inserting it, inverted, into one end of the tube. The other end of the tube was closed with a 50 μm net. These traps were filled with washed and sieved gravel (0.3–1.0 cm). Traps were baited with meat, placed at the sediment–water interface, and covered with stones to keep them in place for 7–8 days. Two pairs of traps were deployed in each spring: one pair at the source and the other 2 m downstream. For each pair, one trap was placed with the opening in the flow direction and the other in the opposite direction.
Vegetation washing and macroinvertebrate trapping were performed two weeks after the net sampling.
In the laboratory, collected material was washed through a 255-µm sieve and fixed with 90% ethanol. Macroinvertebrates were identified to the family level for Plecoptera, Trichoptera, Ephemeroptera, Coleoptera, Diptera, and Crustacea but to a coarser taxonomic level for Hirudinea, Gastropoda, Collembola, Hydrachnidiae, Odonata, and Oligochaeta.
Differences in organism abundance between the three methods were tested with an analysis of variance (ANOVA). Logarithmic transformation was used to obtain normal distributions and homogeneity of data, as determined by Shapiro and Bartlett tests (Legendre & Legendre Citation2012). Non-metric multidimensional scaling (NMDS, Legendre & Legendre Citation2012) was performed to evaluate possible differences in community structures determined by different methods. Centroids of methods were fitted on NMDS plots in order to identify these differences and then tested with Permanova (Anderson & Walsh Citation2013). Differences between methods were assessed by considering both the three methods individually (net, vegetation washing, and traps) and the combination of vegetation washing and traps versus net. Differences were also tested for each of the most diverse insect orders (Trichoptera, Coleoptera, and Diptera). Statistical analyses were performed using R software (version 3.0.0, R Development Core Team Citation2013) and Vegan (version 2.0-7, Oksanen et al. Citation2013).
Results
Ten different methods were found in the literature. The use of hand or kick net was by far the most commonly used protocol (). Overall, 32 taxa were collected in our survey (). Insect orders with the greatest number of families were Diptera (9), Trichoptera (7), and Coleoptera (6). Chironomidae was the most abundant taxon collected with the net (1029 specimens) and vegetation washing (60), whereas traps collected the greatest number of Niphargidae (293). Lepidostomatidae, Chironomidae, Ceratopogonidae, Hirudinea, and Gastropoda were found in all samples collected by the net. The maximum number of taxa collected in one sampling session was 8 using the net, and 11 combining traps and vegetation washing. Thirteen taxa were collected by all methods; net and the trap samplings shared seven taxa, whereas net and vegetation washing shared eight taxa. Finally, Hydropsychidae, Limnephilidae, and Hydrophilidae were only found in net samples, and Empididae was exclusively collected with traps ().
Table 1. Sampling methods used in springs by other researchers.
Table 2. List of taxa and their abundances collected using different methods.
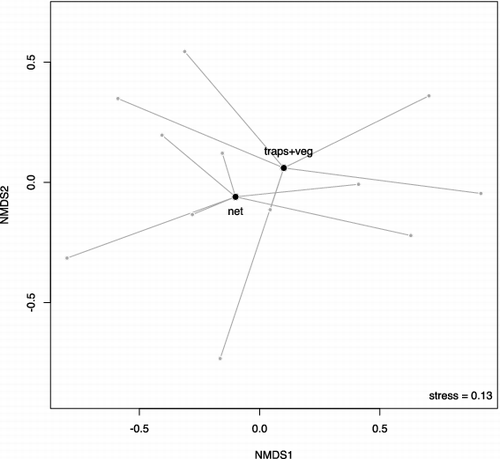
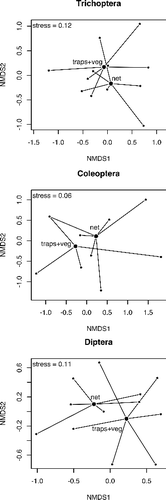
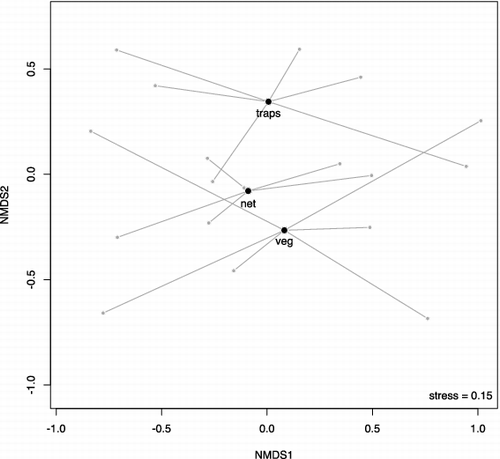
Differences in taxa abundance between methods were significant, both considering the three distinct methods (; F = 16.18; p < 0.001) and when merging the traps and the vegetation washing (; F = 9.46; p = 0.012). Sampled communities formed three distinct groups near their centroids in the NMDS plot (stress = 0.15; ). This indicates differences in macroinvertebrate assemblages according to the methods, as confirmed by the Permanova test (R2 = 0.223; p = 0.010). Stress was 0.13 in the plot of NMDS ordination obtained by merging data collected with traps and vegetation washing (). Differences between the two different methods (net versus traps plus washing vegetation) were less detectable. Permanova test (R2 = 0.136; p = 0.134) indicated that there was not a significant difference between communities sampled with these two methods. Net sampling and combined traps and vegetation samples showed differences for Coleoptera (R2 = 0.219; p = 0.030) and Diptera (R2 = 0.250; p = 0.005), but not for Trichoptera (R2 = 0.056; p = 0.826) ().
Figure 1. Logarithm of taxa abundances for the three methods. Differences in abundances were significant (ANOVA, F = 16.18; p < 0.001).
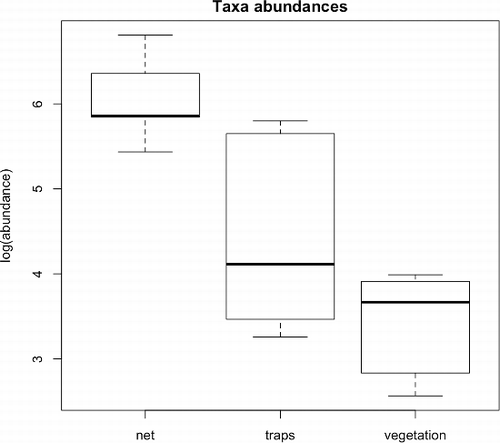
Figure 2. Comparison of taxa abundances (log transformed) using net sampling or a combination of traps and vegetation washing. Differences in abundance between methods were significant (F = 9.46; p = 0.012).
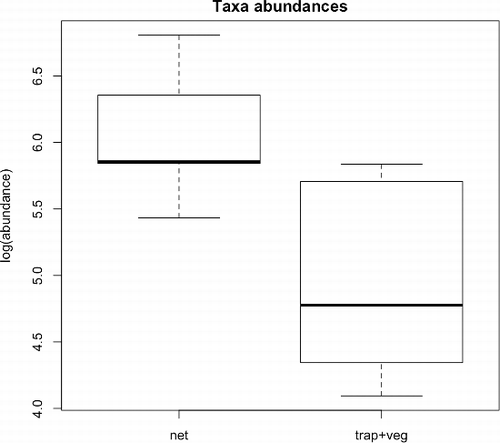
Figure 3. NMDS ordination of the three methods (stress = 0.15). Bigger points are the centroids of methods (veg = vegetation washing). Smaller points are sampled communities.
Discussion
The lack of a standardized sampling protocol for springs has led to the use of a wide variety of methodologies. Standard Surber samplers (sampling area: 0.09–0.1 m2) have been rarely used (Smith et al Citation2003; Barquín & Death Citation2008). More frequently, smaller samplers were preferred (Erman & Erman Citation1995; Erman Citation1998; Zollhöfer Citation1999; von Fumetti et al. Citation2006; Gerecke et al. Citation2011). The mesh size of Surber, kick, or hand nets varies from 100 µm to 1 mm. Although Gerecke et al. (Citation2007) recommended sampling different microhabitats in springs, at their relative microhabitat proportion, few studies have used a proportional multi-habitat approach (Crema et al. Citation1996; Zollhöfer Citation1999; Martin & Brunke Citation2012) or sampled all available substrates (Bonettini & Cantonati Citation1998; Mezzanotte & Sambugar Citation2004; Ilmonen et al. Citation2012). In addition, combined methods have been frequently used in the same study (Williams Citation1991; Erman & Erman Citation1995; Crema et al. Citation1996; Bonettini & Cantonati Citation1998; Erman Citation1998; Myers & Resh Citation2002; Sambugar et al. Citation2006; Staudacher & Füreder Citation2007; Bottazzi et al. Citation2011; Gerecke et al. Citation2011; Spitale Citation2012).
Each method has advantages and disadvantages that may be dependent on the specific aims of the study. For example, the use of sweep nets or emergence traps allows to sample only organisms with aerial stages, whereas drift tubes/nets underestimate taxa with low tendency to drift. Also, methods that require collection by sight could be biased against small, less-mobile, and less-visible organisms. The Surber net, Bou–Rouch pump, and core sampler may allow the collection of quantitative data but the Surber net is usually too large to be used in springs (see Gerecke et al. Citation2007) and the Bou–Rouch pump and core sampler only collect sediment and interstitial samples.
Our results showed that macroinvertebrate community structure estimated by the combination of traps and washing vegetation can be considered comparable to those obtained with net. The four taxa exclusively collected by the net, Hydropsychidae, Limnephilidae, Tipulidae, and Hydrophilidae, have body sizes larger than the opening of the traps (Tachet et al. Citation2000). Furthermore Hydropsychidae, Limnephilidae, and Tipulidae rarely can be found aquatic vegetation, and Hydrophilidae organisms are very mobile and could escape during vegetation collection (Tachet et al. Citation2000). Although similar communities were collected by both net sampling and combined vegetation washing and trap sampling, there were some differences. The two methods produced different results for Diptera and Coleoptera. This result was probably related to issues with single-habitat protocols. For example, traps and washing vegetation probably underestimated the presence of taxa not associated with vegetation or not attracted by meat. The abundances of organisms collected by traps and vegetation washing were significantly lower than those collected by net. Therefore, the impact of these protocols on spring fauna would be expected to be lower, at least on some taxa. In addition, net sampling requires brushing, scraping, digging, and squeezing of different microhabitats and substrata, causing disturbance of the spring habitat with unknown recovery time. The use of traps is more time consuming than other methods, because they require an additional visit to the springs to be removed. Finally, some sampling methods would not be suitable in particular habitat types. For example, some springs lack any kind of vegetation, and traps cannot be placed in hygropetric springs, where the sediment layer is too thin, or in helocrene springs, that often are too deep.
Since spring fauna show evident habitat-preferences (von Fumetti et al. Citation2006), single microhabitat protocols should be used only to survey specific target taxa or habitats. In contrast, a multi-habitat methodology allows a better estimation of the overall biodiversity. In order to obtain more comparable results, Gerecke et al. (Citation2007) recommended sampling all available habitats, using proportional sampling time for each substratum and including transitional zones among different substrata since they may host specialized taxa. In addition, the multi-habitat proportional sampling is considered by the Water Framework Directive (Directive 2000/60/EC) as the best approach for assessing macroinvertebrate diversity.
Proposed methods could be improved in order to be more effective. Traps with different opening dimensions may be able to collect the whole range of organism body sizes. The volume (or number of replicates) of vegetation-washing samples can be increased whenever possible.
In conclusion, the net and the vegetation washing with traps have different characteristics and effectiveness, even though both protocols give very similar qualitative results. The use of the net in a multi-habitat proportional approach provides more accurate and complete information, but also significantly impacts the biotic and abiotic components of springs. For these reasons, this method is only recommended for biodiversity inventories. On the other hand, traps and vegetation washing are reliable methods with fewer negative effects on spring ecosystems; thus, they are more suitable for ecological studies focused on the analysis of the community structure.
Acknowledgements
The authors thank the anonymous reviewers for their helpful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anderson MJ, Walsh DCI. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monog. 83:557–574.
- Barquín J, Death RG. 2008. Physical and chemical differences in karst springs of Cantabria, northern Spain: do invertebrate communities correspond? Aquat Ecol. 43:445–455.
- Bonettini AM, Cantonati M. 1998. Le sorgenti del Parco Adamello-Brenta: ricerche idrobiologiche su fonti non captate [Springs from Adamello-Brenta Park: hydrobiological research about unexploited springs]. Strembo: Parco. Chapter 2, Macrozoobenthos; p. 125–143.
- Bottazzi E. 2010. Indagini ecologiche su sorgenti e headwaters dell'alto Appennino parmense [Ecological research on springs and headwaters from upper Apennines surrounding Parma] [dissertation]. Parma: University of Parma.
- Bottazzi E, Bruno MC, Pieri V, Di Sabatino A, Silveri L, Carolli M, Rossetti G. 2011. Spatial and seasonal distribution of invertebrates in Northern Apennine rheocrene springs. J Limnol. 70:77–92.
- Cantonati M, Bertuzzi E, Spitale D. 2007. The spring habitat: biota and sampling methods. Trento: Monografie del Museo Tridentino di Scienze Naturali.
- Cantonati M, Füreder L, Gerecke R, Jüttner I, Cox EJ. 2012. Crenic habitats, hotspots for freshwater biodiversity conservation: toward an understanding of their ecology. Freshw Sci. 31:463–480.
- Carter JL, Resh VH. 2001. After site selection and before data analysis: sampling, sorting, and laboratory procedures used in stream benthic macroinvertebrate monitoring programs by USA state agencies. J N Am Benthol Soc. 20:658–682.
- Crema S, Ferrarese U, Golo D, Modena P, Sambugar B, Gerecke R. 1996. Ricerche sulla fauna bentonica ed interstiziale di ambienti sorgentizi in area alpina e prealpina [Research on benthic and interstitial fauna from Alpine and pre-Alpine springs]. Trento: Report Centro Ecologia Alpina.
- Davies A. 2001. The use and limits of various methods of sampling and interpretation of benthic macro-invertebrates. J Limnol. 60:1–6.
- Dumnicka E, Galas J, Koperski P. 2007. Benthic invertebrates in karst springs: does substratum or location define communities? Int Rev of Hydrobiol. 92:452–464.
- Erman NA, Erman DC. 1995. Spring permanence, Trichoptera species richness and the role of drought. J Kans Entomol Soc. 68:50–64.
- Erman NA. 1998. Studies in crenobiology: the biology of springs and springbrooks. Leiden: Backhuys Publishers. Chapter 8, Invertebrate richness and Trichoptera phenology in Sierra Nevada (California, USA) cold springs: sources of variation; p. 95–108.
- Gathmann FO, Manne LL, Williams DD. 2009. Spatial patterns in insect community composition in coldwater springs. Aquatic Ecol. 43:501–512.
- Gerecke R, Cantonati M. 1998. Le sorgenti del Parco Adamello-Brenta [Springs from Adamello-Brenta Park]. Strembo: Parco Adamello-Brenta. Chapter 5, Gli idracari [Water mites]; p. 145–150.
- Gerecke R, Cantonati M, Spitale D, Stur E, Wiedenbrug S. 2011. The challenges of long-term ecological research in springs in the northern and southern Alps: indicator groups, habitat diversity, and medium-term change. J Limnol. 70:168–187.
- Gerecke R, Di Sabatino A. 2007. The spring habitat: biota and sampling methods. Trento: Monografie del Museo Tridentino di Scienze Naturali. Chapter 16, Water mites (Hydrachnidia and Halacaridae) in spring habitats: a taxonomical and ecological perspective; p. 265–274.
- Gerecke R, Maiolini B, Cantonati M. 2007. The spring habitat: biota and sampling methods. Trento: Monografie del Museo Tridentino di Scienze Naturali. Chapter 20, Collecting meio- and macrozoobenthos in springs; p. 265–274.
- Gerecke R, Meisch C, Stoch F, Acri F, Franz H. 1998. Studies in crenobiology: the biology of springs and springbrooks. Leiden: Backhuys Publishers. Chapter, Eucrenon-hypocrenon ecotone and spring typology in the Alps of Berchtesgaden (Upper Bavaria, Germany). A study of microcrustacea (Crustacea: Copepoda, Ostracoda) and water mites (Acari: Halacaridae, Hydrachnellae); p. 167–182.
- Gooch JL, Glazier DS. 1991. Temporal and spatial patterns in mid-Appalachian springs. Mem Entomol Soc Can. 155:29–49.
- Hahn HJ. 2000. Studies on classifying of undisturbed springs in southwestern Germany by macrobenthic communities. Limnologica. 30:247–259.
- Ilmonen J, Mykrä H, Virtanen R, Paasivirta L, Muotka T. 2012. Responses of spring macroinvertebrate and bryophyte communities to habitat modification: community composition, species richness, and red-listed species. Freshw Sci. 31:657–667.
- Koperski P, Dumnicka E, Galas J. 2011. Abiotic parameters determining fauna composition in karstic springs. Pol J Ecol. 59:153–163.
- Kubíková L, Simon OP, Tichá K, Douda K, Maciak M, Bílý M. 2012. The influence of mesoscale habitat conditions on the macroinvertebrate composition of springs in a geologically homogeneous area. Freshw Sci. 31:668–679.
- Legendre P, Legendre LFJ. 2012. Numerical ecology: Vol. 24. Amsterdam: Elsevier.
- Lencioni V. 2007. The spring habitat: biota and sampling methods. Trento: Monografie del Museo Tridentino di Scienze Naturali. Chapter 19, Chironomids (Diptera, Chironomidae) in Alpine and pre-Alpine springs; p. 265–274.
- Maiolini B, Carolli M, Silveri L. 2011. Ephemeroptera, Plecoptera and Trichoptera in springs in Trentino (south-eastern Alps). J Limnol. 70:122–133.
- Martin P, Brunke M. 2012. Faunal typology of lowland springs in northern Germany. Freshw Sci. 31:542–562.
- Mezzanotte E, Sambugar B. 2004. Contributo alla conoscenza della fauna delle sorgenti [Contribution to the knowledge of spring fauna]. Memorie del Museo Civico di Storia Naturale di Verona – 2° serie – Monografie naturalistiche. 1:283–292.
- Mori N, Brancelj A. 2006. Macroinvertebrate communities of karst springs of two river catchments in the Southern Limestone Alps (the Julian Alps, NW Slovenia). Aquatic Ecol. 40:69–83.
- Myers MJ, Resh VH. 2002. Trichoptera and other macroinvertebrates in springs of the Great Basin: species composition, richness, and distribution. West N Am Naturalist. 62:1–13.
- Oksanen, J, Blanchet FG, Kindt R, Legendre P. 2013. R package [Version 2.0–7]. Vegan: Community Ecology Package.
- R Core Team. 2013. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Rader RB, Keleher MJ, Billman E, Larsen R. 2012. History, rather than contemporary processes, determines variation in macroinvertebrate diversity in artesian springs: the expansion hypothesis. Freshw Biol. 57:2475–2486.
- Sambugar B, Dessi G, Sapelza A, Stenico A, Thaler B, Veneri A. 2006. Fauna sorgentizia in Alto Adige [Spring fauna from South Tyrol]. Bolzano: Provincia Autonoma di Bolzano.
- Smith H, Wood PJ, Gunn J. 2003. The influence of habitat structure and flow permanence on invertebrate communities in karst spring systems. Hydrobiologia. 510:53–66.
- Spitale D. 2012. A comparative study of common and rare species in spring habitats. Ecoscience. 19:80–88.
- Spitale D, Leira M, Angeli N, Cantonati M. 2012. Environmental classification of springs of the Italian Alps and its consistency across multiple taxonomic groups. Freshw Sci. 31:563–574.
- Staudacher K, Füreder L. 2007. Habitat complexity and invertebrates in selected Alpine springs (Schütt, Carinthia, Austria). Int Rev Hydrobiol. 92:465–479.
- Stoch F, Valenti D, Chiesi M, Tomasin G. 2008. Monitoraggio biologico delle sorgenti salse di Poiano (Reggio Emilia)[Biological monitoring of saline springs of Poiano (Reggio Emilia)]. Mem Ist Speleol. 21:113–120.
- Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P. 2000. Invertébrés d'eau douce: systématique, biologie, écologie [Freshwater invertebrates: systematics, biology, and ecology]. Paris: CNRS éditions.
- Takhteev VV, Galimzyanova AV, Ambrosova EV, Kravtsova LS, Rozhkova NA, Okuneva GL, Semernoi VP, Pomazkova GI, Lopatovskaya OG. 2010. Zoobenthos communities and their seasonal dynamics in nonfreezing springs of Baikal region. Biol Bull. 37:638–646.
- Tichá K, Simon OP, Douda K, Kubiková L. 2012. Detrital components in submontane organogenic springs in relation to their morphology, microhabitat and macroinvertebrates. Pol J Ecol. 60:163–175.
- von Fumetti S, Nagel P, Scheifhacken N, Baltes B. 2006. Factors governing macrozoobenthic assemblages in perennial springs in north-western Switzerland. Hydrobiologia 568:467–475.
- Williams NE. 1991. Geographical and environmental patterns in caddisfly (Trichoptera) assemblages from coldwater springs in Canada. Mem Entomol Soc Can. 155:107–124.
- Worthington Wilmer J, Elkin C, Wilcox C, Murray L, Niejalke D, Possingham H. 2008. The influence of multiple dispersal mechanisms and landscape structure on population clustering and connectivity in fragmented artesian spring snail populations. Mol Ecol. 17:3733–3751.
- Zollhöfer JM. 1999. Spring biotopes in Northern Switzerland: habitat heterogeneity, zoobenthic communities and colonization dynamics [dissertation]. Zurich: Swiss Federal Institute of Science and Technology.