ABSTRACT
Energy production in the Williston Basin, USA, results in the coproduction of highly saline, sodium chloride-dominated water (brine). The Prairie Pothole Region (PPR) overlies the northeastern portion of the Williston Basin. Although PPR wetlands span a range of salinity, the dominant salt is sodium sulfate, and salinities are much lower than brine. Introduction of brine to wetlands can result in pronounced water-quality changes; however, the ecological effects of such contamination are poorly understood. We examined the effects of brine contamination on primary productivity, emergent macrophyte tissue chemistry, and invertebrate communities from 10 wetlands in the PPR. Based on a recognized Contamination Index (CI) used to identify brine contamination in the PPR, water-quality samples indicated that six wetlands were uncontaminated while four were contaminated. Across this gradient, we observed a significant decrease in above-ground biomass and a significant increase in tissue chloride concentrations of hardstem bulrush (Schoenoplectus acutus) with increased CI values. Additionally, a significant decrease in macroinvertebrate taxonomic richness with increased CI values was observed. These findings provide needed insight on the biological effects of brine contamination on PPR wetlands.
Introduction
The Williston Basin has been a leading source of domestic energy production since the 1950s with rapid new development occurring since the early 2000s. As of 1 January 2016, approximately 40,000 petroleum-related wells have been drilled in the Williston Basin and it is estimated that over 50,000 new wells will be required to reach full well capacity. Energy development in the Williston Basin results in the coproduction of large volumes of saline water (brine). The ratio of brine to oil varies by well, well age, and target formation; however, ratios of 10:1 are not uncommon for older wells (Wanty Citation1997). Numerous pathways exist for brine to be introduced into the environment, including historic storage and disposal practices, pipeline breaks, spills, well failures, and illegal discharges.
Overlying the northeastern portion of the Williston Basin is the Prairie Pothole Region (PPR). The PPR is a glacial drift plain characterized by an abundance of highly productive depressional wetlands that provide critical breeding habitat for many species of North American waterfowl. Energy development throughout the PPR has occurred in close proximity to wetlands. As of 2011, 34% of wetlands in the US portion of the PPR underlain by the Williston Basin were within 1600 m of a petroleum-related well (Gleason & Tangen Citation2014). Since then, more than 2000 new wells have been drilled, potentially increasing the percentage of wetlands with nearby wells.
Previous studies identified pronounced effects on water quality from brine contamination of surface water and groundwater (aquatic) resources in the PPR (e.g. Preston & Chesley-Preston Citation2015). Williston Basin brine is dominated by sodium chloride and has some of the greatest total dissolved solid (TDS) concentrations in the USA with values >450,000 mg/L (Otton Citation2006). While aquatic resources in the PPR naturally exhibit a wide range of salinity, the dominant salt is sodium sulfate and TDS concentrations are generally <10,000 mg/L. Only 10% of PPR wetlands have naturally elevated chloride concentrations (Gleason & Tangen Citation2014). Brine contamination increases the salinity and relative chloride concentrations in PPR wetlands. Reiten and Tischmak (Citation1993) created a Contamination Index (CI) to identify the presence and magnitude of brine contamination to aquatic resources across the range of salinities in the PPR. Although several studies have identified brine contamination in the PPR, there is a paucity of literature pertaining to the ecological effects of such contamination (Farag & Harper Citation2014).
Methods
A total of 10 wetlands on public lands were sampled in the summer and fall of 2012. In each wetland, we characterized water-quality field parameters and documented the above-ground biomass and tissue chloride concentrations of hardstem bulrush (Schoenoplectus acutus) stands. Hardstem bulrush was chosen as it is common to wetlands across the region. Additionally, we documented macroinvertebrate taxonomic richness using the lowest taxonomic level (usually genus). To ensure a contamination gradient, wetland sites included six randomly selected sites and four sites with known contamination.
Water-quality parameters were collected in the field during June, July, and September; wetlands with permanent standing water were sampled multiple times over the season. Chloride concentration and specific conductance were determined from the mean of three Hach QuanTab® chloride titrator strips (accuracy ±10%) and from a YSI®556 conductivity meter (accuracy ±0.5%), respectively. CI values, the ratio of chloride concentration (in mg/L) to specific conductance (in µS/cm), were calculated for each wetland. Reported CI values are from the earliest sample collected as some of the wetlands went dry during the summer.
Hardstem bulrush above-ground biomass was collected using four 0.25-m2 quadrats at each wetland in late September. Quadrats were located in the center of the first monotypic strand encountered in each of four transects radiating from the wetland center along the cardinal directions. All living plant materials in the quadrat were clipped at ground level and processed as in Ray et al. (Citation2012). In brief, the plant material was oven-dried and weighed, and reported biomass values are the mean of all four quadrats. Next, inflorescences were removed and dried shoots were mixed and passed through a 1-mm sieve. Elemental tissue analysis was conducted by the University of Missouri Extension, Columbia, MI, using an inductively coupled plasma optical emission spectrometer. Chloride content was reported as a percentage of the dry sample weight.
Macroinvertebrate samples were collected from 9 of the 10 wetlands (wetlands with standing water) using eight 0.5-m sweeps with a D-frame sweep net (0.3-m wide, 500-mm mesh) at each wetland in late September. Four sweeps were collected in vegetation near the shore and four were collected in open water near the center. All sweeps were combined together to produce a single composite sample per wetland. A minimum of 200 macroinvertebrates were randomly selected following the methods of Caton (Citation1991) and identified to the lowest taxonomic level possible (often genus; occasionally class or family) at Rhithron Associates Inc., Missoula, MT.
To evaluate effects of brine contamination on emergent macrophyte biomass and tissue chemistry and macroinvertebrate communities, we used linear least squares regressions to explore the relationship to the calculated CI values per wetland. Specifically, we regressed mean biomass (log transformed), mean tissue chloride concentration, and macroinvertebrate taxonomic richness against the mean CI value for each wetland. Due to the small sample size, a significance level of 0.1 was used to indicate the significance for all regressions.
Results and discussion
Water-quality parameters confirmed the sites spanned a contamination gradient (). CI values from six wetlands were <0.035, the threshold used to identify contamination (Reiten & Tischmak Citation1993), while CI values from the remaining four were >0.035.
Table 1. Mean water-quality field parameters and mean hardstem bulrush (Schoenoplectus acutus) above-ground biomass and macrophyte tissue chloride concentrations for 10 wetlands in the Prairie Pothole Region.
Mean above-ground biomass of hardstem bulrush strands significantly decreased with the increased CI values (F(1,8) = 8.319, p = 0.020, with an r2 of 0.51; ; (A)). Increased salinity can reduce the primary production (Neill Citation1993) and the uptake of macronutrients (e.g. calcium, magnesium, nitrogen, and potassium) in plants (Grattan & Grieve Citation1999). Reductions in the uptake of growth-limiting nutrients can limit plant biomass (Koerselman & Meuleman Citation1996) and the overall wetland productivity. Although the contaminated sites sampled in this study were not necessarily the wetlands with the highest natural background levels of salinity (), these sites did have the highest chloride levels. The introduction of brine can dramatically change the chemical composition in wetlands and shift saline systems from ones previously dominated by sulfate to ones dominated by chloride. In addition to reducing above-ground biomass, increased chloride levels can also reduce seed germination (Baskin & Baskin Citation1998), alter plant community composition (Nielsen et al. Citation2003), and overall primary productivity (Neill Citation1993).
Figure 1. Panels A, B, and C show hard-stemmed bulrush above-ground biomass, macroinvertebrate richness, and plant tissue chloride concentrations plotted against Contamination Index values for 10 wetlands in the Prairie Pothole Region. Error bars in Panels A and B indicate the standard error.
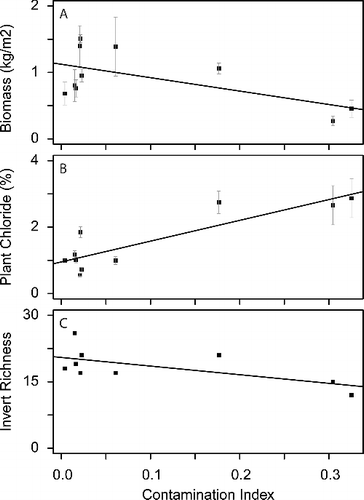
Plant tissue chloride concentration significantly increased with the increased CI values (F(1,8) = 26.84, p < 0.001, with an r2 of 0.77; ; (B)). The use of plant tissue chemistry has been used as a tool for comparing vegetative components in nutrient-enriched reference and restored wetlands (Ray et al. Citation2012). To date, brine contamination in the PPR has largely been described by documenting impacts to surface water or groundwater (Reiten & Tischmak Citation1993). Unfortunately, surface water levels in the PPR are variable at multiple time scales (e.g. seasonal, annual, and decadal) and the absence of water may limit the ability to identify contamination using traditional water sampling methods. Moreover, surface water samples provide temporally discrete data; however, they may not be representative of long-term conditions. In contrast, wetland plants have the ability to integrate nutrient and salinity fluxes from surface water and groundwater over periods extending from days to years and provide a living synthesis of site and ecological condition (Lopez & Fennessy Citation2002). Perennial wetland plants can act as biological indicators of anthropogenic stress (DeKeyser et al. Citation2003), and these results indicate that wetland plants may provide a proxy for the presence and potential magnitude of brine contamination.
Macroinvertebrate taxonomic richness significantly decreased with the increased CI values (F(1,7) = 4.661, p = 0.068, with an r2 of 0.40; (C)). Macroinvertebrates are widely used indicators of biological integrity and aquatic ecosystem health and are also recommended for wetland assessments in the PPR (Adamus Citation1996). Overall, invertebrates represent a taxa-rich group in wetlands and generally serve as the principal link between aquatic macrophytes and other primary producers (e.g. bacteria and algae), litter/detritus, and higher order consumers (e.g. amphibians, fish, and waterfowl; Murkin & Ross Citation2000). Invertebrate assemblages in the PPR are thought to be less rich than other freshwater wetlands (Euliss et al. Citation1999); however, despite this relative lack of richness, macroinvertebrates are a critical part of PPR food webs and serve as a primary food source for breeding female ducks and pre-fledgling ducklings, as well as juvenile and adult amphibians (Zimmer et al. Citation2000). The macroinvertebrate samples yielded 56 distinct taxa across 14 orders with individuals from the order diptera (true flies) being the most common macroinvertebrates in the majority of wetlands sampled (). Due to the large number of taxa and limited number of wetlands sampled, we were unable to identify systematic changes in major taxa with increased CI values; however, the decrease in taxonomic richness with increased CI values suggests that changes in water quality from the introduction of brine may selectively restrict the use of contaminated wetlands by some macroinvertebrate species.
Table 2. Contamination Index values and macroinvertebrate counts, by order, for 10 wetlands in the Prairie Pothole Region. The number in parentheses, if present, indicates the number of distinct taxa recorded within each order or wetland.
These results indicate the introduction of brine to PPR wetlands likely has deleterious ecological effects related to primary productivity and benthic biota; however, additional work is necessary to better understand and quantify these effects. For example, wetland plant species differ in their ability to accumulate nutrients and convert nutrients into biomass (Ray et al. Citation2012); therefore, it remains unclear if the reductions in biomass with increased CI values observed for hardstem bulrush would be representative of other wetland plant species. Additionally, the long-term consequences of tissue chloride accumulation, and biomass reduction, remain unknown. While this work illustrated the potential to use plant tissue chloride as an indicator of brine contamination, our sample size is currently insufficient to identify a threshold concentration indicative of contamination. Lastly, the PPR produces up to 50% of North America's waterfowl and aquatic invertebrates are a key food source for breeding and fledgling waterfowl. Thus, decreases in plant biomass and shifts in macroinvertebrate composition could have effects on higher trophic levels. We hope that the focus on macrophyte and macroinvertebrate metrics will help advance public understanding of brine contamination by translating the effects in terms of important ecosystem services.
Acknowledgments
We thank Rick Sojda, Colleen Charles, and Mike Borgreen for administrative and logistical assistance. Scott Owen and Jill Frankforter collected field samples and Caitlyn Brendal prepared plant tissues.
Disclosure statement
Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Additional information
Funding
Notes on contributors
Todd M. Preston
Todd M. Preston is a Geologist at the U.S. Geological Survey Northern Rocky Mountain Science Center where he examines the ecological effects of energy development in the Williston Basin.
Andrew M. Ray
Andrew M. Ray is an Aquatic Ecologist with the National Park Service Greater Yellowstone Network where he works on long-term wetland and water quality monitoring programs.
References
- Adamus PR, Hairston AJ. 1996. Bioindicators for assessing ecological integrity of prairie wetlands. Washington (DC): US Environmental Protection Agency. 216 p. ( EPA/600/R/082).
- Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego (CA): Academic Press. 665 p.
- Caton LW. 1991. Improving subsampling methods for the EPA's ‘rapid bioassessment’ benthic protocols. Bull N A Benthol Soc. 8:317–319.
- DeKeyser ES, Kirby DR, Ell MJ. 2003. An index of plant community integrity: development of the methodology for assessing prairie wetland plant communities. Ecol Indic. 3:119–133.
- Euliss Jr NH, Wrubleski DA, Mushet DM. 1999. Wetlands of the Prairie Pothole Region: invertebrate species composition, ecology, and management. In: Batzer DP, Rader RB, Wissinger SA, editors. Invertebrates in freshwater wetlands of North America: ecology and management. New York (NY): Wiley; p. 471–514
- Farag A, Harper DD. 2014. A review of environmental impacts of salts from produced waters on aquatic resources. Int J Coal Geol. 126:157–161.
- Gleason RA, Tangen BA. 2014. Brine contamination to aquatic resources from oil and gas development in the Williston Bain, United States. Reston (VA): U.S. Geological Survey. 127 p. (Scientific Investigations Report 2014–2017).
- Grattan SR, Grieve CM. 1999. Mineral nutrient acquisition and response by plants grown in saline environments. In: Pessarakli M, editor. Handbook of plant and crop stress. New York (NY): Marcel Dekker; p. 203–229.
- Koerselman W, Meuleman AF. 1996. The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol. 14:41–50.
- Lopez RD, Siobhan Fennessy M. 2002. Testing the floristic quality assessment index as an indicator of wetland condition. Ecol App. 12:487–97.
- Murkin HR, Ross LC. 2000. Invertebrates in prairie wetlands. In: Murkin HR, Van der Valk AG, Clark WR, editors.Prairie wetland ecology. Ames (IA): Iowa State University Press; p. 201–248.
- Neill C. 1993. Seasonal flooding, soil salinity and primary production in northern prairie marshes. Oecologia. 95:499–505.
- Nielsen DL, Brock MA, Rees GN, Baldwin DS. 2003. Effects of increasing salinity on freshwater ecosystems in Australia. Aust J Bot. 51:655–665.
- Otton JK. 2006. Environmental aspects of produced water salt releases in onshore and coastal petroleum-producing areas of the conterminous U.S. – a bibliography. Reston (VA): U.S. Geological Survey. 223 p. ( Open-File Report 2006-1154).
- Preston, TM, Chesley-Preston TL. 2015. Risk assessment of brine contamination to aquatic resources from energy development in glacial drift deposits: Williston Basin, USA. Sci. Total Environ. 508:534–545.
- Ray AM, Hamilton A, Aquino C, Litts JC. 2012. Using vegetative nutrient stocks to compare restored and reference wetland in the upper Klamath basin, Oregon. Wetlands. 32:827–839.
- Reiten JC, Tischmak T. 1993. Appraisal of oil field brine contamination in shallow groundwater and surface water, eastern Sheridan County, Montana. Billings (MT): Montana Bureau of Mines and Geology. 296 p. ( Montana Bureau of Mines and Geology Open-File Report 260).
- Wanty RB. 1997. USGS research on saline waters co-produced with energy resources. Reston (VA): U.S. Geological Survey. 3 p. ( U.S. Geological Survey Fact Sheet 003-97).
- Zimmer KD, Hanson MA, Butler MG. 2000. Factors influencing invertebrate communities in prairie wetlands: a multivariate approach. Can J Fish Aquat Sci. 57:76–85.
