ABSTRACT
The current study examined differences in sediment stability preferences for two federally threatened and a locally common mussel species’ habitats in the Choctawhatchee River watershed located in southeastern Alabama and northwestern Florida. Relative shear stress (RSS), the ratio of shear stress to critical shear stress, was calculated for individuals of two threatened mussel species, Fusconaia burkei (N = 94), and Pleurobema strodeanum (N = 201), and a common mussel species, Elliptio pullata (N = 94). Relative shear stress is related to sediment movement and deposition as sediment movement takes place when RSS > 1. Mussels were collected and RSS measured at each individual mussel location (N = 389). The Kruskal–Wallis H-test and/or Mann–Whitney U-test found that RSS for the target-threatened species’ habitats were significantly higher than that for E. pullata at all sites (p < 0.05). Spearman's rank correlation found significant negative correlations between shear stress and total mussel abundance at all sites (p < 0.05). Streams selected in our study were typical representatives of high-quality southeastern Coastal Plain streams. Therefore, we suggest that our findings of differences in hydraulic instability of mussel habitats among species may represent similar conditions for other rare and endangered species. Sediment movement and deposition may be linked to the decline and consequent federal listing of the target-threatened species under our study.
Introduction
Biodiversity is under threat, and freshwater mussels are no exception. Unionids are among the most imperiled groups of organisms in North America, and habitat impairment has been documented as the most widespread cause (Wilcove & Master Citation2005). Unstable habitats in riverine systems have been identified as a major factor limiting adult mussel abundance, as well as juvenile mussel dispersal, all over North America (Gangloff & Feminella Citation2006; Allen & Vaughn Citation2010; Daraio et al. Citation2010; Davis et al. Citation2013; French & Ackerman Citation2014). Freshwater mussels tend to aggregate in stable streambed microhabitats that experiences low sediment mobilization even at high flows (Strayer Citation1999; Johnson & Brown Citation2000; Gangloff & Feminella Citation2006). In stream microhabitats with high rates of sediment transport and accumulation, mussels may not be able to maintain stable positions due to sediment instability, which may threaten survival (Arbuckle & Downing Citation2002).
Stability of sediment can be determined using complex hydraulic variables such as shear stress (tangential force of water on streambed), Reynold's number (ratio of inertial to viscous forces), and Froude's number (ratio of inertial to gravitational forces) (Morales et al. Citation2006; Steuer et al. Citation2008; Allen & Vaughn Citation2010). Studies examining hydraulics and substrate stability for mussels apply the shear stress approach, and have determined shear stress to be the major predictor of mussel abundance and distribution (Morales et al. Citation2006; Allen and Vaughn Citation2010; Daraio et al. Citation2010). Shear stress is a major factor determining sediment stability at high-flow conditions and is a direct function of shear velocity (Morales et al. Citation2006; Allen & Vaughn Citation2010). Shear stress increases at higher flow conditions creating less suitable mussel habitat (Steuer et al. Citation2008; Allen & Vaughn Citation2010). Since excessive shear stress may initiate substrate movement, mussels tend to aggregate in patches of streambed that experience low shear stress even at high-flow conditions (Allen & Vaughn Citation2010). Critical shear stress is the level required to set the sediment in motion (Gordon et al. Citation2004). Morales et al. (Citation2006) proposed relative shear stress (RSS, ratio of observed shear stress of flowing water on sediment to the critical shear stress of the sediment, is a nondimensional parameter) as a measure of sediment stability; where, if RSS < 1, there is no substrate movement, and if RSS > 1, substrate movement takes place. Shear stress conditions at the microhabitat level may be even more important than a mesohabitat level shear stress as the overall sediment in motion may not reflect the stability of sediment at the exact location where individual mussels are found (Di Maio & Corkum Citation1995).
Generally, substrate stability studies on freshwater mussels examine the relationship between shear stress and mussel abundance (Hardison & Layzer Citation2001; Morales et al. Citation2006; Allen & Vaughn Citation2010). These studies have established that substrate stability is one of the major predictors of mussel abundance, and that shear stress and mussel density/abundance are strongly negatively correlated at high flows. Even though substrate stability at a given flow within a stream system may vary among mussel species depending on the variability in habitat use, studies examining substrate stability for individual mussel species and differences among them are lacking. Our study addresses differential microhabitat stability among two recently federally listed and a locally common mussel species in the Choctawhatchee River watershed. Sampling for rare and endangered mussel species using transects and quadrats has not yielded statistically useful sample sizes, and sampling using this strategy has not been able to statistically establish differences among species. Due to the rarity of the two target-threatened mussel species, necessary data were collected for each individual encountered to obtain a greater sample size compared to quadrat sampling.
In the Choctawhatchee River basin, Fusconaia burkei (Walker, 1922), and Pleurobema strodeanum (Wright, 1898) have been recently listed as threatened under the Endangered Species Act (U.S. Fish and Wildlife Service Citation2012). The objective of this study was to determine differences in relative sediment stability of respective habitats among the target-threatened species and a common mussel species, Elliptio pullata (Lea, 1856), and to determine the relationship between shear stress and total mussel abundance. The null hypothesis (Ho) for the study was that there was no relationship between total mussel abundance and shear stress, and those areas where threatened mussel species and common mussel species had no difference in relative shear stress preferences among the species. The alternative hypothesis (H1) was that there is a negative relationship between total mussel abundance and shear stress and that threatened and common mussel species had different relative shear stress.
Materials and methods
Study area
The Choctawhatchee River watershed lies within the Southeastern Plains (65) Level IV ecoregion, and drains 12,297 km2 in southeast Alabama and the Florida Panhandle (Heath et al. Citation2010). Three sites in the Choctawhatchee River watershed were selected based on previous knowledge of presence of target species in abundance high enough to be useful in statistical analyses (Pilarczyk et al. Citation2006). The first site, Blue Springs (BS, 31.66379°, −85.50523°), a fourth order stream, was located on the West Fork of the Choctawhatchee River. The stream reach was 100 m in length with an average width of 11.8 m starting immediately upstream of the bridge on State Highway 10, Barbour County, AL, USA. The second and third sites were located on Eightmile Creek (a second order tributary of Flat Creek), which drains into the Pea River, Choctawhatchee River watershed, Walton County, FL, USA; the second site (8M1, 30.98064°, −86.17930°) began immediately upstream of the bridge on County Road 181 and had an average width of 6.31 m, and the third site (8M2, 30.97965°, −86.17927°) began 141 m upstream of the bridge with an average width of 6.29 m. Both streams were predominantly sand-bottomed with extensive riparian vegetation and canopy cover. Blue Springs and 8M2 had some deeply incised banks, while 8M1 was devoid of any incision.
Habitat data collection
Eight samples were taken at each site over a four-month period from June to October 2012; two samples per month, usually on consecutive days. On the first visit to each site, sampling was completed over five person-hours moving upstream at each site, and stream reaches were marked. All subsequent samplings were performed on the same stream segments. As individual mussels were identified as a species of interest, a color-coded flag with a number assigned to each individual was inserted in the sediment at the exact location of collection. Data were only collected for individuals not previously collected (previously collected individuals were identified by the presence of a unique tag, and tagging was done as a part of another study). Data for approximately 30 individuals were taken for E. pullata at each site and used for comparative purposes, as it is a common mussel species in the Choctawhatchee River watershed (Pilarczyk et al. Citation2006).
All variables needed to compute shear stress and critical shear stress were measured. Depth and current velocity were measured at each flag marking where each individual mussel had been located using a Pygmy Current Meter (USGS Pygmy Meter Model 6205). One measurement of each variable was taken and considered the same for individuals that were ≤5 cm from each other assuming negligible differences within such distance. Mean current velocity was measured at 0.6 × depth downwards from the water surface for depths less than 0.5 m. In depths greater than 0.5 m, mean current velocity was determined by averaging current velocities at 0.8 × depth and 0.2 × depth downwards from the water surface (Gordon et al. Citation2004; Allen & Vaughn Citation2010).
Sediment samples were collected using a 5.08-cm-diameter PVC pipe at each flag marking the mussel location. The pipe was pushed into the sediment to approximately 8 cm, and upon withdrawal, one hand was placed to seal the top, while another hand was placed at the bottom of the pipe to prevent the loss of the sediment sample upon removal. The sample was then emptied into a sealable plastic bag marked with the species’ name and flag number, and transported to the laboratory on ice for sediment particle size analysis. The sediment sample was oven dried at 105 °C for 24 hours (Gordon et al. Citation2004). A subsample of the thoroughly mixed sample (∼100 g) was then sieved through six sieves of mesh sizes including 2 mm (no. 10), 1 mm (no. 18), 0.5 mm (no. 35), 0.25 mm (no. 60), 0.125 mm (no. 120), and 0.063 mm (no. 230) (U.S.A. Standard Test Sieves, Cole-Parmer®) with a Humboldt motorized sieve shaker (H-4325) for 15 minutes. Percentage sediment remaining on each sieve was used to determine the particle size at which x% of the sample was finer (Dx), which was used to determine bed roughness. The greater the roughness, the lower the shear velocity and shear stress. Density of sediment was assumed to be 2.65 g/cm3, and Shield's parameter was assumed to be 0.065 as substrates from all sites were mostly composed of sand with a small amount of fines (Gordon et al. Citation2004; Allen & Vaughn Citation2010).
Calculations
Shear stress, critical shear stress, and RSS were calculated using the following formulas (Gordon et al. Citation2004; Allen & Vaughn Citation2010):
Shear stress
Critical shear stress (τc, dynes/cm2)
Relative shear stress
Shear velocity
Bed roughness
where is the substrate particle size at which x% of the sample is finer; U is the mean current velocity (cm/s);
is the water depth (cm); ρ is the density of water (0.998 g/cm3);
is the Shield's parameter (0.065);
is the acceleration due to gravity (980 cm/s2); and
is the density of substrate (2.65 g/cm3).
If RSS was less than one, there was no substrate movement. If RSS was greater than one, water entrained the substrate making mussel microhabitats unstable (Gordon et al. Citation2004).
Statistical analysis
Tests were performed for normality and equality of variances for RSS for each species at each site. For sites where data were normal and variances were equal, one-way ANOVA was used to determine the difference in RSS among species’ respective habitats. If either of these assumptions were violated, a nonparametric equivalent, the Kruskal–Wallis H test was performed to determine the difference in RSS among the species’ habitats. In the cases of significant difference, Bonferroni's correction was applied to the value of α, i.e. level of significance was reduced by the times equal to number of treatments to reduce the probability of Type I error. Multiple comparison tests were then performed using the Mann–Whitney U-test between each two species at a site with the reduced level of significance to determine which two species were different (Sokal & Rohlf Citation2012). Correlation between shear stress and total mussel abundance was determined using Spearman's rank correlation coefficient at each site. For correlation analyses, shear stress values for individual mussels were rounded to the nearest decimal place to obtain number of mussel species in different categories of shear stress values (abundance at zero shear stress due to no measurable current velocities were excluded from correlation analyses).
Results
Null hypotheses for correlation between total mussel abundance and shear stress and differences in relative shear stress among threatened and common mussel species were rejected.
Site – Blue Springs (BS)
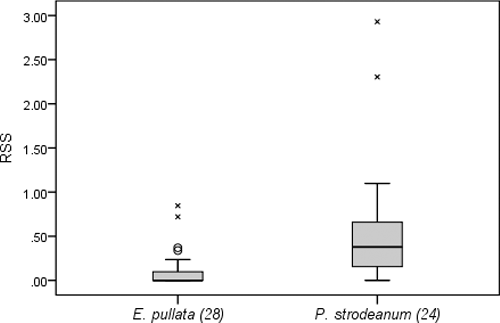
RSS at locations where E. pullata were collected had a mean of 0.10 and ranged from 0 to 0.85 (). Mean RSS at locations where P. strodeanum were found was 0.57 and RSS ranged from 0 to 2.93. Three individuals (12.5%) of P. strodeanum were found at locations with RSS > 1, while all locations where E. pullata were found had RSS < 1. Relative shear stress was significantly different between the two species (p < 0.001). Significant negative correlation was found between shear stress and total mussel abundance (ρ = −0.53, p = 0.03) ().
Table 1. Number of individuals of each species (N), range and mean of relative shear stress (RSS), p-value for the difference in RSS among the species’ respective habitats, Spearman's rank correlation coefficient (ρ) between shear stress and total abundance and its significance for each site sampled from June to October, 2012, in the Choctawhatchee River watershed (*indicates significant and ** indicates highly significant difference or correlation).
Site – Eightmile Creek, Site 1 (8M1)
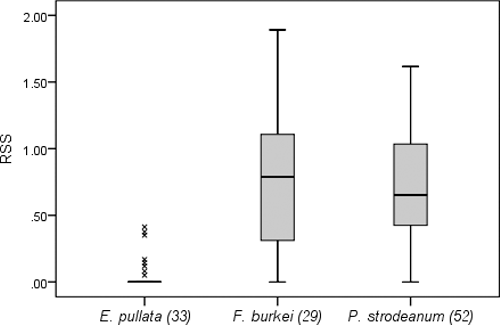
Mean RSS where E. pullata were found was 0.05 and RSS ranged from 0 to 0.41 (). Individuals of F. burkei were collected where RSS ranged from 0 to 1.89 and mean RSS was 0.73. Locations where P. strodeanum were collected had a mean RSS of 0.69 and ranged from 0 to 1.62. Twenty five individuals (31%) of the two target-threatened species under study were found at locations with RSS > 1, compared to no E. pullata at such locations. Relative shear stress was significantly different among the mussel species (p < 0.001). Multiple comparison tests showed that RSS for E. pullata was significantly lower than that for F. burkei (p < 0.001) and P. strodeanum (p < 0.001), whereas F. burkei and P. strodeanum did not have significantly different RSS (p = 0.918) (). There was a significant negative correlation between total mussel abundance and shear stress (ρ = −0.69, p < 0.001) ().
Figure 1. Relative shear stress (RSS) calculated for each individual of the two mussel species collected at BS from June to October, 2012 (ᴼ indicates outliers that are values between 1.5 X interquartile range (IQR) and 3 X IQR and x indicates extreme outliers that are values greater than 3 X IQR. Outliers and extreme outliers indicate that data can be variable and individuals can be distributed in a highly variable range of relative shear stress conditions).
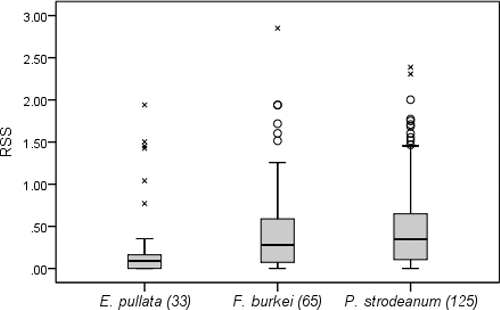
Site – Eightmile Creek, Site 2 (8M2)
E. pullata were collected from locations that had an RSS range of 0–1.94 and a mean RSS of 0.30 (). Mean RSS where F. burkei were collected was 0.48 and ranged from 0 to 2.85. Locations where P. strodeanum were collected had a mean RSS of 0.51 and ranged from 0 to 2.39. Five individuals (15%) of E. pullata were found with RSS > 1, and 29 (15%) individuals of the target threatened species were found with RSS > 1. Relative shear stress was significantly different among the mussel species (p = 0.002). Multiple comparison tests showed that RSS for E. pullata was significantly lower than for F. burkei (p = 0.007) and P. strodeanum (p = 0.001). Similar to 8M1, the two threatened species did not have significantly different RSS (p = 0.358) (). The total mussel abundance was significantly negatively correlated with shear stress (ρ = −0.84, p < 0.001) ().
Discussion
Similar to most studies on habitat stability of freshwater mussels (Hardison & Layzer Citation2001; Morales et al. Citation2006; Allen & Vaughn Citation2010), we found negative correlations between shear stress and total mussel abundance at each site. This suggests that locations that experience higher shear stress possess fewer mussels. Davis et al. (Citation2013) examined habitat variables at landscape, mesohabitat, and microhabitat scales at 82 sites in the Klamath River Basin of northwestern California. Predictors of mussel abundance were different across the scales examined; however, they suggested that mussel distribution was primarily driven by the influence of substrate stability and hydraulics.
In general, mussel beds are present at locations that experience low sediment mobilization even at high flows, and sediment stability should not affect mussel distribution at low flows (Strayer Citation1999; Morales et al. Citation2006). However, streams in the Choctawhatchee watershed and others with a very high proportion of sand are vulnerable to sediment mobility even at low-flow conditions, which makes sediment stability of vital importance when mussel assemblages are concerned. In streams with gravel, cobble, pebble, and boulder substrates, either sediments are difficult to set in motion by flowing water, or mussels may find refuge at high-flow events. Stream systems without these components lack adequate refuge against flowing water (except for large woody debris and log, if present (Niraula et al. Citation2015)), and are much more vulnerable to sediment movement and transportation creating unstable habitats.
As several habitat data points for threatened species had an RSS greater than one, the current study suggested that many microhabitats where the threatened species were found were unstable. Moreover, several individuals of the threatened species were found in locations where RSS was greater than 1.25, a number used by Morales et al. (Citation2006) as the maximum tolerable RSS for mussels. Shear stress at high-flow conditions is more limiting to mussel species richness and abundance than at low-flow conditions (Allen & Vaughn Citation2010). Since samples in the current study were taken during low flows, more unstable and extreme habitat conditions for the threatened species can be expected during high-flow conditions.
Differences in microhabitat stability among species
Our study is possibly the first one to ever demonstrate significant differences in RSS among mussel species, especially among threatened and common species. E. pullata at all three sites were associated with microhabitats that had lower RSS compared to threatened species’ microhabitats, likely due to its occurrence in slack water regions or toward the stream banks. As a majority of E. pullata was found along the stream banks, current velocity for E. pullata was much lower compared to threatened species, which lowered the shear stress exerted on the bed. However, occurrence of several individuals of threatened species in unstable microhabitats is unlikely due to active selection or preference for instability, since no study has found increased mussel abundance with higher shear stress. Fast flow allows for constant renewal of water, transport of excess organic matter, dissolution of atmospheric oxygen, and a lower water temperature which increases solubility of atmospheric oxygen in the water. Therefore, we speculate that the occurrence of P. strodeanum and F. burkei at locations with higher RSS is possibly related to physicochemical water properties at those locations and not an active preference for unstable habitat.
Additionally, calculated shear stress values and the baseflow sampling conditions suggest that higher RSS at locations where threatened species were found is not likely due to excessive shear stress exerted by flowing water on streambed. Instead, low critical shear stress of the substrate predominantly composed of sand yielded higher values of RSS. Therefore, even the suitable mussel microhabitats, such as deep water (∼1 m) with normal currents in these coastal plain streams experience sediment mobility due to a very low critical shear stress of substrate. We believe that the high-quality habitats, such as those occupied by the target-threatened mussel species, are deteriorating in coastal plain streams due to the sensitive nature of the substrate and streambed. Depth is directly and current velocity is inversely proportional to shear stress. At high-flow conditions, both current velocity and depth generally increase. If both increase in the same proportion, shear stress also increases creating more unstable microhabitats during high-flow events as current velocity affects shear stress more than depth does (see formulae in Calculations). Di Maio and Corkum (Citation1995) suggested that mussels may burrow deeper into the sediment to avoid dislodgement at extreme flow conditions, and sediment entrainment and instability may be of little relevance to mussels with this ability. Whether or not a burrowing response to sediment instability exists for the target threatened mussel species has yet to be determined. However, given their declining populations, it is unlikely that the threatened species under our study are doing well in unstable sediments.
Impacts of sedimentation on microhabitat stability
Sedimentation has been documented as a major issue in the Choctawhatchee River watershed (Morris et al. Citation2003), and mussel survival is threatened in streams with high rates of sediment transport and accumulation (Arbuckle & Downing Citation2002). Streams selected in our study are typical representatives of high-quality southeastern coastal plain streams. Therefore, we suggest that our findings of hydraulic instability of mussel species’ habitat also represent similar conditions for other rare and endangered species within the Coastal Plains. Constant addition of sediments into these coastal plain streams prevents streambeds from settling and results in unstable habitats. Fusconaia burkei is endemic to the Choctawhatchee River basin, and P. strodeanum is endemic to the Escambia, Yellow, and Choctawhatchee River basins of Alabama and Florida. (U.S. Fish and Wildlife Service Citation2012). Since the two streams studied in the current study are strongholds for the target-threatened species in the watershed, continued sedimentation, resulting in unstable habitats, may cause the target threatened species’ populations to continue to decline and possibly disappear in this watershed. We also believe that most watersheds and freshwater mussels in the Southeastern Plains are likely affected by heavy sedimentation and unstable habitats. A majority of the watersheds in Alabama are affected by siltation and habitat alteration, and streams designated for ‘fish and wildlife’ use are listed in the 2014 303(d) list of impaired waterways (Alabama Department of Environmental Management Citation2014).
Aid in microhabitat stabilization
Stability measured under the current study accounts only for the stability of sediments. Instream habitat structures, such as leaf packs, root mats, root wads, woody debris, and logs, form a major component of mussels’ microhabitat (Niraula et al. Citation2015). Harriger et al. (Citation2009) suggested that woody debris helps to stabilize mussel habitat. The majority (67%–96%) of the individuals of all species at all three sites were found in close proximity to at least one such instream habitat structure. Sediment stability for mussels at these sites may be somewhat higher than indicated due to the presence of such habitat forming structures. In addition to instream cover as refuge, mussel survival in habitats where RSS was greater than one may be attributed to avoidance behaviors, such as vertical migration or migration to areas where RSS < 1, such as stream banks, during high shear stress conditions. Future studies need to examine the existence and type of avoidance behaviors in the target-threatened species. Despite the aid due to instream habitat and avoidance behaviors, entrained and transported sediments may cause shell erosion. Studies also need to be undertaken to determine the degree of association between sediment movement and shell erosion that eventually lead to mortality.
Conclusion
Our study not only examined the relationship between stability and mussel abundance but also determined differences in sediment stability among mussel species. Differences found in our study suggest that even though mussel abundance and shear stress are generally negatively correlated, some species may experience unstable sediments more than the others due to the differences in habitat use. E. pullata appeared to avoid unstable microhabitats in the streams by inhabiting slack water regions and stream banks, whereas 15% at BS and 8M2, and up to one-third of the threatened species’ individuals at 8M1 were found at locations with more unstable sediments. For streams with beds composed mainly of sand, sediment stability is of much greater importance compared to streams with gravel, cobble, pebble, etc., as instability can be caused even at low flows in sandy streams. Extrinsic sedimentation is the source of the predominating proportion of sand in streambeds of the Choctawhatchee River watershed (Cook Citation2007), and potentially linked to declining populations of the threatened mussel species under the current study and their recent (November 2012) federal listing. However, abilities of mussel species to avoid unstable habitat conditions via migration to stable areas or digging deeper into the substrate should be examined to ensure that the effects of unstable microhabitats such as, burial and dislodgement are not being overestimated. Instream structures, such as woody debris, root mat, root wad, and logs, may help stabilize microhabitats; however, entrained and suspended particles are still capable of shell abrasion, an aspect of mussel–sediment association that is in much need of quantification.
Acknowledgments
We thank Evelyn Reatégui-Zirena for field assistance, Kesley Gibson for review of the manuscript, and Sandy Pursifull, Adam Kaeser (U.S. F&WS, Panama City Field Office), and Jeff Garner (Alabama Department of Conservation and Natural Resources) for ideas associated with this project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Bijay B. Niraula
Bijay Niraula completed his M.S. from Troy University, where he conducted research on mussels and crayfish. Currently, Mr. Niraula works as a Lead Ecologist at Moreland Altobelli Associates, Inc., Duluth, GA, USA.
J. Murray Hyde
J. Murray Hyde is a PhD candidate at Virginia Tech where he monitors freshwater mussel populations and is developing guidelines for assessing injuries to freshwater mussels.
Jonathan M. Miller
Jonathan M. Miller is a lecturer at Troy University where he researches stream ecology related to mussels, crayfish, and fish assemblages.
Paul M. Stewart
Dr Paul M. Stewart is a professor and endowed chair at Troy University where he researches streams ecology related to mussels, crayfish, and fish assemblages.
References
- Alabama Department of Environmental Management, 2014. Alabama 2014 303(d) List. Available from: http://www.adem.state.al.us/programs/water/wquality/2014AL303dList.pdf
- Allen DC, Vaughn CC. 2010. Complex hydraulic and substrate variables limit freshwater mussel species richness and abundance. J North Am Benthol Soc. 29:383–394.
- Arbuckle KE, Downing JA. 2002. Freshwater mussel abundance and species richness: GIS relationships with watershed land use and geology. Can J Fish Aquat Sci. 59:310–316.
- Cook MR. 2007. Analysis of sediment loading rates and impacts of land-use change on the D'Olive and Tiawasee Creek watersheds, Baldwin County, Alabama. Tuscaloosa: Geological Survey of Alabama. Available from: http://www.mobilebaynep.com/images/uploads/library/DOlive-and-Tiawasee-Creeks-Sedimentation-Final-Report.pdf.
- Daraio JA, Weber LJ, Newton TJ. 2010. Hydrodynamic modeling of juvenile mussel dispersal in a large river: the potential effects of bed shear stress and other parameters. J North Am Benthol Soc. 29:383–851.
- Davis EA, David AT, Norgaard KM, Parker TH, McKay K, Tennant C, Soto T, Rowe K, Reed R. 2013. Distribution and abundance of freshwater mussels in the mid Klamath subbasin, California. Northwest Sci. 87:189–206.
- Di Maio J, Corkum LD. 1995. Relationship between the spatial distribution of freshwater mussels (Bivalvia: Unionidae) and the hydrological variability of rivers. Can J Zool. 73:663–671.
- French SK, Ackerman JD. 2014. Responses of newly settled juvenile mussels to bed shear stress: implications for dispersal. Freshw Sci. 33:46–55.
- Gangloff MM, Feminella JW. 2006. Stream channel geomorphology influences mussel abundance in southern Appalachian streams, USA. Freshw Biol. 52:64–74.
- Gordon ND, McMahon TA, Finlayson CJ, Gippel CJ, Nathan RJ. 2004. Stream hydrology: an introduction for ecologists. 2nd ed. West Sussex: John Wiley and Sons.
- Hardison BS, Layzer JB. 2001. Relations between complex hydraulics and the localized distribution of mussels in three regulated rivers. Reg Riv Res Mgmt. 17:77–84.
- Harriger K, Moerke A, Badra P. 2009. Freshwater mussel (Unionidae) distribution and demographics in relation to microhabitat in a first-order Michigan stream. Mich Acad. 39:149–162.
- Heath WW, Stewart PM, Simon TP, Miller JM. 2010. Distributional survey of crayfish (Crustacea: Decapoda) in wadeable streams in the coastal plains of southeastern Alabama. Southeast Nat. 9:139–154.
- Johnson PD, Brown KM. 2000. The importance of microhabitat factors and habitat stability to the threatened Louisiana pearl shell, Margaritifera hembeli (Conrad). Can J Zool. 78:271–277.
- Morales Y, Weber LJ, Mynett AE, Newton TJ. 2006. Effects of substrate and hydrodynamic conditions on the formation of mussel beds in a large river. J North Am Benthol Soc. 25:664–676.
- Morris CC, Sawyer JA IV, Bennett HH, Robinson CD. 2003. Effects of sediment quantity on the health of aquatic ecosystems: a case study on depth of fines in Coastal Plain streams in Alabama. In: Simon TP, editor. Biological response signatures: indicator patterns using aquatic communities. Boca Raton (FL): CRC Press; p. 113–124.
- Niraula, BB, Miller JM, Hyde, JM, Stewart, PM. 2015. Instream habitat associations among three federally threatened and a common freshwater mussel species in a southeastern watershed. Southeast Nat. 14:221–230.
- Pilarczyk MM, Stewart PM, Shelton DN, Blalock-Herod HN, Williams JD. 2006. Current and recent historical freshwater mussel assemblages in the Gulf Coastal Plains. Southeast. Nat. 5:205–226.
- Sokal RR, Rohlf FJ. 2012. Biometry: the principles and practice of statistics in biological research. 4th ed. New York: W.H. Freeman and Co.
- Steuer JJ, Newton TJ, Zigler SJ. 2008. Use of complex hydraulic variables to predict the distribution and density of unionids in a side channel of the Upper Mississippi River. Hydrobiology. 610:67–82.
- Strayer, DL. 1999. Use of flow refuges by unionid mussels in rivers. J North Am Benthol Soc. 18:468–476.
- United States Fish and Wildlife Service. 2012. Federal Register, 77 (196):61663-61719. Available from: http://www.gpo.gov/fdsys/pkg/FR-2012-10-10/html/2012-24161.htm.
- Wilcove DS, Master LL. 2005. How many endangered species are there in the United States? Front Ecol Environ 3:414–420.