ABSTRACT
Experimental acidification of a tropical stream was conducted to measure the effects of declining pH on aquatic macroinvertebrates. The mechanisms by which anthropogenic acidification occur in a freshwater stream system are relatively well understood, while little is known about the natural phenomena of acidification or the corresponding effects on macroinvertebrate assemblages. Previous studies have attempted to model stream acidification using strong acids; however, this is one of the first studies which models stream acidification using the addition of gaseous CO2. This method is a more natural means of modeling stream acidification conditions arising from increased levels of dissolved CO2. We hypothesized that if experimental acidification was expressed most strongly at the injection site and produced a pH gradient downstream, macroinvertebrates should respond to the gradient and employ an escape mechanism to avoid the adverse conditions. Three macroinvertebrate sampling strategies were used: drift nets, leaf pack samples and benthic Surber samples. Samples were evaluated in the lab for macroinvertebrate abundance and taxonomic richness per m3 for drift net samples and benthic samples, and per g leaf material in the leaf pack samples. A maximum decline of 2 units in pH along a gradient was observed associated with the injection of CO2. Results obtained from drift net and benthic sample analysis were inconclusive, possibly because of low stream flow, although analysis of the leaf pack samples indicates lower macroinvertebrate composition at areas of lower pH. The leaf pack samples also show significant macroinvertebrate sensitivity to the most severe pH decline at the injection site.
1. Introduction
Stream acidification has been shown to have negative effects on freshwater ecosystems (Malmqvist & Rundle Citation2002; Dangles et al. Citation2004; Camargo & Alonso Citation2006). Freshwater may be acidified either through the impact of dilution of acid neutralizing capacity, atmospheric deposition (i.e. sulfur and nitrogen deposition), oxidation–reduction (redox) reactions, organic acid inputs, or elevated concentrations of dissolved carbon dioxide (CO2). The mechanisms by which anthropogenic acidification occur are relatively well understood, while little is known about the natural phenomena of acidification. Small et al. (Citation2012) observed that an increase in labile carbon storage in the forest soil associated with the most severe drought during ENSO-1998 produced a pH decline of ∼3 units in some streams at La Selva (Costa Rica). Small et al. (Citation2012) suggested that the pulse of CO2-saturated runoff when the rains returned contributed to higher levels of dissolved CO2, lowering the pH of shallow streams. Similar findings have been observed by Johnson et al. (Citation2008) in groundwater springs, where high soil CO2 concentrations contribute to a pH of 4.65, compared with first- and second-order streams with lower CO2 concentrations and a neutral pH.
According to the hypothesis of acidification mediated by CO2, global climate change could intensify natural acidification events due to a redistribution of precipitation across different regions, which would result in changes in rainfall patterns. Seasonal wet and dry patterns are expected to be intensified in a given area as the effects of global warming increase, and droughts are predicted to have both earlier onset and greater severity (Trenberth et al. Citation2014). These droughts could increase carbon concentration in the forest soil due to increased microbial respiration and tree root death. By these mechanisms, more severe and frequent droughts are expected to stimulate strong and unpredictable pH changes in streams. These unusual acidification events can cause significant stress on aquatic communities, with detrimental effects on both stream biota and ecological functions (e.g. Ramírez et al. Citation2006).
Macroinvertebrate responses to acidification events in tropical streams are poorly understood (Ramírez et al. Citation2006; Small et al. Citation2012), but have been well documented in temperate streams (e.g. Rosemond & Reice Citation1992; Lepori et al. Citation2003; Lepori & Ormerod Citation2005). Stream acidification simplifies macroinvertebrate assemblages and reduces taxonomic richness (Lepori & Ormerod Citation2005; Durance & Ormerod Citation2007), particularly among Ephemeroptera and grazers in general (e.g. Rosemond & Reice Citation1992). A common response of macroinvertebrates to decreases in pH is entering into drift and allowing the current to transport them to a new area. Ardón et al. (Citation2013) observed increased macroinvertebrate drift in response to experimental acidification in tropical lowland streams; the drift was dominated by Ephemeroptera and Chironomidae. Some experimental studies have attempted to demonstrate the effects of acidification on stream macroinvertebrates using strong acids; some examples are the use of nitric acid by Dangles and Guérold (Citation2000), and hydrochloric acid in a study on tropical streams (Ardón et al. Citation2013). Strong acids are good alternatives for the simulation of acid rain or the effects of mining, but not for the study of the effects of CO2 pulses related to organic matter decomposition.
The goal of this study was to understand the effects of episodic acidification events on macroinvertebrates in a tropical stream. In order to model stream conditions resulting from increased dissolved CO2, gaseous CO2 was added directly to the stream to cause an experimental decline in pH. It is well known that a major stream buffering system is the carbonate-bicarbonate system, in which gaseous CO2 exchange at the water surface reacts with water molecules to form H2CO3, a weak acid which protonates easily to produce H+ and HCO3– ions (Small et al. Citation2012; Ardón et al. Citation2013; Hasler et al. Citation2016). With the addition of injected gaseous CO2, the equilibrium of this reaction may be driven to produce more H2CO3 and cause the stream to become acidified. We hypothesized that (1) the injected CO2 would react with the water and cause a decline in stream pH, which could result in adverse conditions for aquatic organisms, and (2) macroinvertebrates exposed to the increased acidity of the environment would respond by entering into drift or employing an escape mechanism. Consequently, we predicted to find (1) the experimental acidification expressed most strongly at the CO2 injection site and a pH gradient to be formed downstream, and (2) if the acidification effect was acute enough to induce changes in macroinvertebrate composition, the macroinvertebrate assemblages would respond to the pH gradient and have the most organisms in drift (or in fewer numbers in the benthic layer and leaf litter) near to the CO2 injection site.
2. Site description
The study was performed in July 2014 at El Verde Field Station, which is within the Luquillo Experimental Forest (latitude 18°19′ N, longitude 65°45′ W). El Verde Field Station is located at an elevation of 350 m and is part of El Yunque National Forest. El Yunque is a subtropical wet forest under the Holdridge Life Zone classification which encompasses 113.32 km2 and has mountains that reach 1076.9 m asl (Ewel & Whitmore Citation1973). The annual rainfall at El Verde averages over 3460 mm and is evenly distributed throughout the year. The average pH of rainfall measured at El Verde was found to be 4.92 (Gioda et al. Citation2013). The driest period begins in January and ends in April with a relative humidity ranging from 60% to 100%. The mean temperature is 22.8 °C, while mean maximum temperature is 27 °C and mean minimum temperature is 21.1 °C.
The Buruquena stream, a first-order stream at El Verde, was chosen as the study site. The stream is a tributary in the Espiritu Santo watershed that drains a small, steep catchment and is characterized by a series of pools interspersed with boulder-lined riffles. The stream is bordered by dense riparian vegetation dominated by Dacryodes excelsa (tabonuco), Guarea guidonia, Cecropia sp. and Prestoea montana (sierra palm). Stream temperature ranges from 22.1 °C to 23.5 °C (unpublished data). Discharge is variable, and is strongly influenced by local storm conditions. Dominant substrates were bedrock, large boulders, and cobble, as well as some gravel and sand found mainly in pools.
3. Methods
3.1. Experimental acidification
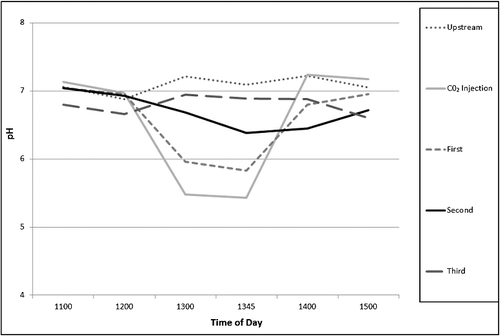
The Buruquena stream was acidified by bubbling compressed gaseous CO2 from a tank through a hose submerged in the stream. Acidification was for one hour, beginning at 1255 when the CO2 tank was opened and ending at 1355 when the tank was closed. The whole experiment took place during the same day. The stream pH was measured at 1100, 1200, 1300, 1345, 1400 and 1500. Measurements were taken at the CO2 injection site and at four stations located over a total distance of 35 m. One station was located 4 m upstream from the CO2 injection site (Upstream) and the other stations were located at 4 m (First station), 10 m (Second station) and 29.5 m downstream from the injection site (Third station), respectively.
3.2. Macroinvertebrate assemblage characterization
3.2.1. Surber samples
To characterize the aquatic macroinvertebrate assemblages in the Buruquena stream before the experiment, three Surber samples (area: 0.036 m2; mesh size: 500 µm) were taken prior to the CO2 injection at each sample site except the injection site. The same procedure was used the next day after the CO2 injection to assess any change caused by the decline in pH. All material collected was preserved in the field with 95% ethanol and transported to the laboratory, where aquatic macroinvertebrates were separated using a stereoscopic microscope. All macroinvertebrates collected were identified to the family level for aquatic insects using Merritt et al. (Citation2008), Springer et al. (Citation2010), and Gutiérrez-Fonseca et al. (Citation2013). Specimens were deposited in the UPRRP El Verde Field Station insect collection.
3.2.2. Leaf pack samples
Another measure of macroinvertebrate response to acidification was taken using leaf packs. Each pack was composed of approximately 7 g of G. guidonia (Meliaceae) leaves, a key riparian tree widely used in leaf-litter decomposition experiments in the Luquillo Experimental Forest (e.g. Rincón & Covich Citation2014). Six packs were placed at the Upstream station, directly at the injection site, and at the First and Second stations, with an additional seventh pack at each station in case a pack was lost due to some environmental disturbance. The leaf packs were left in the stream to develop for 5 days before the acidification experiment. Before the CO2 injection, three packs were collected from each station and preserved in the field with 95% ethanol. The CO2 tank was then opened for 1 hour from 1220 to 1320 of the same day. After the acidification, three more leaf packs were collected from each of the stations and preserved in the same way. Macroinvertebrates were separated from each sample using a stereoscopic microscope and preserved according to the procedure given for the Surber samples. The leaf pack samples were evaluated in the laboratory for abundance and taxonomic richness per g.
3.3. Drift response
Macroinvertebrate samples were collected using three small drift nets (mouth: 0.06 m2; length: 0.5 m; mesh size: 250 µm) attached to a board 0.32 m apart. Samples were collected before the acidification at 1100 and 1200, and after the acidification at 1300 (beginning of CO2 injection), 1400 (end of CO2 injection), and 1500. For each sample taken, the drift net was completely submerged and the water column was filtered for 15 min. All samples were processed in the same manner as above.
Stream velocity was measured by a Marsh McBirney® current meter for each drift net. Macroinvertebrate drift density and taxonomic richness were calculated by dividing the number of individuals in a sample by the volume of water sampled. Water volume was calculated by multiplying net area, current velocity, and sampling time. Each sample was evaluated for abundance and taxonomic richness per m3.
4. Statistical analyses
For the macroinvertebrate drift experiment, a mixed model analysis of variance (ANOVA) was used to compare the macroinvertebrate assemblage structure at each station before and after the acidification. Separate analyses were run for macroinvertebrate abundance and richness. Time was specified as a random factor. The independent factors incorporated in the between effects design were station, treatment (before/after acidification), and the interaction between station, treatment, and time. The Euclidean distance was used as the distance measure.
To analyze changes in the Surber samples and the leaf pack samples, a mixed model ANOVA was also used to compare the macroinvertebrate structure at each station before and after the acidification. Separate analyses were run for macroinvertebrate abundance and richness and for each individual station. Station was specified as a random factor to account for the lack of independence in letting each station serve as its own control before and after the acidification. The independent factor incorporated in the between effects design was treatment (before/after acidification). All analyses were performed using Statistica v12 (Statsoft Inc., Tulsa, OK, USA).
5. Results
5.1. Experimental acidification
During the CO2 injection experiment, we observed a decline in stream pH. The Upstream station maintained a constant pH over the experiment, remaining very near 7 with fluctuations between 6.88 and 7.22. The injection site experienced the largest drop in pH: the initial measurement was 7.13 before the injection, and after the CO2 tank was opened at 1255 and the pH measured at 1300, it had dropped to 5.48. This low pH persisted and was measured as 5.43 at 1345 until the CO2 tank was closed at 1355, at which point the pH began to rise to neutral levels of 7.24 at 1400 and 7.17 at 1500. The same trend may be seen with less severity at the other stations and follows a gradient that lessens in acidity farther away from the source of injection. The First station experienced a pH decrease from ∼7 to 5.96 at 1300 and 5.83 at 1345 during the time the tank was open, the Second station dropped from ∼7 before the injection to 6.68 at 1300 and 6.38 at 1345, and the Third station experienced little change during the injection and fluctuated between 6.94 and 6.6 ().
5.2. Macroinvertebrate assemblage characterization
5.2.1. Surber samples
The most prevalent groups in the benthic samples were Leptophlebiidae (Ephemeroptera), Chironomidae (Diptera), and Hydropsychidae (Trichoptera). No significant changes in macroinvertebrate abundance or taxonomic richness were observed associated with acidification for the Upstream station (F1,4 = 0.39, p > 0.05, n.s.; F1,4 < 0.01, p > 0.999, n.s., respectively), First station (F1,4 = 2.40, p > 0.05, n.s.; F1,4 = 4.00, p > 0.05, n.s., respectively), Second station (F1,4 = 0.06, p > 0.05, n.s.; F1,4 = 0.01, p > 0.05, n.s., respectively), or Third station (F1,4 = 1.93, p > 0.05, n.s.; F1,4 = 2.50, p > 0.05, n.s., respectively).
5.2.2. Leaf pack samples
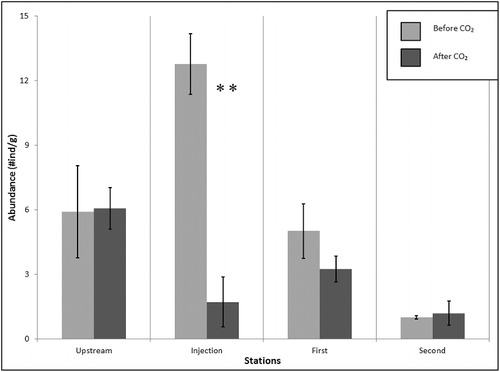
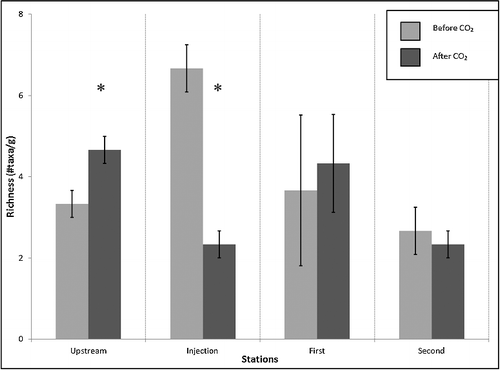
Leaf pack analysis showed the clearest macroinvertebrate response to the CO2 acidification. At all stations the dominant group collected was Leptophlebiidae (Ephemeroptera), followed by Coenagrionidae (Odonata). No significant difference was found in macroinvertebrate abundance before and after acidification at the Upstream station (F1,4 > 0.01, p > 0.05, n.s.; ); however, a significant difference was found in the taxonomic richness at the Upstream station associated with acidification (F1,4 = 8.00, p = 0.047; ). At the injection site, there was a highly significant difference between the macroinvertebrate abundance (F1,4 = 36.89, p = 0.004; ) and taxonomic richness (F1,4 = 21.13, p = 0.010; ) before and after the CO2 injection. At the First station, no significant difference was found in macroinvertebrate abundance (F1,4 = 1.58, p > 0.05, n.s.; ) or taxonomic richness (F1,4 = 0.29, p > 0.05, n.s.; ) associated with acidification. Similarly, no significant differences were found in macroinvertebrate abundance (F1,4 = 0.11, p > 0.05, n.s.; ) or taxonomic richness (F1,4 = 0.20, p > 0.05, n.s.; ) at the Second station. Although significant differences were not observed in the First and Second stations in either measure of macroinvertebrate composition, a trend showing a decline of macroinvertebrates in terms of abundance () and taxonomic richness () was observed after the addition of CO2 at these stations. A trend was also observed at the Upstream station showing an increase in macroinvertebrate abundance after CO2 was added ().
5.3. Drift response
Macroinvertebrate drift density was consistently low and the effects of acidification were not clear. The drift samples were dominated by Baetidae (Ephemeroptera). No significant change in macroinvertebrate abundance (F1,15 = 3.09, p > 0.05, n.s.) or taxonomic richness (F1,15 = 3.41, p > 0.05, n.s.) was observed associated with acidification. A significant difference was found in the taxonomic richness measured between stations (F1,15 = 3.69, p = 0.036), although no significant differences were detected between stations using the Tukey HSD post hoc comparison. For abundance between stations, no significant differences were detected (F1,15 = 2.81, p > 0.05, n.s.).
6. Discussion
The hypothesis that the injection of CO2 in the Buruquena stream should decrease the pH and strongly affect the composition and structure of aquatic macroinvertebrates was supported by a decline of up to 2 pH units at the site of injection and a change in richness and abundance of aquatic organisms. The pH decline observed in this study is consistent with the hypothesis put forward in Small et al. (Citation2012) and Ardón et al. (Citation2013) which place increased concentrations of CO2 as one of the primary factors driving acidification in streams. This study further demonstrated that acidic conditions could be created in a stream with the addition of CO2, and that the injection would create severe enough conditions to induce a macroinvertebrate response. We did not observe an increase in drift during the drift experiment, which could be because of low stream flow due to a drought that affected the study site when it was conducted. Nevertheless, we observed a clear effect on the macroinvertebrate composition and structure in the leaf pack samples as a product of the acidification.
Leaf pack analysis showed the clearest and most compelling decrease in macroinvertebrate composition. At the site of the CO2 injection, a vast difference was seen in insect abundance and a similarly large difference was seen in taxonomic composition after the stream was acidified. These provide good evidence that some kind of escape is being made at the site where the pH decrease is most severe. Possibly, increased CO2 concentrations may have an effect on leaf litter decomposition in streams and the development of microorganism communities, which could be why the leaf pack samples showed the most change due to acidification. This would corroborate the results of Mulholland et al. (Citation1987), who showed that both microorganism communities and leaf litter decomposition are negatively impacted by decreased pH. According to Sterner and Elser (Citation2002), leaf litter exhibits a wider range of C:P:N ratios than living leaves, making it a strong factor in nutrient cycling. The increase in macroinvertebrate composition present at the Upstream station after the CO2 injection in the leaf pack samples could also be the result of upstream taxis, another mechanism by which insects can escape disturbance (Berghahn et al. Citation2012). These data reinforce the view that a significant macroinvertebrate response was caused by the acidification.
The dominance of Leptophlebiidae (Ephemeroptera), Chironomidae (Diptera), and Hydropsychidae (Trichoptera) in the Surber samples is understandable since these insects most commonly make their habitat in the gravel and leaf litter at the stream bottom. The primary escape mechanism of these families, particularly Leptophlebiidae, is burrowing or hiding under rocks to avoid the disturbance (Ramírez & Hernández-Cruz Citation2004), so their presence in the benthic level of the stream is not surprising. Leptophlebiidae also predominated in the leaf pack samples, which was expected as they can be commonly found in larger masses of decomposing vegetation (Jandry et al. Citation2014). Studies have shown that mayflies prefer to feed on algae and cyanobacteria (Dudgeon Citation2008), and the microorganisms present on the surface of the leaf substrata would make the leaf packs a good environment for Leptophlebiidae. The overwhelming presence of Baetidae in the drift net samples concurs with a study by Berghahn et al. (Citation2012) and can be explained because the primary escape mechanism of this family is entering into drift to avoid adverse conditions. The behavior of the shrimp at the First station below the injection site was very interesting, because once the CO2 tank had been opened during the acidification experiment they began swimming downstream and leaping out of the water to avoid the pH change. The next day when we returned to conduct the post-acidification Surber samples, we found several dead shrimp at the First station, which we believed was due to the acidification experiment the previous day. The behavior of the shrimp in response to acidification would make a good subject for a future study.
It was expected that the number of insects collected in drift nets at each station throughout the acidification experiment would reflect the changing pH. A decrease in pH at a station ought to have corresponded to a proportional increase in specimens collected. However, the drift results for this experiment were inconclusive, especially as compared with the results of Ardón et al. (Citation2013). For the drift experiment in this study, a significant difference in macroinvertebrate richness was detected after the injection of CO2. However, this result was only barely statistically significant and post hoc comparisons could not detect significant differences between the stations. The macroinvertebrate drift measured in response to short-term acidification by HCl by Ardón et al. (Citation2013) showed significant results below the HCl injection site, where the macroinvertebrate drift was found to be higher than above the injection site. The drift results were significant not only at the station 10 m below the injection but also 100 m below in both the well-buffered and poorly-buffered streams (Ardón et al. Citation2013). A possible reason for this is that unlike dissolved CO2, HCl has no way of leaving the stream once it is introduced, while CO2 is able to dissolve back out of solution by passive diffusion when the atmospheric concentration becomes lower than the concentration in the stream. According to Cole et al. (Citation2007), excess CO2 dissolved in the stream in the form of HCO3– is respired as CO2 ‘meters to kilometers downstream of its origin when it evades across the stream surface’, which is consistent with the formation of a pH gradient we observed corresponding to the distance downstream from the CO2 injection site. The macroinvertebrate response observed using HCl could be very different from the response caused by increased CO2 (Ardón et al. Citation2013), possibly because no mechanism is present which would allow HCl to leave solution.
One assumption held from the beginning of the experiment was that a consistent population of insects existed along the extent of stream being studied. One explanation for our inconclusive drift and benthic data is the possibility that this assumption was not met and there were varying existing insect populations at each site. In addition, the summer during which this study was conducted was considered abnormally dry, so the low streamflow present could explain the low drift samples collected. According to Berghahn et al. (Citation2012), under normal and low-flow stream conditions, macroinvertebrate drift must be considered a minor downstream shift within a range of only a few decimeters to meters. The macroinvertebrates that entered into drift could have been unable to drift far enough downstream to be caught in the sampling nets, or could have not entered into drift at all and used another mechanism to escape the acidic conditions. The low stream flow experienced over the course of this study, while impeding our understanding of macroinvertebrate drift, was beneficial however for facilitating a more pronounced drop in pH than would have been possible in a stream with greater volume.
In summary, the results obtained from this study are noteworthy because the correlation between CO2 injection and decreased pH could indicate the possible effects that could result from an increase in dissolved CO2 due to climate change. Decreased pH has been shown by this study to be linked to increased concentrations of gaseous CO2 dissolving into the stream. There is also now empirical data associated with the tolerance of macroinvertebrate taxa, particularly insects, to deviations from environmental CO2 levels, an area Hasler et al. (Citation2016) identify as a knowledge gap. According to Small et al. (Citation2012), seasonal pH patterns are amplified by drought years, with lower pH drops experienced during the ensuing wet season. Therefore, the pH in the Buruquena Stream may be expected to drop more significantly when the wet season returns to Puerto Rico because of the dry summer experienced during this study. The in situ nature of our study allows inferences to be made within a natural ecological system with its many levels and factors in play, and stream acidification was mediated by a well-recognized buffering system rather than by the addition of strong acids in order to put episodic acidification in the context of CO2 pulses. The Earth's carbon cycle is projected to continue to respond to climate change and increasing atmospheric CO2 (Ciais et al. Citation2013), which will increasingly impact overall ecosystems into the future. The acidic conditions created with the injection of CO2 in this study were shown to have an effect on macroinvertebrate assemblages, and the role of increased dissolved CO2 on these communities may now be better understood.
Acknowledgements
This research was made possible by the Research Experience for Undergraduates (REU) program funded by the National Science Foundation (NSF-DBI1062769, Ramírez). In particular, the authors wish to thank the REU Site: Tropical Ecology and Evolution at El Verde Field Station, and Alonso Ramírez, for providing the opportunity, facilities and support needed to complete this project. Thanks are also extended to Alonso Ramírez for editing the manuscript, Pedro J. Torres and Bethany Vázquez for helping with sampling, and all the 2014 REU students and mentors for their input.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Crystal C. Klem
Crystal C. Klem received her biology BS from University of Dallas in 2015, and is currently pursuing an entomology PhD from Purdue University.
Pablo E. Gutiérrez-Fonseca
Pablo E. Gutiérrez-Fonseca received his ecology and systematics PhD in June 2016. He is currently a post-doctoral associate with the Luquillo LTER program at University of Puerto Rico, Rio Piedras.
References
- Ardón M, Duff JH, Ramírez A, Small GE, Jackman AP, Triska FJ, Pringle CM. 2013. Experimental acidification of two biogeochemically-distinct neotropical streams: buffering mechanisms and macroinvertebrate drift. Sci Total Environ. 443:267–277.
- Berghahn R, Mohr S, Hubner V, Schmiediche R, Schmiedling I, Svetich-Will E, Schmidt R. 2012. Effects of repeated insecticide pulses on macroinvertebrate drift in indoor stream mesocosms. Aquat Toxicol. 122–123:56–66.
- Camargo JA, Alonso A. 2006. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int. 32(6):831–849.
- Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, et al. 2013. Carbon and Other Biogeochemical Cycles. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge (UK): Cambridge University Press.
- Cole JJ, Prairie YT, Caraco NF, McDowell WH, Tranvik LJ, Striegl RG, Duarte CM, Kortelainen P, Downing JA, Middelburg JJ, Melack J. 2007. Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems. 10:171–184.
- Dangles OJ, Gessner MO, Guérold FA, Chauvet E. 2004. Impacts of stream acidification on litter breakdown: implications for assessing ecosystem functioning. J Appl Ecol. 41(2):365–378.
- Dangles OJ, Guérold FA. 2000. Structural and functional responses of benthic macroinvertebrates to acid precipitation in two forested headwater streams (Vosges Mountains, northeastern France). Hydrobiologia. 418(1):25–31.
- Dudgeon D (ed). 2008. Tropical stream ecology. London (UK): Academic Press.
- Durance I, Ormerod SJ. 2007. Climate change effects on upland stream macroinvertebrates over a 25-year period. Glob Change Biol. 13:942–957.
- Ewel JJ, Whitmore JL. 1973. The ecological life zones of Puerto Rico and the U.S. Virgin Islands: USDA Forest Service, Institute of Tropical Forestry. Research Paper ITF-018.
- Gioda A, Mayol-Bracero OL, Scatena FN, Weathers KC, Mateus VL, McDowell WH. 2013. Chemical constituents in clouds and rainwater in the Puerto Rican rainforest: potential sources and seasonal drivers. Atmos Environ. 68:208–220.
- Gutiérrez-Fonseca PE, Rosas KG, Ramírez A. 2013. Aquatic insects of Puerto Rico: a list of families. Dugesiana. 20(2):215–219.
- Hasler CT, Butman D, Jeffrey JD, Suski CD. 2016. Freshwater biota and rising pCO2? Ecol Lett. 19(1):98–108.
- Jandry J, Brulin M, Parinet B, Grandjean F. 2014. Ephemeroptera communities as bioindicators of the suitability of headwater streams for restocking with white-clawed crayfish, Austropotamobius pallipes. Ecol Indic. 46:560–565.
- Johnson MS, Lehmann J, Riha SJ, Krusche AV, Richey JE, Ometto JPHB, Couto EG. 2008. CO2 efflux from Amazonian headwater streams represents a significant fate for deep soil respiration. Geophys Res Lett. 35:L17401.
- Lepori F, Barbieri A, Ormerod SJ. 2003. Effects of episodic acidification on macroinvertebrate assemblages in Swiss alpine streams. Freshw Biol. 48:1873–1885.
- Lepori F, Ormerod SJ. 2005. Effects of spring acid episodes on macroinvertebrates revealed by population data and in situ toxicity tests. Freshw Biol. 50:1568–1577.
- Malmqvist B, Rundle S. 2002. Threats to the running water ecosystems of the world. Environ Conserv. 29(02):134–153.
- Merritt RW, Cummins KW, Berg MB, editors. 2008. Aquatic insects of North America. 4th ed. Dubuque (IA): Kendall/Hunt.
- Mulholland PJ, Palumbo AV, Elwood JW, Rosemond AD. 1987. Effects of acidification on leaf decomposition in streams. J N Am Benthol. 6(3):147–158.
- Ramírez A, Hernández-Cruz LR. 2004. Aquatic insect assemblages in shrimp-dominated tropical streams, Puerto Rico. Biotropia. 36:259–266.
- Ramírez A, Pringle CM, Douglas M. 2006. Temporal and spatial patterns in stream physicochemistry and insect assemblages in tropical lowland streams. Freshw Sci. 25:108–125.
- Rincón J, Covich A. 2014. Effects of insect and decapod exclusion and leaf litter species identity on breakdown rates in a tropical headwater stream. Rev Biol Trop. 62:143–154.
- Rosemond AD, Reice SR. 1992. The effects of stream acidity on benthic invertebrate communities in the South-Eastern United States. Freshw Biol. 27:193–209.
- Small GE, Ardón M, Jackman AP, Duff JH, Triska FJ, Ramírez A, Snyder M, Pringle CM. 2012. Rainfall-driven amplification of seasonal acidification in poorly buffered tropical streams. Ecosystems. 15:974–985.
- Springer M, Ramírez A, Hanson P. 2010. Macroinvertebrados de agua dulce de Costa Rica I. Rev Biol Trop. 58(Suppl 4):97–136.
- Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton (NJ): Princeton University Press.
- Trenberth KE, Dai A, van der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J. 2014. Global warming and changes in drought. Nat Clim Change. 4(1):17–22.