Abstract
Elodea nuttallii (Planch.) H. St John is an invasive alien submerged macrophyte, which grows very fast and dominates in many Chinese waters. Bellamya aeruginosa (Reeve) is a widely distributed benthonic organism in China. This snail species rarely grazes submerged macrophytes and may promote their growth by feeding on epiphyte and phytoplankton. The above two species can commonly be found together in nature waters and their interaction may promote the growth of both species, which could disturb the ecological balance. In this paper, effects of different densities of B. aeruginosa at two different nutrient stages on water quality and the growth of E. nuttallii were studied. The results showed that the growth rates (GRs) of E. nuttallii were not significantly affected by different B. aeruginosa densities in the low nutrient (LN) stage. However, in the high nutrient (HN) stage, the GRs of the aboveground parts of E. nuttallii in the high density (HD) groups were considerably higher than the control (CK) and low density (LD) groups. The water chlorophyll (Chl) and nitrate-nitrogen (NO3-N) contents increased substantially with increasing B. aeruginosa density in the LN stage, while the Chl and NO3-N contents in the LD groups were significantly higher than in the HD and CK groups in the HN stage. The results of this paper indicated that B. aeruginosa could promote the growth of E. nuttallii by reducing the Chl contents in the water in high-nutrient environment rather than in low-nutrient environment, which highlighted that B. aeruginosa may strengthen the invisibility of E. nuttallii in eutrophic water caused by human activities.
Introduction
Freshwater snails and submerged macrophytes are both important biological groups in aquatic ecosystem and relate well in many ways (Brönmark and Bronmark Citation1985; Sheldon Citation1987; Zhu et al. Citation2013). Submerged macrophytes are primary producers in aquatic ecosystems. They help to maintain the biodiversity and functions of aquatic ecosystems, mediate the biogeochemical cycles of water nutrients, and maintain clear water state (Carpenter and Lodge Citation1986; Scheffer et al. Citation1992). Submerged macrophytes provide habitat and spawning sites for freshwater snails and can improve their living condition. Freshwater snails can filter phytoplankton in the water and scrape algae attached on submerged macrophytes, which may alleviate the intensity of competition for light and nutrient. The metabolism of freshwater snails have an impact on many physical-chemical indexes of water and sediment, which may change the growth of submerged macrophytes or their community structure (Pieczyńska Citation2003; Li et al. Citation2009; Cao et al., Citation2014).
In this study, we chose the freshwater snail Bellamya aeruginosa (Reeve) and the submerged macrophyte Elodea nuttallii (Planch.) H. St John as our research species. B. aeruginosa is an important benthonic organism, with a wide distribution in waters of Southeast Asia, India and Africa, especially, in waters of Yangtze River basin, Yellow River basin and Yungui Plateau in China (Cai et al. Citation2016; Li et al. Citation2016). It has high genetic diversity and often coexists with aquatic plants (Gu et al. Citation2015). This snail species rarely grazes submerged macrophytes and may promote their growth by feeding on the nutrient and light competitor, epiphyte algae and phytoplankton (Zheng et al. Citation2011; Zhu et al. Citation2013). E. nuttallii was chosen because it is an invasive alien species for Chinese water bodies, which grow very fast and dominate in many waters (Xu et al. Citation2007). These two species can commonly be found together in nature waters and their interaction may promote the growth of both species, which could disturb the ecological balance.
This study investigated effects of the coexistence of B. aeruginosa and E. nuttallii on water quality and the growth of E. nuttallii at two different nutrient levels. The aim of this study is to clarify how B. aeruginosa affects the growth of E. nuttallii and the water quality. We hypothesized that: (a) B. aeruginosa can promote the growth of E. nuttallii and the water quality; (b) the growth of E. nuttallii with B. aeruginosa presence was better than that of E. nuttallii without B. aeruginosa presence when the water was nutrient enriched.
Materials and methods
Experimental design
The experiment was performed in 12 glass tanks (length × width × height: 0.6 × 0.5 × 1.0 m) at the Poyang Lake Model Experimental Research Base in Gongqing city, Jiangxi Province, China. The glass tanks were put in a large greenhouse (180 × 110 × 21 m) whose roof was made of steel frame with large panes of glass, which was very helpful to control light intensity and temperature. The seedlings of E. nuttallii were collected from the aquatic plant nursery in the research base. Healthy macrophytes without branches were selected, which were cut at 15 cm from the top of the branch. The initial fresh and dry weights of E. nuttallii were 0.51 ± 0.06 and 0.055 ± 0.008 g, respectively. The sediment was collected from the Poyang Lake near the base and mixed evenly after removing the stones and debris. The organic matter, total nitrogen and phosphorus contents in the sediment was 5.22%, 1.35 mg/g and 0.91 mg/g, respectively.
The sediment was placed into a plastic rectangular tray (19.5 × 13.5 × 5 cm) to a depth of 4 cm. Eight seedlings of E. nuttallii, which were washed carefully, were planted into each tray at a depth of 3 cm. Six trays containing the planted macrophytes were then placed into the glass tanks. Air aerated tap water was slowly added to the tanks to a height of 0.8 m (120 L water). The 12 glass tanks were divided into three groups with different densities of B. aeruginosa: the control group (CK, no snails), the low-density snail group (low density (LD), 30 snails, density of 125/m3) and the high-density snail group (high density (HD), 90 snails, density of 375/m3). Each group had four replicates.
The experiment was divided into two stages. The first stage was the low nutrient (LN) stage during which no additional nutrients were added, and the main source of nutrients in the system was the sediment. Water quality parameters were measured every 10 d, and this stage lasted for 90 d. After 90 d, three trays of E. nuttallii were collected from each glass tank in order to measure the growth of the macrophytes, and the remaining three trays of E. nuttallii in each tank were used for the second stage of the experiment.
The second stage was the high nutrient (HN) stage. During this stage, ammonium nitrate and potassium dihydrogen phosphate solution were added into each glass tank, so that the initial total nitrogen (N) and phosphorous (P) contents in the water body were approximately 3.2 mg/L and 0.64 mg/L, respectively. Water quality parameters were then measured every 7 d, and this stage lasted for 28 d. At the end of the experiment, the E. nuttallii samples in the remaining three trays were collected from each glass tank for macrophyte growth determination.
Water and macrophyte parameter determination
During the experiment, water temperature (T), dissolved oxygen (DO), pH, conductivity (COND), oxidation-reduction potential (ORP) and chlorophyll (Chl) were measured using a portable multi-parameter water quality analyzer (HQ40D, Hach, USA). The ammonium nitrogen (NH4-N), nitrate-nitrogen (NO3-N) and orthophosphate phosphorus (PO4-P) contents were measured in the laboratory using Chinese national standard methods (Huang Citation2000).
The macrophyte samples were carefully washed with distilled water at least three times and separated into aboveground and belowground parts. They were repeatedly dried with water-absorbing paper until no water dropped by hard shaking. The wet weight of aboveground and belowground biomass of the submerged macrophytes was measured using electronic scale. The growth rate (GR) of the submerged macrophytes was calculated by a formula: GR = (M2 − M1)/dt, where M1 and M2 are the initial wet weight and the postharvest wet weight of submerged macrophytes, respectively, and dt is the macrophyte growth time (days).
Statistical analysis
Data processing, analysis and plotting were completed using SPSS software (SPSS Inc., USA). The effects of different densities of B. aeruginosa on the GRs of aboveground and belowground parts of E. nuttallii were investigated by the one-way analysis of variance method (one-way ANOVA). Post hoc multiple comparisons among treatments were performed using Tukey HSD tests. All data were tested for normality and homogeneity before performing ANOVAs. Data were transformed (Sqrt(x), square(x), artan(x), reciprocal(x) or/and log10(x)) to obtain normality and/or homogeneity if they did not meet the basic assumptions. The threshold for significance differences among groups was at the level of p < 0.05.
Results
The growth of E. nuttallii
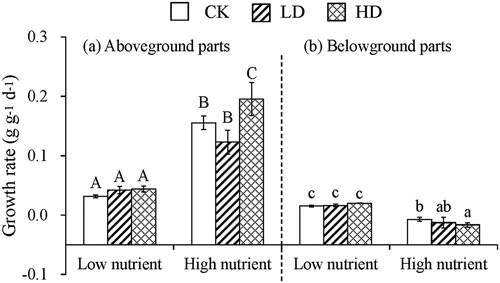
In the LN stage, the GRs of the aboveground and belowground parts of E. nuttallii in the CK group averaged at 0.032 ± 0.002 d−1 and 0.015 ± 0.002 d−1, respectively. There were no significant differences (p > 0.05) for the GRs of the aboveground and belowground parts of E. nuttallii among CK, LD and HD groups. In the HN stage, the GRs of the aboveground parts of E. nuttallii in the HD group (averaged at 0.195 ± 0.028 d−1) were significantly higher (p < 0.05) than the CK and LD groups (averaged at 0.156 ± 0.011 d−1 and 0.123 ± 0.020 d−1, respectively). However, the GRs of the belowground parts were negative with the HD group significantly lower (p < 0.05) than that of the CK group ().
Dynamic changes of the water quality parameters
In the LN stage, the COND in the CK group was relatively stable as time progressed but exhibited an upward trend in the LD and HD groups, with the HD group always being higher than the LD group. In the HN stage, with the exception of Day 111, the COND values of the three groups were in the order of HD > LD > CK and showed a downward trend. The pH values of the CK, LD and HD groups showed an increasing trend and stabilized over time in both the LN stage and HN stage. The water ORP values of the three groups showed an initial downward trend, and then stabilized with time ().
Figure 2. Changes of water parameters (a) COND, (b) pH, (c) ORP, (d) Chl and (e) DO with time at different nutrient stages among experimental treatments.
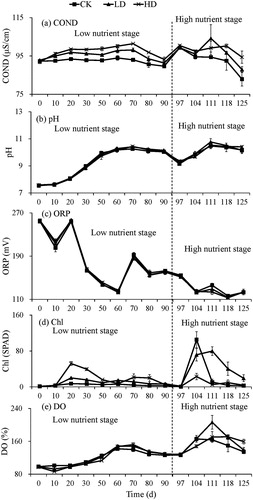
The Chl contents in the water body exhibited a downward trend after an initial increase in both the LN and HN stages. In the LN stage (with the exception of Day 60), the Chl contents in the water body of the three groups were in the order of HD > LD > CK. In the HN stage, the Chl contents of the LD group were generally higher than the HD and CK groups. The water DO of the three groups at both nutrient stages showed a decreasing trend with time following an initial increase ().
The NO3-N contents in the water showed a decreasing trend in both the LN and HN stages and were in the order of HD > LD > CK in the LN stage and in the order of LD > HD > CK in the HN stage. The changes in the NH4− contents were not apparent in the LN stage but showed a decreasing trend in the HN stage.
The PO4-P in the water remained at a low level during the LN stage and decreased with increasing time during the HN stage, and the PO4-P contents in the CK group were always higher than in the LD and HD groups. The changes of the NO3-N, NH4-N, and PO4-P contents altered the N:P in the water. In the LN stage, the N:P was stable over time, although a certain degree of fluctuation was observed, while in the HN stage, the N:P in the water increased with increasing snail density ().
Figure 3. Changes of water parameters (a) NO3-N, (b) NH4-N, (c) PO4-P and (d) N:P with time at different nutrient stages among experimental treatments.
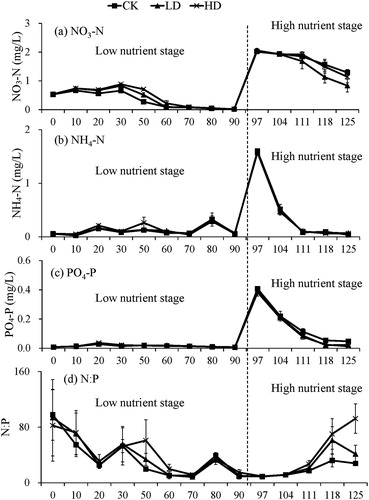
In the HN stage, the NH4-N and PO4-P removal rates of each group were between 88 and 97%, and the NO3-N removal rates were between 30 and 60%. Among them, the NO3-N removal rate of the LD group was significantly higher than the CK and HD groups (p < 0.05); the NH4-N removal rate of the CK group was significantly higher than the LD group and HD group (p < 0.05); and the PO4-P removal rate increased with increasing snail density.
Discussion
A large number of studies have assessed the effects of freshwater snails on the growth of submerged macrophytes, but with no consistent conclusions. This is primarily because the interaction between snails and submerged macrophytes is influenced by numerous factors, such as the species and density of the snails, the species and growth period of the submerged macrophytes, and the nutrient level of the water bodies (Underwood Citation1991; Pinowska Citation2002; Li et al. Citation2009). This study was different from other studies in that the submerged macrophyte was planted with sediment while most of others without sediments (Underwood Citation1991; Pinowska Citation2002; Li et al. Citation2009). The results of this study demonstrated that different densities of B. aeruginosa did not significantly influence the growth of the aboveground and belowground parts of E. nuttallii in the LN stage. Conversely, during the HN stage, the high B. aeruginosa density substantially promoted the growth of the aboveground parts of E. nuttallii, indicating that the metabolism of B. aeruginosa may not be the primary factor affecting the growth of E. nuttallii in the present study. Submersed macrophytes can absorb nutrient from both water and sediment dependent on the nutrient concentrations and relative availabilities in sediment vs. water column (Rattray et al. Citation1991; Madsen and Cedergreen Citation2002; Cao et al. Citation2011). In the LN stage of this study, sediment may be the primary source of nutrient for E. nuttallii. However, during the HN stage, the Chl content in the HD group was significantly lower than the CK and LD groups, suggesting that B. aeruginosa at a HD can promote the growth of E. nuttallii by inhibiting algal growth.
The COND of the water was most significantly affected by B. aeruginosa. In the LN and HN stages, the COND of the water body increased with increasing B. aeruginosa density, suggesting that B. aeruginosa can increase the ion content of the water. However, the parameters which resulted in the changes of COND cannot be determined from this experiment and need to be further explored.
In this experiment, the interactive effects of B. aeruginosa and E. nuttallii led to high ammonia nitrogen and orthophosphate phosphorus removal rates, and a low nitrate-nitrogen removal rate. This may be because ammonia nitrogen and orthophosphate phosphorus are the main nutrient sources for the growth of E. nuttallii. When both ammonia nitrogen and nitrate-nitrogen are present, submerged macrophytes preferentially utilize ammonia nitrogen as the N source (Cao et al. Citation2011). The study of Zhu et al. (Citation2013) demonstrated that the existence of B. aeruginosa can significantly reduce the N:P of water bodies. However, in the present study, the coexistence of B. aeruginosa and E. nuttallii increased the N:P of the water body, which may be attributed to the absorption of a large amount of P in the water by E. nuttallii. In addition, the N and P in the sediment could also be absorbed by submerged macrophyte, which could potentially change the pH and redox potential of the sediment and water, and thus the N:P of the water, which need further studies.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Wei Li
Wei Li is a PhD at the Research Institute of Ecology and Environmental Science, Nanchang Institute of Technology, who has worked on water ecological environmental protection and restoration for many years.
Yujie Li
Yujie Li is a bachelor degree candidate who is studying in the School of Hydraulic and Ecological Engineering, Nanchang Institute of Technology.
Weihua Nie
Weihua Nie is a bachelor degree candidate who is studying in the School of Hydraulic and Ecological Engineering, Nanchang Institute of Technology.
Guiqing Gao
Guiqing Gao is an associate professor in the School of Civil and Architectural Engineering, Nanchang Institute of Technology, who has worked on water ecological environmental protection and restoration for many years.
Houbao Fan
Houbao Fan is a professor at the Research Institute of Ecology and Environmental Science, Nanchang Institute of Technology, who has worked on water ecological environmental protection and restoration for many years.
Jiayou Zhong
Jiayou Zhong is a professor engineer in Jiangxi Institute of Water Sciences, who has worked on water ecological environmental protection and restoration for many years.
Huijun Ding
Huijun Ding is a senior engineer in Jiangxi Institute of Water Sciences, who has worked on water ecological environmental protection and restoration for many years.
References
- Brönmark C, Bronmark C. 1985. Interactions between macrophytes, epiphytes and herbivores: An experimental approach. Oikos. 45(1):26–30.
- Cai Y, Xue Q, Xu J, Zhang L, Gong Z, Acharya K. 2016. Widespread natural intraspecific variation in tissue stoichiometry of two freshwater molluscs: Effect of nutrient enrichment. Ecol Indic. 66:583–591.
- Cao T, Ni L, Xie P, Xu J, Zhang M. 2011. Effects of moderate ammonium enrichment on three submersed macrophytes under contrasting light availability. Freshwater Biol. 56(8):1620–1629.
- Cao Y, Li W, Jeppesen E. 2014. The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: A microcosm approach. Hydrobiologia. 738(1):49–59.
- Carpenter SR, Lodge DM. 1986. Effects of submersed macrophytes on ecosystem processes. Aquat Bot. 26:341–370.
- Gu QH, Husemann M, Ding B, Luo Z, Xiong BX. 2015. Population genetic structure of Bellamya aeruginosa (Mollusca: Gastropoda: Viviparidae) in China: weak divergence across large geographic distances. Ecol Evol. 5(21):4906–4919.
- Huang X. 2000. Survey, observation and analysis of lake ecology. Beijing (In Chinese): Standards Press of China.
- Li D, Erickson RA, Tang S, Zhang Y, Niu Z, Liu H, Yu H. 2016. Structure and spatial patterns of macrobenthic community in Tai Lake, a large shallow lake, China. Ecol Indic. 61:179–187.
- Li K, Liu Z, Gu B. 2009. Density-dependent effects of snail grazing on the growth of a submerged macrophyte, Vallisneria spiralis. Ecol Complex. 6(4):438–442.
- Madsen TV, Cedergreen N. 2002. Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich stream. Freshwater Biol. 47(2):283–291.
- Pieczyńska E. 2003. Effect of damage by the snail Lymnaea, (Lymnaea) stagnalis, (L.) on the growth of Elodea canadensis, Michx. Aquat Bot. 75(2):137–145.
- Pinowska A. 2002. Effects of snail grazing and nutrient release on growth of the macrophytes Ceratophyllum demersum and Elodea canadensis and the filamentous green alga Cladophora sp. Hydrobiologia. 479:83–94.
- Rattray MR, Howard-Williams C, Brown JMA. 1991. Sediment and water as sources of nitrogen and phosphorus for submerged rooted aquatic macrophytes. Aquat Bot. 40(3):225–237.
- Scheffer M, Redelijkheid MR, Noppert F. 1992. Distribution and dynamics of submerged vegetation in a chain of shallow eutrophic lakes. Aquat Bot. 42(3):199–216.
- Sheldon SP. 1987. The effects of herbivorous snails on submerged macrophyte communities in Minnesota Lakes. Ecology. 68(6):1920–1931.
- Underwood GJC. 1991. Growth enhancement of the macrophyte Ceratophyllum demersum in the presence of the snail Planorbis planorbis: the effect of grazing and chemical conditioning. Freshwater Biol. 26(2):325–334.
- Xu J, Li W, Liu G, Zhang L, Liu W. 2007. Inter-specific competition between two submerged macrophytes, Elodea nuttallii and Hydrilla verticillata. J Plant Ecol. 31:83–92.
- Zheng Z, Lv J, Lu K, Jin C, Zhu J, Liu X. 2011. The Impact of snail (Bellamya aeruginosa) bioturbation on sediment characteristics and organic carbon fluxes in an eutrophic pond. Clean Soil Air Water. 39(6):566–571.
- Zhu J, Lu K, Liu X. 2013. Can the freshwater snail Bellamya aeruginosa (Mollusca) affect phytoplankton community and water quality? Hydrobiologia. 707(1):147–157.