Abstract
Lake Chaohu is a representative lake in China that suffers from severe eutrophication and algal blooms. In this study, the composition of bacterial communities in the water and sediment from one of the inlets and outlets of Lake Chaohu in response to spatiotemporal changes was analyzed by Illumina MiSeq sequencing of the V4 region of the 16S rRNA gene. Substantially distinct bacterial community composition was observed between the water column and sediments in both the Zhaohe (the inlet, C1) and Yuxihe Rivers (the outlet, C2). The phyla of the water samples were Cyanobacteria, Proteobacteria, Actinobacteria and Bacteroidetes, whereas the sediment samples were dominated by Proteobacteria, Acidobacteria, Chloroflexi and Nitrospirae. The sediment exhibited higher diversity and richness than did the water column at the two sites, with the sediment samples from the Yuxihe River showing higher richness and lower diversity than those from the Zhaohe River. The pattern of spatiotemporal changes indicated the seasonal factor, rather than the region, to be the primary cause of the observed variation in the bacterial structure in Lake Chaohu. In water samples from the two study areas, unclassified genera of Cyanobacteria dominated in winter, with members of unclassified FamilyI and unclassified Cyanobacterium. Moreover, redundancy analysis indicated that water temperature (T), total phosphorus (TP), organic carbon (OC) and total nitrogen (TN) were all determined to be related to bacterial community structure. And T and TP were the main factors influencing the water bacterial structure and that OC and TN were the main indicators determining the sediment bacterial structure. The results of this study may be useful for indicating the tendency of bacteria to respond to environmental changes in Lake Chaohu.
Introduction
In recent years, global climate change and human activities have profoundly affected aquatic ecosystems as well as their functional activity in terms of biogeochemical cycling (Ma et al. Citation2018). Eutrophication and algal blooms have become the most obvious and troublesome phenomena related to environmental issues that alter ecosystems and threaten human lives (Paerl Citation2014; Li et al. Citation2015), especially in China, where the representative lakes are Lake Chaohu and Lake Taihu. Given that bacteria are highly sensitive to environmental changes (Williamson et al. Citation2009), and the microbial community structure as well as its functions may react separately or simultaneously to natural or artificial disturbances (Ma et al. Citation2018), bacteria have been widely used to monitor water quality changes and pollution (Kurilkina et al. Citation2016). However, the changes in bacterial structures that will occur in different environments and how environmental factors determine community structure and associated functional characteristics remain unclear.
Sediment contains a high abundance of microorganisms and is the main destination of lake nitrogen, phosphorus, organic matter and heavy metals; it can provide a unique record of the natural and anthropogenic inputs of contaminants in aquatic environments (IP et al. Citation2004; Roske et al. Citation2012). When the environmental conditions of the sediment–water interface change with the pH level, redox potential, oxygen concentration and organic matter content, pollutants, especially heavy metals, may be released, affecting the trophic status of the overlying water body and directly or indirectly threatening the ecosystem through bioaccumulation (Zhong et al. Citation2006; Laing et al. Citation2008). Because microorganisms have prominent roles in energy conversion, nutrient biogeochemical cycles, pollutant degradation and organic matter biotransformation, bacteria are frequently used as indicators of watershed ecosystem health (Wei et al. Citation2008; Chen et al. Citation2018). Lake water and sediment comprise two different habitats, both of which play indispensable roles in aquatic ecosystems and these different roles attributed to lake water and sediment may be responsible for the microbial biogeography in lakes (Yang et al. Citation2016). Thus, characterizing bacterial community composition (BCC) in sediment and water and respective responses to a changing environment would provide valuable information to aid in understanding aquatic microbial ecology and assessing ecological risk (Psenner et al. Citation2008; Wang et al. Citation2016).
Lake Chaohu (31°25′–31°43′ N, 117°16′–117°51′ E), located in Anhui Province in southeastern China, is one of the five largest freshwater lakes in China. The lake serves as a main water transport hub and is also important for supporting local development (Yang et al. Citation2013). Unfortunately, because of the rapid development of industrial and agricultural production in the basin, an excess of concentrated organic matter and nutrient salts have been imported into the lake via industrial and agricultural wastewater (Xu et al. Citation2005; Yang et al. Citation2013). Coupled with intensive anthropogenic activities in the basin, the lake has deteriorated and suffered from eutrophication (Zan et al. Citation2011), causing severe damage to the ecological environment. To control this situation, the Water Transfer Project from the Yangtze River to Lake Chaohu has been implemented as a measure to improve the water capacity and self-purification ability of Lake Chaohu. Some scholars have conducted studies on the relationship between protozoa and eutrophication (Xu et al. Citation2005), implications for benthic biodiversity conservation (Cai et al. Citation2012) and the capacity of phytoplankton communities to indicate water quality (Jiang et al. Citation2014). However, analysis of community composition and possible regulating factors of bacteria within the context of spatiotemporal changes in both aquatic and sediment environments have scarcely been investigated. Nonetheless, studying the main factors that may cause changes in bacterial community composition is crucial for ameliorating pollution, controlling eutrophication and searching for associations between microbes and their environment.
In this study, we selected locations where the Yangtze River enters and exits Lake Chaohu. We collected surface sediments and overlying water, and used Illumina MiSeq 16S rRNA gene sequencing to evaluate the bacterial community composition across spatiotemporal gradients. The main objective of the present study is to determine the association between environmental factors and bacteria by comparing physicochemical indexes of samples obtained at the locations where the Yangtze River enters into and exists from Lake Chaohu. We also assessed changes in the microflora structure over time and space and discuss the possible mechanisms underlying these variations.
Materials and methods
Study site and sampling
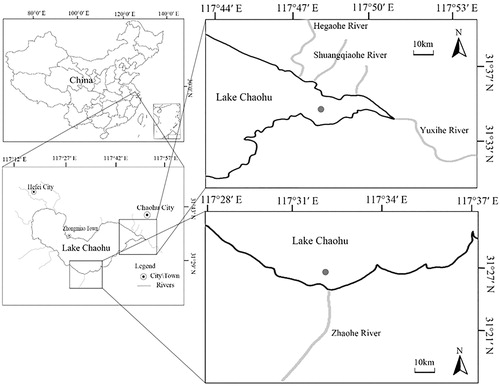
Lake Chaohu between the Yangtze River Delta and the Huaihe River watershed is fed by 33 tributaries and only a tributary of the lake that connects Lake Chaohu and Yangtze River. The Zhaohe River belongs to one of the seven major inflowing rivers and is in the impact zone that introduces the Yangtze River into Lake Chaohu in the Water Transfer Project; the Yuxihe River is the only channel by which the water of Lake Chaohu flows into the Yangtze River (Jiang et al. Citation2014). In this study, two sampling points were set up in the eastern half of Lake Chaohu is located in the estuary of the Zhaohe River (C1:117°33' E, 31°26' N) and the exit of the Yuxihe River (C2:117°48' E, 31°35' N), as shown in . At each station, sampling was carried out in summer (August 2016) and winter (February 2017). Surface water (top 50 cm) and sediment (0–5 cm deep) at each site were collected three times in parallel. The water was collected with a 5-L Schindler sampler, and the sediment was collected with a Petersen grab sampler. All samples were sealed with plastic bags and stored at 4 °C in a car refrigerator and then immediately shipped to the laboratory. A total of eight samples were marked as follows: SC1W (Zhaohe River water sample in summer), SC2W (Yuxihe River water sample in summer), WC1W (Zhaohe River water sample in winter), WC2W (Yuxihe River water sample in winter), SC1S (Zhaohe River sediment sample in summer), SC2S (Yuxihe River sediment sample in summer), WC1S (Zhaohe River sediment sample in winter), WC2S (Yuxihe River sediment sample in winter).
Physicochemical analysis
The water temperature (T), dissolved oxygen (DO) and pH were measured at the time of sample collection using a multiparameter water quality sonde (YSI 6600V2, Ohio, USA). Total nitrogen (TN), total phosphorus (TP) and ammonium nitrogen (NH4+-N) were measured in the laboratory according to standard methods (Jin and Tu Citation1990). The organic carbon (OC) content was determined using an H2SO4–K2Cr2O7 oxidation method (Nelson Citation1982).
DNA extraction and quality detection
Before extracting microbial genomic DNA, 500 mL of water samples was passed through sterile polycarbonate membrane filters (0.22 µm), and cells retained on the filters were stored at -80 °C until subsequent analysis. Freeze-dried sediment (0.25 g) was used for DNA extractions after homogenization under aseptic conditions. Triplicate DNA extractions for each of the eight samples were performed separately using a PowerSoil® DNA Isolation Kit (MoBio, USA) following the manufacturer’s instructions. The quality of the extracted DNA was verified by 0.8% agarose gel electrophoresis, and the DNA was quantified using an ultraviolet spectrophotometer (Eppendorf, Germany). The DNA was stored at −20 °C until further processing (Keshri et al. Citation2018).
Polymerase chain reaction amplification, library construction and sequencing
Polymerase chain reaction (PCR) amplification was performed using universal primers targeting the V4 region of the bacterial 16S rRNA gene, which is approximately 280 bp in length. The forward amplification primer 515 F (5′-GTGCCAGCMGCCGCGGTAA-3′) and reverse primer 907 R (5’-CCGTCAATTCMTTTRAGTTT-3’) were used. PCR was carried out in a total volume of 25 μL containing 0.25 μL Q5 high-fidelity DNA polymerase, 5 μL 5 × Reaction Buffer, 5 μL High GC Buffer, 0.5 μL dNTP (10 mM), 1 μL template DNA, 1 μL forward and reverse primers (10 μM) and 11.25 μL water. The PCR conditions were pre-denaturation at 98 °C for 30 s, followed by 27 cycles of denaturation at 98 °C for 15 s, annealing at 50 °C for 30 s and elongation at 72 °C for 30 s with a final extension occurred for 5 min at 72 °C and hold at 4 °C.
The PCR products were detected using 2% Invitrogen agarose gels, and the target fragments were recovered with an AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA, USA). The products were used for preliminary fluorescence quantification via electrophoresis. Quant-iT PicoGreen dsDNA Assay Kit was used for fluorescent staining, and fluorescence was quantified with a microplate reader (BioTek, FLx800, USA). Based on the fluorescence quantitative results, each sample was mixed in a corresponding ratio according to amount of sequencing required for each sample. Purified products were sent to Personal Biotechnology Co., Ltd., Shanghai, China for sequencing.
Sequencing data optimization and OTU clustering
To integrate the original sequencing data, the sliding window method was first used to screen the quality of the FASTQ format sequence; the average quality of the bases in the window was required to be ≥ Q20 (the average sequencing accuracy of the base was ≥ 99%), the sequence length was required to be ≥ 150 bp and the ambiguous base N was not allowed. Subsequently, FLASH software (v1.2.7, http://ccb.jhu.edu/software/FLASH/) (Magoc and Salzberg Citation2011) was used to pair the sequences that passed the mass screening according to overlapping bases; the overlapping base lengths of the two sequences were required to be ≥ 10 bp, and base mismatch was not allowed. Finally, the valid sequence for each sample was obtained.
Using QIIME-UCLUST, which is a sequence alignment tool (Edgar Citation2010), to merge the high-quality sequences by 97% sequence similarity and to divide operational taxonomic units (OTUs), the most abundant sequence in each OTU was selected as the representative sequence. In QIIME, the OTU representative sequences were compared with Greengenes Database (Desantis et al. Citation2006) to obtain the taxonomic information corresponding to each OTU. The OTUs were then streamlined, and any OTU with an abundance of less than 0.001% of the total number of sequences was removed (Bokulich et al. Citation2013) to obtain a simplified OTU matrix.
Bacterial diversity and statistical analysis
Using QIIME software, the total number of sequences of each sample in the OTU abundance matrix was randomly sampled at different depths, and a rarefaction curve was drawn using the number of sequences extracted at each depth and its corresponding OTU number, thereby judging whether the current sequencing depth of each sample was sufficient to reflect the microbial diversity present in the community sample. The alpha diversity of bacterial communities of each sample was measured (QIIME software) with Chao 1 (Chao Citation1984), ACE (Chao and Yang Citation1993), Shannon (Citation1948) and Simpson (Citation1949) indices. The NMDS were conducted in R with the vegan package. Redundancy analysis (RDA) was employed with the software CANOCO (version 4.5) to explain relationships between environmental parameters and BCC (Ter Braak and Smilauer Citation2002). The Illumina MiSeq sequences were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under accession NO.SRP200812.
Results
Physicochemical characteristics
Some differences in environmental parameters in surface water and sediment samples were found at C1 and C2 (Supplementary Table S1). The average temperatures of the surface water in summer and winter were 32.2 °C and 2.95 °C, respectively, and pH values changed slightly between 8.05 and 8.37. Expressed ammonium nitrogen concentrations in the water were lower in summer than in winter, ranging from 0.029 to 0.127 mg/L, of which SC1W (0.029 mg/L) showed the lowest concentration. TN and TP contents were measured in both water and sediment, with both being higher in the latter. In sediment samples, the average OC content was 3.52 mg/g, and the WC1S content was relatively low at 1.49 mg/g.
Bacterial community diversity
To explore the microbial community structure of eight samples from Lake Chaohu, Illumina MiSeq sequencing was utilized to sequence the bacterial 16S rRNA gene V4 region. A total of 194,403 quality sequences from four water samples and 144,483 quality sequences from four sediment samples were obtained (). OTUs were determined for calculating the richness, diversity and rarefaction curves of the microbial communities (Sheng et al. Citation2016). The rarefaction curves revealed that the OTU number of the water samples was almost saturated with increasing sequence number. In sediment, the OTUs essentially tended to be stable, indicating coverage of the majority of the total microbial structure and diversity ().
Figure 2. Rarefaction curves of OTUs clustered at 97% sequence identity across eight samples in Lake Chaohu.
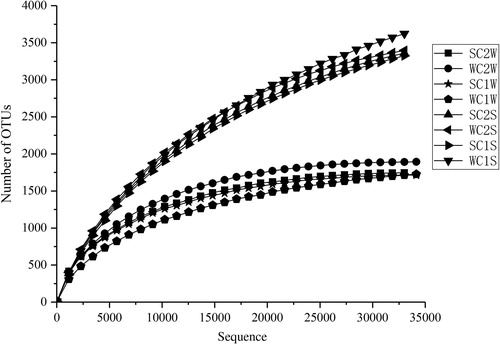
Table 1. Bacterial index of community diversity in Lake Chaohu.
Specifically, the Chao 1, Ace, Shannon and Simpson indices of the water samples from both the Zhaohe River and Yuxihe River were lower than those of the sediment samples. When comparing diversity between the stations, the Chao 1 and Ace indices of the sediment at site C1 were slightly higher than those of the sediment at site C2, though the results for Simpson and Shannon indices were exactly the opposite results. In terms of seasonal factors, Chao 1 and Ace indices were lower in summer than in winter at the two stations, except for the results obtained for site C2 sediment samples. However, the Simpson and Shannon indices of the water were high in summer, whereas these two indices of the sediment were lower in summer than in winter (). Overall, the analysis indicated that the microbial richness and diversity of sediment were higher than those of water and that the richness observed was relatively high in station C1, but that its diversity was relatively low compared to C2. In addition, the richness and diversity did not change regularly with seasonal changes in two different environments.
The spatiotemporal composition of bacterial communities
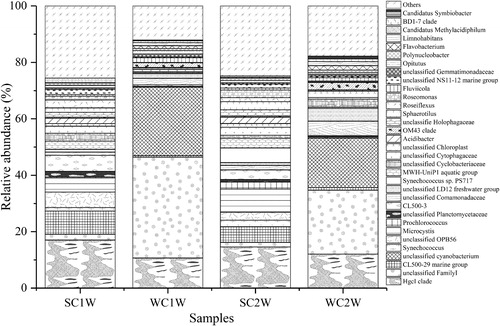
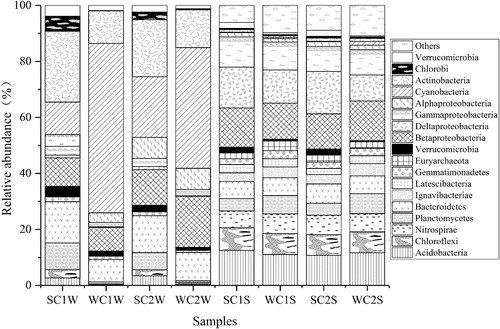
At the phylum level, the sediment bacterial communities of C1 and C2 displayed a similar distribution with regard to variation both in season and in station (). The predominant phyla between the water and sediment samples showed a number of differences; the water samples were dominated by Cyanobacteria (34.6 ± 22.3%), Proteobacteria (17.9 ± 6.4%), Actinobacteria (17.9 ± 6.4%) and Bacteroidetes (11.6 ± 3.1%), whereas the sediment samples were dominated by Proteobacteria (37.4 ± 2.9%), Acidobacteria (11.5 ± 0.8%), followed by Chloroflexi (7.6 ± 0.3%) and Nitrospirae (6.6 ± 0.5%). The bacterial community composition of the water samples displayed some different trends with seasonal variation. For instance, Cyanobacteria in winter increased compared to in summer both in C1 and C2. In contrast, Actinobacteria and Bacteroidetes were higher in proportion in summer than in winter.
Figure 3. Relative abundance of bacterial communities at the phylum level in the water and sediment samples of Lake Chaohu.
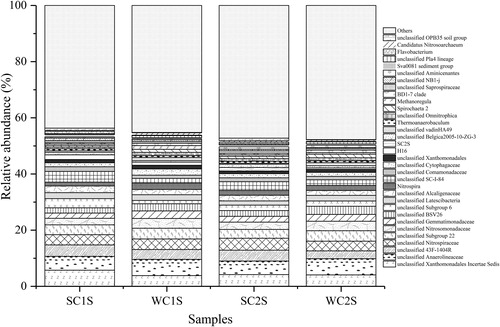
Among the water samples, the relative abundance of unclassified FamilyI (15.5 ± 16.7%) and unclassified Cyanobacterium (10.5 ± 12.4%) in winter was higher and almost undetectable in summer (). The HgcI clade (13.4 ± 2.7%) was uniformly distributed among the four water samples, with a slightly lower relative abundance in winter. Similar to the phylum level, the distribution of genera in the sediment samples changed slightly with season and space (). The relative abundance of each dominant genus was relatively low, and the proportion of Others was up to 46.0 ± 1.9%, indicating relatively high diversity among the sediment samples was. The dominant genera were all unclassified, including unclassified Anaerolineaceae (5.3 ± 0.5%), unclassified Xanthomonadales Incertae sedis (4.5 ± 0.9%), unclassified Nitrospiraceae (3.8 ± 0.3%) and unclassified 43 F-1404R (3.6 ± 0.6%).
Redundancy analysis
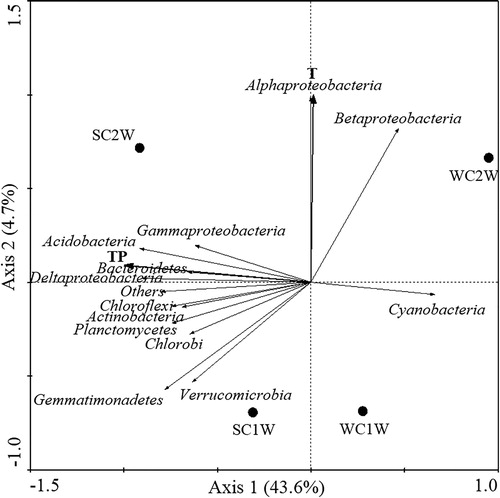
RDA was conducted to determine correlations among physicochemical indicators and bacterial communities in the water and sediment samples of the Zhaohe and Yuxihe. The RDA model showed that T and TP played indispensable roles in determining the distribution of bacterial communities in the water column, as shown in . The locations of the four samples were relatively dispersed, indicating that disparate bacterial community compositions. Relationships and contact strength can be determined according to the angle between phyla and environmental factors, and it can be seen that almost all of the phyla were positively correlated with the TP except for Betaproteobacteria and Cyanobacteria. T had a positive effect on Alphaproteobacteria and Betaproteobacteria, which were abundant at WC2W.
Figure 6. Redundancy analysis (RDA) of bacterial community composition in water samples and environmental factors in Lake Chaohu.
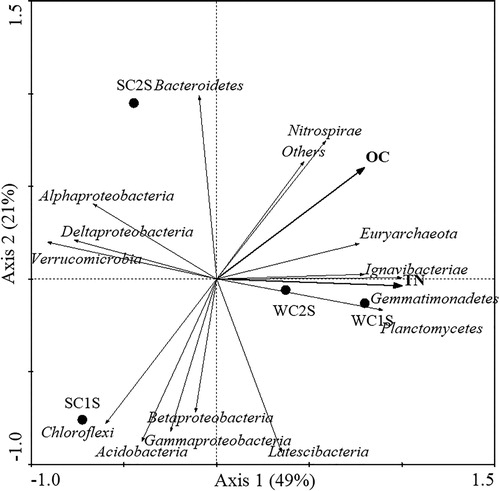
For sediment bacterial communities, interpretation of the rates of variation in bacterial community composition on the first two axes were 49.0% and 21.0%. The first axis revealed a high canonical correlation with the content of OC and TN. As shown in , the bacterial community structure was similar between WC1S and WC2S, which was slightly different from the two. TN and OC had a positive effect on Euryarchaeota, Ignavibacteria and Gemmatimonadetes. The dominant bacteria group included Deltaproteobacteria, Acidobacteria and Gammaproteobacteria, which were negatively correlated with TN and OC.
Discussion
This study revealed the existence of large differences in diversity and bacterial community composition in the sediment and water of the rivers Zhaohe and Yuxihe, and these sediment and aquatic bacterial communities were subject to spatiotemporal changes (, ). The NMDS (Supplementary Figure S1) showed that the bacterial community composition of samples SC1W and WC1W is similar, and sediment samples WC1S and WC2S had similar bacterial community composition. Previous studies have confirmed that environmental parameters exert a potential effect on influencing BCC, such as nutrients (Xing and Kong Citation2007), salinity (Swan et al. Citation2010) and pH (Ganzert et al. Citation2014). Therefore, to further understand their mutual correlation in the present study, we measured physicochemical factors of sediment and water samples in the Zhaohe River and Yuxihe River (Supplementary Table S1). The bacterial distribution of water samples across time and space was notably affected, especially by seasonal elements. It can be seen from Supplementary Table S1 that the DO in winter water is higher than that in summer both in two sampling sites. RDA indicated that T and TP were the most influential parameters contributing to BCC of the water samples at the collection sites (). In addition, OC and TN appear to be significant factors shaping the sediment bacterial distribution at both sites, despite no marked changes (). Although different types of physiochemical parameters lead to particular bacterial communities, whether a single factor or comprehensive set of factors is responsible and how these factors regulate the bioflora.
Biodiversity is indispensable for monitoring and determining the quality of aquatic environments because higher biodiversity is thought to increase ecosystem defence and resilience capacity to disturbance (Zinger et al. Citation2012). Dai et al. (Citation2016) studied the spatiotemporal variation of bacteria in plateau freshwater lakes, and the conclusions suggested that sediments had higher bacterial community richness and diversity than did water. Our results also show that bacterial diversity at C1 and C2 was higher in sediment than in water and that the diversity of the water samples in summer was relatively higher than that in winter (). Such a distribution of bacterial diversity with seasonal shifts may be accounted for by the phenomenon of algal blooms which frequently occur in summer. According to previous studies, bacterial diversity increases in proportion to algal density, and the bacterial community may vary with the phytoplankton growth in water (Woodhouse et al. Citation2016). The higher bacterial diversity in sediment can be explained by the potentially greater adaptive abilities of sediment bacteria. There is also evidence that in freshwater sediments, nutrients promote higher diversity and richness via increased niche partitioning (Dykhuizen Citation1998). Sheng et al.’s (Citation2016) results showed that increases in TN and TP may lead to high bacterial richness and diversity. Our results indicate that, the levels of TN and TP were relatively high at SC2S and WC2S compared to levels in the other sediment samples, and the diversity indices for these samples were also relatively high. However, the richness indices did not match the lower values, which shows that these two indicators did not well explain bacterial diversity in our study. In addition, OC, heavy metals and other physicochemical indices may also affect sediment bacterial (Liu et al. Citation2015; Yu et al. Citation2015).
Many studies have investigated the effects of changes in space and time on bacterial communities. For instance, a study on the spatiotemporal distribution of sediment microorganisms in Erhai Lake found that the distribution of microbes is associated with many factors (Zhang et al. Citation2015). Wan et al. (Citation2017) studied five regions of Lake Taihu and revealed that, compared with region, season was a dominant factor in altering bacterial community structure. In the present study, a distinct difference in bacterial structure was observed between water and sediment samples from the Zhaohe River and Yuxihe River. Although the composition of phyla in the water and sediment was very similar, the distribution was quite different. For example, Actinobacteria and Cyanobacteria were taxa particular to water, whereas Nitrospirae, lgnavibacteria, Latescibacteria and Euryarchaeota were unique in the sediment of Lake Chaohu but were not found in its water. In general, the relative abundance of each phylum in the sediments was relatively stable over time and space in the two study areas. In water, the relative abundance of Actinobacteria, Cyanobacteria, Planctomycetes, Chloroflexi and Acidobacteria clearly varied with season, which suggests that seasonal factors played an indispensable role in affecting the bacterial community structure in the water of C1 and C2 in Lake Chaohu.
We observed a phylum with a relatively high abundance of Proteobacteria in both water and sediment, with relative abundances of 17.9 ± 6.4% and 37.4 ± 2.9%, respectively. Proteobacteria often comprise major components of water and sediment bacterial communities, and the proportion of this phylum in sediments was generally higher than that in water. Similar conclusions have been reported by Dai et al. (Citation2016). Our results indicated Betaproteobacteria, Deltaproteobacteria and Gammaproteobacteria were representative classes of Proteobacteria in the sediment from the Zhaohe River and Yuxihe River, and Betaproteobacteria and Alphaproteobacteria were the dominant classes of Proteobacteria in the water samples from the two sites. Overall, Betaproteobacteria are more prevalent in water columns and sediments than are other classes of Proteobacteria (Spring et al. Citation2000). Betaproteobacteria comprises many types of aerobic or facultative bacteria (Freitag et al. Citation2006), most of which can be found in aquatic ecosystems. Members of Deltaproteobacteria are thought to be more common in freshwater sediments than in water bodies (Roske et al. Citation2012). This study supports this opinion because the abundance of Deltaproteobacteria was much higher in the sediment than in water samples.
As shown in , Cyanobacteria were dominant in water samples from Lake Chaohu, especially in winter. And the pH values in winter at C1 (8.37) and C2 (8.26) were relatively high compared to in summer at C1 (8.05) and C2 (8.12). Bacteria belonging to Cyanobacteria, unclassified FamilyI and unclassified Cyanobacterium accounted for a relatively high percentage at C1 and C2 during the winter period, and the genera Microcystis and Synechococcus, which contributed small proportions of additional cyanobacterial taxa, were slightly more abundant in summer. The abundance of Cyanobacteria is related to nutrient levels and the previous study showed that pH is related to the relative abundance of Cyanobacteria (Kong and Gao Citation2005; Staley et al. Citation2013); however, it is unclear why Cyanobacteria were more abundant in winter in our study, as the mechanism that results in algal blooms frequently occurs in summer. As the previous study showed, DO, pH and nitrate were negatively related with Cyanobacteria, which may related to eutrophication (Wei et al. Citation2014). Considering that the genus Microcystis is sensitive to temperature changes and has an optimal growth rate at relatively high temperatures (Paerl and Otten Citation2013), it was not surprising that this genus was predominant in summer. Although Synechococcus is generally considered to be a unique and dominant taxon in lakes, it has been argued that Microcystis and Synechococcus have competitive or antagonistic relationships (Ji et al. Citation2018). On the basis of the relationship between the environmental factors and bacterial community above, the importance of controlling the input of nutrients to prevent algal blooms can be found.
Our results indicated that members of Actinobacteria are a major fraction of the bacterial community during periods of high cyanobacterial biomass, which is similar to findings of previous research (Woodhouse et al. Citation2016). Bacteroidetes is also associated with cyanobacterial blooms (Wu et al. Citation2017), and the relative abundance of these bacteria was stable with time and space in the water column and sediment. The abundance of Actinobacteria and Bacteroidetes declined with increasing proportions of Cyanobacteria, which provides evidence that the latter can inhibit the growth of the former (Ji et al. Citation2018). In the summer period, the phylum Actinobacteria in water samples was represented mainly by CL500-29 marine group and the HgcI clade, and the genera present in the HgcI clade were determined by the low dissolved OC content and temperature (Keshri et al. Citation2018). Among the Bacteroidetes observed in the Zhaohe River and Yuxihe River water, multiple uncultured bacteria, including unclassified Cyclobacteriaceae, unclassified Cytophagaceae and unclassified NS11-12 marine groups, were found.
In sediment, Acidobacteria was the second largest bacterial group but was present at much lower proportions in the water column, especially in winter. As reported in previous studies, Acidobacteria bacteria commonly exist in soils and sediments (Ganzert et al. Citation2014; Huang and Jiang Citation2016). The proportions of Acidobacteria in SC1W and SC2W were high, which might be explained by the fact that these bacteria Acidobacteria predominate at lower pH values (Griffiths et al. Citation2011). In addition, RDA indicated an inverse correlation between Acidobacteria and TN, supporting previous reports that the distribution of these bacteria is inversely associated with low nutrient availability (Ward et al. Citation2009). These phenomena indicate that the pH value and nutrient level may affect the presence of Acidobacteria. At the genus level, unclassified genera and Others comprised the majority of the entire population of bacteria at C1 and C2; the remaining species identifiable to the genus level accounted for an extremely small proportion. Unclassified Xanthomonadales Incertae sedis, unclassified Anaerolineaceae, unclassified 43 F-1404R, unclassified Nitrospiraceae and unclassified Subgroup 22, belonging to the phyla Gammaproteobacteria, Chloroflexi, Deltaproteobacteria, Nitrospirae and Acidobacteria, respectively, were the main groups of sediment bacteria in both the water column and sediment. A large number of bacteria were found in the sediment samples, though the reason is poorly understood and requires additional research.
This study reports the bacterial communities of sediment and water in Lake Chaohu, while taking into account spatiotemporal factors, with the aim of estimating the effects of the changes in living conditions on the bacterial structure. We found that there was a large difference in bacterial community composition between the water and sediment in the Yangtze River inlet and outlet. The composition of sediment bacterial showed similar patterns with spatial changes; the effects of seasonal factors on bacterial structure of water were observed. The sediment bacterial communities were more diverse than were the water bacterial communities. Nutrients may contribute to the higher diversity in sediments, and algal blooms may explain why the diversity of the water samples in summer was higher. Our results confirm that environmental factors have a certain impact on the distribution of bacterial communities and that competition exists between bacteria. Furthermore, our results show that the growth of Cyanobacteria might restrain the growth of Actinobacteria and Bacteroidetes. In addition, large quantities of unclassified genera were observed in the sediment at the two sampling sites.
Notes on contributors
Lei Zhang is an associate professor of Chuzhou Univeristy, China. Skills and Expertise: water treatment technology, environmental microorganisms.
Tingting Zhao is a student in Chuzhou University, China.
Qiao Wang is a student in Chuzhou University, China.
Li Li is a student in Chuzhou University, China.
Tingting Shen is a student in Chuzhou University, China.
Guang Gao is a professor of Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences. Skills and Expertise: microbiology, lake microbial ecology, molecular ecology.
Acknowledgements
Anonymous reviewers are acknowledged for their constructive comments and helpful suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. 2013. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 10(1):57–59.
- Cai YJ, Gong ZJ, Xie P. 2012. Community structure and spatiotemporal patterns of macrozoobenthos in Lake Chaohu (China). Aquat Biol. 17(1):35–46.
- Chao A. 1984. Nonparametric-estimation of the number of classes in a population. Scand J Stat. 11:265–270.
- Chao A, Yang MCK. 1993. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika. 80(1):193–201.
- Chen J, Wang PF, Wang C, Wang X, Miao LZ, Liu S, Yuan QS. 2018. Bacterial communities in riparian sediments: A large-scale longitudinal distribution pattern and response to dam construction. Front Microbiol. 9:999.
- Dai Y, Yang YY, Wu Z, Feng QY, Xie SG, Liu Y. 2016. Spatiotemporal variation of planktonic and sediment bacterial assemblages in two plateau freshwater lakes at different trophic status. Appl Microbiol Biotechnol. 100(9):4161–4175.
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microb. 72(7):5069–5072.
- Dykhuizen DE. 1998. Santa Rosalia revisited: why are there so many species of bacteria? Anton Leeuw Inter J G. 73(1):25–33.
- Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26(19):2460–2461.
- Freitag TE, Chang L, Prosser JI. 2006. Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ Microbiol. 8(4):684–696.
- Ganzert L, Bajerski F, Wagner D. 2014. Bacterial community composition and diversity of five different permafrost-affected soils of Northeast Greenland. FEMS Microbiol Ecol. 89(2):426–441.
- Griffiths RI, Thomson BC, James P, Bell T, Bailey MJ, Whiteley AS. 2011. The bacterial biogeography of British soils. Environ Microbiol. 13(6):1642–1654.
- Huang W, Jiang X. 2016. Profiling of sediment microbial community in Dongting Lake before and after impoundment of the three gorges dam. Int J Environ Res Public Health. 13(6):617.
- Ip CCM, Li XD, Zhang GS, Farmer JG, Wai OWH, Li YS. 2004. Over one hundred years of trace metal fluxes in the sediments of the Pearl River Estuary, South China. Environ Pollut. 132(1):157–172.
- Ji B, Qin H, Guo SD, Chen W, Zhang XC, Liang JC. 2018. Bacterial communities of four adjacent fresh lakes at different trophic status. Ecotoxicol Environ Saf. 157:388–394.
- Jiang Y-J, He W, Liu W-X, Qin N, Ouyang H-L, Wang Q-M, Kong X-Z, He Q-S, Yang C, Yang B, et al. 2014. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol Indic. 40:58–67.
- Jin XC, Tu QY. 1990. The standard methods for observation and analysis in lake eutrophication. Beijing: Chinese Environmental Science Press; p. 240.
- Keshri J, Ram AS, P, Sime-Ngando T. 2018. Distinctive patterns in the taxonomical resolution of bacterioplankton in the sediment and pore waters of contrasted freshwater lakes. Microb Ecol. 75(3):662–673.
- Kong FX, Gao G. 2005. Hypothesis on cyanobacteria bloom-forming mechanism in large shallow eutrophic lakes. Acta Ecol Sin. 25:589–595.
- Kurilkina MI, Zakharova YR, Galachyants YP, Petrova DP, Bukin YS, Domysheva VM, Blinov VV, Likhoshway YV. 2016. Bacterial community composition in the water column of the deepest freshwater Lake Baikal as determined by next-generation sequencing. FEMS Microbiol Ecol. 92:fiw094.
- Laing GD, Vos RD, Vandecasteele B, Lesage E, Tack F, Verloo M. 2008. Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuar Coast Shelf S. 77:589–602.
- Li JF, Zhang JY, Liu LY, Fan YC, Li LS, Yang YF, Lu ZH, Zhang XD. 2015. Annual periodicity in planktonic bacterial and archaeal community composition of eutrophic Lake Taihu. Sci Rep. 5(1):15488.
- Liu LX, Xu M, Qiu S, Shen RC. 2015. Spatial patterns of benthic bacterial communities in a large lake. Internat Rev Hydrobiol. 100(3–4):97–105.
- Ma YT, Li JQ, Wu J, Kong ZY, Feinstein LM, Ding X, Ge G, Wu L. 2018. Bacterial and fungal community composition and functional activity associated with lake wetland water level gradients. Sci Rep. 8(1):760.
- Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27(21):2957–2963.
- Nelson D. 1982. Dry combustion method using medium temperature resistance furnace. Chemical and Microbial Properties, 2nd edn. Soil Science Society of America and American Society of Agronomy Book Series No.9: 539–579.
- Paerl HW. 2014. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life (Basel). 4(4):988–1012.
- Paerl HW, Otten TG. 2013. Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol. 65(4):995–1010.
- Psenner R, Alfreider A, Schwarz A. 2008. Aquatic microbial ecology: water desert, microcosm, ecosystem. What's Next?. Internat Rev Hydrobiol. 93(4–5):606–623.
- Roske K, Sachse R, Scheerer C, Roske I. 2012. Microbial diversity and composition of the sediment in the drinking water reservoir Saidenbach (Saxonia, Germany). Syst Appl Microbiol. 35:35–44.
- Shannon CE. 1948. A mathematical theory of communication. Bell Syst Tech J. 27(3):379–423.
- Sheng P, Yu YZ, Zhang GH, Huang JL, He L, Ding JN. 2016. Bacterial diversity and distribution in seven different estuarine sediments of Poyang Lake, China. Environ Earth Sci. 75(6):479.
- Simpson EH. 1949. Measurement of diversity. Nature. 163(4148):688.
- Spring S, Schulze R, Overmann J, Schleifer K-H. 2000. Identification and characterization of ecologically significant prokaryotes in the sediment of freshwater lakes molecular and cultivation studies. Fems Microbiol Rev. 24(5):573–590.
- Staley C, Unno T, Gould TJ, Jarvis B, Phillips J, Cotner JB, Sadowsky MJ. 2013. Application of –generation sequencing to characterize the bacterial community of the Upper Mississippi River. J Appl Microbiol. 115(5):1147–1158.
- Swan BK, Ehrhardt CJ, Reifel KM, Moreno LI, Valentine DL. 2010. Archaeal and bacterial communities respond differently to environmental gradients in anoxic sediments of a California Hypersaline Lake, the Salton Sea. Appl Environ Microb. 76(3):757–768.
- Ter Braak CJF, Smilauer P. 2002. CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power (Ithaca NYUSA).
- Wan Y, Bai Y, He J, Zhang YP, Li RF, Ruan XH. 2017. Temporal and spatial variations of aquatic environmental characteristics and sediment bacterial community in five regions of Lake Taihu. Aquat Ecol. 51(3):343–358.
- Wang J, Li Y, Wang P, Niu L, Zhang W, Wang C. 2016. Response of bacterial community compositions to different sources of pollutants in sediments of a tributary of Taihu Lake, China. Environ Sci Pollut Res Int. 23(14):13886–13894.
- Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Xie G, Haft DH, Sait M, Badger J, et al. 2009. Three genomes from the Phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl and Environ Microb. 75(7):2046–2056.
- Wei CL, Bao S, Zhu XY, Huang XX. 2008. Spatio-temporal variations of the bacterioplankton community composition in Chaohu Lake, China. Prog Nat Sci. 18(9):1115–1122.
- Wei GS, Li J, Wang NX, Gao Z. 2014. Spatial abundance and diversity of bacterioplankton in a typical stream-forming ecosystem, Huangqian Reservoir, China. J Microbiol Biotechnol. 24(10):1308–1318.
- Williamson CE, Saros JE, Vincent WF, Smol JP. 2009. Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol Oceanogr. 54(6):2273–2282.
- Woodhouse JN, Kinsela AS, Collins RN, Bowling LC, Honeyman GL, Holliday J, Neilan BA. 2016. Microbial communities reflect temporal changes in cyanobacterial composition in a shallow ephemeral freshwater lake. ISME J. 10(6):1337–1351.
- Wu H, Li Y, Zhang J, Niu L, Zhang W, Cai W, Zhu X. 2017. Sediment bacterial communities in a eutrophic lake influenced by multiple inflow-rivers. Environ Sci Pollut Res Int. 24:19795–19806.
- Xing P, Kong FX. 2007. Intra-habitat heterogeneity of environmental factors regulating bacterioplankton community composition in Lake Taihu, China. Aquat Microb Ecol. 48:113–122.
- Xu MQ, Cao H, Xie P, Deng DG, Feng WS, Xu J. 2005. The temporal and spatial distribution, composition and abundance of Protozoa in Chaohu Lake, China: relationship with eutrophication. Eur J Protistol. 41(3):183–192.
- Yang J, Jiang H C, W, G, Liu W, Zhang GJ. 2016. Distinct factors shape aquatic and sedimentary microbial community structures in the lakes of Western China. Front Microbiol. 7:1782.
- Yang LB, Lei K, Meng W, Fu G, Yan WJ. 2013. Temporal and spatial changes in nutrients and chlorophyll-α in a shallow lake, Lake Chaohu, China: an 11-year investigation. J Environ Sci (China). 25(6):1117–1123.
- Yu C, Zhang J, Wu L, Liu YZ, Ge G. 2015. Effects of heavy metal and nutrients on benthic microbial communities in freshwater sediment of Poyang Lake (China). JRST. 12(2):105–111.
- Zan FY, Huo SL, Xi BD, Li QQ, Liao HQ, Zhang JT. 2011. Phosphorus distribution in the sediments of a shallow eutrophic lake, Lake Chaohu, China. Environ Earth Sci. 62(8):1643–1653.
- Zhang L, Wang SR, Li YP, Zhao HC, Qian WB. 2015. Spatial and temporal distributions of microorganisms and their role in the evolution of Erhai Lake eutrophication. Environ Earth Sci. 74(5):3887–3896.
- Zhong AP, Guo SH, Li FM, Li G, Jiang KX. 2006. Impact of anions on the heavy metals release from marine sediments. J Environ Sci (China). 18(6):1216–1220.
- Zinger L, Gobet A, Pommier T. 2012. Two decades of describing the unseen majority of aquatic microbial diversity. Mol Ecol. 21(8):1878–1896.