ABSTRACT
Purpose: The concept of tissue-dependent cytokine hierarchy has been demonstrated in a number of diseases, but it has not been investigated in ophthalmic diseases. Here, we evaluated the functional hierarchy of interleukin-1β (IL-1β), IL-6, IL-17A, and tumor necrosis factor (TNF) in the induction of ocular inflammation.
Materials and Methods: We delivered adeno-associated virus (AAV) vectors expressing IL-1β, IL-6, IL-17A, or TNF intravitreally in naïve C57/BL6 mice and compared and contrasted the inflammatory effects in the eye 5 weeks after AAV-mediated gene transfer. We also used an in vitro human system to test the effect of cytokines on barrier function.
Results: We found that IL-1β had the highest ability to initiate ocular inflammation. The continuous overexpression of IL-1β resulted in a significant upregulation of additional proinflammatory mediators in the eye. Using scanning laser ophthalmoscope and optical coherence tomography imaging techniques, we showed that a low dose of AAVIL-1β was sufficient and was as pathogenic as a high dose of TNF in inducing vascular leakage, retinal degeneration, and cellular infiltration. Furthermore, only a marginal increase in IL-1β was enough to cause cellular infiltration, thus confirming the highly pathogenic nature of IL-1β in the eye. Contrary to our expectation, IL-6 or IL-17A had minimal or no effect in the eye. To examine the clinical relevance of our findings, we used an impedance assay to show that IL-1β alone or TNF alone was able to cause primary human retinal endothelial cell barrier dysfunction in vitro. Again, IL-6 alone or IL-17A alone had no effect on barrier function; however, in the presence of IL-1β or TNF, IL-17A but not IL-6 may provide additive proinflammatory effects.
Conclusions: Our studies demonstrate the existence of a functional hierarchy of proinflammatory cytokines in the eye, and we show that IL-1β is the most pathogenic when it is continuously expressed in the eye.
Introduction
Conventional treatments such as glucocorticoids, methotrexate, and azathioprine for chronic inflammatory diseases are aimed at targeting a broad spectrum of immune responses. The clinical success of immune pathway selective therapies such as the inhibition of tumor necrosis factor-alpha (TNF-α) in patients with autoimmune diseases like rheumatoid arthritis has challenged the utility of conventional treatments.Citation1 Moreover, the success of anti-TNF therapy puts forward the question of whether there are other important nodes in the inflammatory cascade that could be targeted to provide clinical benefits in patients with chronic diseases. Clinical findings suggest that the critical node, hence the critical proinflammatory cytokine, differs depending on the disease and the target tissue. For example, anti-TNF therapy is efficacious in psoriasis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, juvenile arthritis but not multiple sclerosis, which worsens upon inhibition of TNF.Citation2 Inhibition of interleukin-6 (IL-6) signaling is effective in treating rheumatoid arthritis, yet this strategy has shown limited efficacy or even failed in other chronic diseases.Citation3,Citation4 IL-1β inhibitors were developed for the treatment of rheumatoid arthritis, but they have so far shown no convincing benefits. Furthermore, although data are limited, IL-1β inhibition does not benefit patients with diseases mentioned above. In contrast, IL-1β inhibition does provide a drastic benefit to patients suffering from cryopyrin-associated periodic syndrome.Citation5
IL-1β, IL-6, IL-17A, and TNF may be involved in inflammatory ocular diseases such as uveitis,Citation6 proliferative diabetic retinopathy (PDR),Citation7 and diabetic macular edema (DME).Citation8 For example, the vitreous level of IL-1β is significantly higher in patients with ocular sarcoidosis than in nonsarcoid individuals.Citation9 Similarly, the vitreous level of IL-6 is significantly higher in PDR compared to noninflammatory retinopathy. In contrast, the vitreous level of TNF was not statistically different between proliferative retinopathy and noninflammatory retinopathy.Citation10 IL-1β is a potent proinflammatory cytokine with diverse effects on the function of ocular parenchymal cells such as Müller cells.Citation11 It was recognized earlier on that corneal cells responded to IL-lβ by increasing the synthesis of thromboxane, a vasoconstrictor and a potent hypertensive agent that facilitated platelet aggregationCitation12 and the release of chemokine monocyte chemotactic protein-1 (MCP-1 or CCL2). MCP-1 mediates the recruitment of monocytes to the eye,Citation13 which contribute to corneal neovascularization.Citation14 IL-6 is a multifunctional, pleiotropic cytokine involved in hematopoiesis and inflammation.Citation15 IL-6 is secreted by T cells and macrophages to stimulate proinflammatory immune responses against infectious agents.Citation16 T cells that predominantly produce IL-17A are termed Th17 cells.Citation17 IL-17A acts on a myriad of immune and parenchymal cells. These cells respond to IL-17A stimulation by upregulating the expression of proinflammatory cytokines, chemokines, and metalloproteases. Cells activated by IL-17A become highly pathogenic in a number of autoimmune diseases. TNF plays a significant role in the induction of cytokine synthesis and extracellular matrix protein synthesis. In addition, TNF augments monocyte and fibroblast chemotaxis by increasing the expression of soluble adhesion molecules like vascular cell adhesion protein 1 (VCAM-1 or CD106) in retinal blood vessels.Citation18 Many of these cytokines are simultaneously detected in patient eyes as well as in rodent models of ocular disease. However, it is unclear whether some of these cytokines play a redundant role or there is a hierarchical order in their ability to induce inflammation in the eye. In order to examine the hierarchy of proinflammatory cytokines in the eye, we induced continuous overexpression of IL-1β, IL-6, IL-17A, or TNF by intravitreal (IVT) injection of adeno-associated virus (AAV) vectors expressing the different cytokines. We found that only the continuous overexpression of IL-1β or TNF caused an increase in additional proinflammatory cytokines and chemokines as well as cell adhesion molecule VCAM-1. To our surprise, overexpressing IL-6 or IL-17A had minimal or no effects on the expression of other proinflammatory mediators in the eye. A continuous overexpression of IL-1β or TNF in the eye initiated a proinflammatory cascade that led to ocular inflammation characterized by cellular infiltrates, retinal degeneration, and vascular leakage. Interestingly, we found that IL-1β was more pathogenic than TNF in that it induced significantly more cellular infiltrates and a higher degree of retinal degeneration. To test the functional effect of these proinflammatory cytokines in humans, we used an impedance assay to demonstrate that IL-1β and TNF but not IL-6 or IL-17A decreased the ability of primary human retinal microvascular endothelial cells (HRMECs) to maintain barrier function. Our results suggest that, similar to other tissue-specific inflammatory conditions, there is a hierarchical order of proinflammatory cytokines in the eye. IL-1β and/or TNF may be the key nodes in the initiation of ocular inflammation.
Materials and methods
Animals
This study was performed according to animal protection guidelines issued by the Novartis Animal Welfare Policy. C57BL/6J mice (8- to 10-week-old females and males) were purchased from The Jackson Laboratory (JAX, Bar Harbor, ME, USA). Animals were housed in groups of two to five under temperature- and humidity-controlled conditions in individually ventilated cages with a 12-hour light/12-hour dark cycle. Mouse blood was collected into ethylenediaminetetraacetic acid (EDTA)-coated tubes (Sarstedt, Nümbrecht, Germany). Mouse plasma was prepared by centrifugation at 775 × g for 10 min at 4°C.
AAV vectors expressing cytokines and IVT administration of AAVs
The coding sequences for the mature peptides of mouse IL-1β, IL-6, IL-17A, and TNF were synthesized (Genewiz, LLC, MA, USA) to include an upstream Ig kappa signal sequence which is intended to facilitate protein secretion. The synthesized sequences were cloned into AAV shuttle plasmids containing a truncated CMV promoter and a SV40 polyadenylation signal sequence. The AAV-null shuttle plasmid contained DNA sequence with no promoter and no substantial open-reading frames. All shuttle sequences were flanked by wildtype AAV2 3ˊ inverted terminal repeats (ITRs) and mutant 5ˊ AAV2 ITRs which confer self-complementary viral genomes. Self-complementary, serotype AAV2 vectors were produced at the UMASS Medical Vector Core using the above shuttle vectors. For IVT administration of AAVs, AAV vector stocks were thawed from storage at −80°C on the day of injection. Vectors were diluted to a titer of 1 × 108 vg/µl (1E8) or 5 × 108 vg/µl (5E8) in 1× phosphate-buffered saline (PBS) containing 0.001% Pluronic F68 and placed on ice until the injection. Fluorescein was added to each vector dilution vial at a final 1:500 dilution (Alcon, Fort Worth, TX, USA). Mouse pupils were dilated by applying 1 drop (approximately 40 μL) of 1% cyclopentolate (Alcon). Immediately before anesthetic cocktail administration, pupil dilation was maximized with 1 drop of 2.5–10% phenylephrine (Alcon). Mice were then anesthetized with an intraperitoneal (IP) injection of a mixture of ketamine and xylazine at doses of 40 to 80 mg/kg and 5 to 10 mg/kg, respectively. Mice were kept on heating pads until animals were fully recovered from anesthesia. Prior to IVT injection, 1 drop of 0.5% proparacaine hydrochloride (Akorn, Lake Forest, IL, USA) as a local anesthetic was applied to both eyes. The anesthetized mouse was placed under a surgical microscope and a sclerotomy was made with a 30-gauge needle approximately 1.5 mm below the limbus. A 33-gauge blunt-tipped needle attached to a 10 μL syringe (Hamilton Co., Reno, NV, USA) was then inserted into the vitreous space through the sclerotomy and 1 μL of the test article was administered. The procedure was repeated on the fellow eye. After IVT injection, antibiotic ointment (tobramycin ophthalmic ointment, Akorn) was applied to both eyes. Mice were returned to the cage on a heating pad until they were fully recovered from the anesthesia.
Eye tissue preparation
Whole eyes were collected into 2 ml Eppendorf tubes, frozen on dry ice, and then stored at −80°C. To obtain eye lysate, a total of 220 μl lysis buffer consisting of radio immuno precipitation assay (RIPA) buffer (ThermoFisher Scientific) and Halt Protease and Phosphatease Inhibitor Cocktail (ThermoFisher Scientific, Grand Island, NY, USA) was added to each eye. Eye lysates were prepared using Qiagen TissueLyser II followed by centrifugation at 3000 × g for 10 min at 4°C. The protein concentration of each lysate preparation was determined by Lowry assay (BIO-Rad Labs, Hercules, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
The levels of cytokine and other secreted proteins in the eye were detected using CiraplexTM immunoassay kits from Aushon (Billerica, MA, USA). Each well of the 96-well microplate was pre-spotted with protein-specific antibodies. Protein levels were measured according to the manufacturer’s instructions. Luminescent signal was detected using a CirascanTM Imaging System.
Optical coherence tomography (OCT)
OCT imaging was performed using a spectral domain OCT imaging system (Bioptigen Envisu R2210, Leica Microsystems, Wetzlar, IL, USA). Prior to imaging, pupils were dilated using 1–2 drops of 1% cyclopentolate hydrochloride (Alcon) followed by 1–2 drops of 2.5% phenylephrine hydrochloride (Akorn) applied topically. 0.5% proparacaine hydrochloride (Akorn) was also applied topically as a local anesthetic. Mice were anesthetized by IP injection of a ketamine/xylazine (40–80 mg/kg and 5–10 mg/kg, respectively) cocktail and subsequently maintained at sternal recumbency on a heating pad during imaging. Corneal hydration was maintained through application of 0.3% hypromellose lubricating drops (Alcon). OCT b-scans centered at the optic nerve were acquired in the inferior–superior and nasal–temporal directions from each eye. Ten 1.8 mm b-scans were acquired at each position and then subsequently aligned and averaged for analysis.
Assessment of retinal vessel leakage
Retinal vessel leakage was assessed with fluorescence angiography. Images were obtained with a scanning laser ophthalmoscope (SLO) (HRA2, Heidelberg Engineering, Franklin, MA, USA) equipped with a 55-degree lens centered on the optic nerve. Retinal vessel permeability was assessed via IP injection of 200 µL of a combination of indocyanine green (ICG) (15 mg/kg, Akorn) and fluorescein (100 mg/kg, Akorn) approximately 3 minutes prior to anesthesia administration. Images were acquired approximately 4–6 minutes after dye injection. Mice were maintained under anesthesia for imaging as described in the OCT section. Data analysis was performed on retinal vessel images that were each composed of an average of up to 40 registered individual SLO images. Images were masked and randomized for the measurement of vascular leakage. Vascular permeability was quantified by processing the ICG and fluorescein images using a software routine developed in MATLAB® (MathWorks, Natick, MA, USA). ICG and fluorescein retinal images were first co-registered using an image processing algorithm. Normalized, co-registered images were subsequently subtracted from each other on a pixel-by-pixel basis. The ICG image contained labeled retinal vessels, whereas the fluorescein image contained signal in the retinal vessels in addition to extravasated dye. Therefore, subtraction of the ICG image from the fluorescein image produced an image containing only the extravasated fluorescein. The resulting fluorescein intensity per unit area quantified from the subtracted image pair was reported as “leakage” for each eye.
Histology
Animal eyes were harvested and fixed in 10% neutral buffered formalin for 2 days. Eyes were embedded in paraffin wax, serially sectioned at a thickness of 5 μm through the optic nerve, and processed for hematoxylin and eosin staining. 40× images were taken by Aperio AT2 scanner. Hematoxylin and eosin (H&E) slides were viewed using the Aperio eSlide system. Retina thickness was measured by the ruler tool in the system.
Impedance assay
Cell impedance was monitored in real time using the xCELLigence system E-Plate (ACEA Biosciences, San Diego, CA, USA). HRMECs were initiated by elutriation from dissociated normal human retinal tissue (Neuromics, Edina, MN, USA). About 40 000 primary HRMECs were seeded in each well and cultured until 100% confluent and then recombinant cytokines (R&D Systems, Minneapolis, MN, USA) were added. Culture medium with 0.5% fetal bovine serum (FBS) was used. The impedance value of each well was expressed as a cell index (CI) value. Data for cell impedance were normalized from the time of cytokine addition. Normalized CI was calculated using the software provided by the vendor.
RNA isolation and real-time PCR
Cultured HRMECs were lyzed and mRNA prepared using TurboCapture 96 mRNA kit (Qiagen, Germantown, MD, US) according to the manufacturer’s protocol. mRNA was used directly in cDNA synthesis by high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Real-time polymerase chain reaction (PCR) reaction was carried out on a ViiA 7 machine (Applied Biosystems) with TaqMan Gene Expression Assays using TaqMan Fast Advanced Master mix from Applied Biosystems. All data normalized to 18s rRNA expression. The 2−ΔΔCt method for relative quantification of gene expression was used to determine the level of miRNA expression. ΔCt was calculated by subtracting the Ct value of 18s rRNA from the Ct value of the mRNA of interest. The relative expression was generated using the equation 2−ΔΔCt.
Statistical analysis
Statistical analysis was carried out using one-way or two-way analysis of variance followed by Tukey’s and/or Dunnett’s multiple comparison tests. Results with p values of <0.05 were considered to be significant. All results are expressed as mean ± SEM. The exact p values are provided or expressed as *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001.
Results
Transduction of AAV vectors expressing proinflammatory cytokines significantly increased the proinflammatory cytokines in the eye
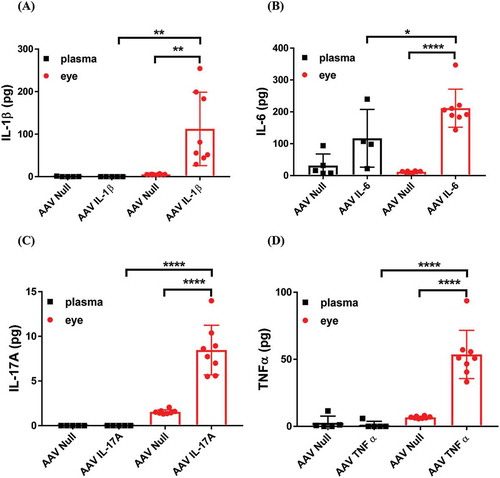
IL-1β, IL-6, IL-17A, and TNF have been widely shown to be upregulated in the eyes of animals as well as patients with different inflammatory ocular diseases such as PDR, uveitis, and dry eye. However, the individual contribution to ocular inflammation by each one of these cytokines in the absence of disease contributing factors has not been examined and contrasted. Here, we used AAV technology to overexpress the individual cytokines in the eye by IVT injection. We allowed the intraocular expression of cytokines for 5 weeks and then we measured the level of each cytokine in the eye lysate. shows that the levels of all four cytokines were upregulated following AAV cytokine transduction compared to controls that received AAV-null vectors. However, the absolute amount of cytokine detected in the eye varied between the different cytokines. The lowest average amount of cytokine was IL-17A and the highest average amount of cytokine was IL-6. We do not yet know if this is due to the difference in the intrinsic turnover of each cytokine or this is due to the efficiency of AAV transduction in the eye by IVT injection. Nonetheless, we detected a significant increase in the level of all four cytokines in the eye 5 weeks after in vivo AAV transduction. We also measured the systemic level of cytokines in mouse plasma following IVT injection of AAV vectors. shows that apart from an insignificant increase in IL-6 ()), IVT injection of AAV vectors did not lead to detectable elevations of cytokines in the plasma. The data suggest that AAV is a reliable tool to induce long-term overexpression of cytokines in the eye.
Figure 1. The level of cytokines in the plasma and in the eye following intraocular AAV transduction. The levels of (A) IL-1β, (B) IL-6, (C) IL-17A, and (D) TNF-α in mouse plasma and eye lysate was measured by ELISA. Mice were injected IVT with 5 × 108 vg/eye and 5 weeks later plasma and eye samples were collected for analysis. Whole eye lysates were used for cytokine measurement. We calculated the total amount of cytokines in plasma based on 1.5 ml total blood volume in a mouse. Each symbol represents a plasma sample or an eye sample.
AAV–IL-1Β or AAV–TNF but not AAV–IL-6 or AAV–IL-17A induced additional proinflammatory cytokines as well as chemokines in the eye
To study the individual contribution of cytokines to ocular inflammation, we first profiled the level of other cytokines and chemokines as well as adhesion molecule VCAM-1 and metalloproteinase MMP-9 in the eye as a result of overexpressing the individual cytokines. We chose to measure interferon-γ, IL-12p70, and IL-10 as these cytokines have been implicated in a number of ophthalmic diseases.Citation6,Citation19,Citation20 Chemokines are a family of small, secreted polypeptides that are typically released by endothelial cells and leukocytes in response to an inflammatory signal such as an elevation of TNF and IL-1β. Chemokines contribute to ocular inflammation by regulating the activation of leukocytes, the expression of adhesion molecules, and chemotaxis.Citation21
Our results show that only the overexpression of IL-1β or TNF induced additional proinflammatory cytokines in the eye. Overexpression of IL-1β induced IL-6 as well as IL-17A ()). In contrast, overexpression of TNF induced a significant upregulation of IL-1β but not the other cytokines we measured ()). We also measured a number of inflammatory mediators in response to continuous overexpression of IL-1β, IL-6, IL-17A, or TNF. Similar to cytokines, we found that AAV–IL-1β-injected eyes had significantly elevated levels of chemokine (c-x-c motif) ligand 1 (KC) (CXCL1), MCP-1, vascular endothelial growth factor (VEGFA), VCAM-1, and MMP-9 ()). We did not observe a significant effect on chemokines as a result of overexpressing IL-6 or TNF. However, we did find the overexpression of IL-6 or TNF induced a significantly higher level of VCAM-1 in the eye ()–), respectively).
Figure 2. Initiation of an inflammatory cascade by the continuous overexpression of IL-1β or TNF-α. The levels of cytokines (A–D) and inflammatory mediators (E–H) in mouse eye lysates following IVT injection of AAV–IL-1β (A and E), AAV–IL-6 (B and F), AAV–IL-17A (C and G), or AAV–TNF-α (D and H) were measured by ELISA. Mice were injected IVT with 5 × 108 vg/eye, and 5 weeks later whole eyes were collected for analysis. Each symbol represents an eye sample.
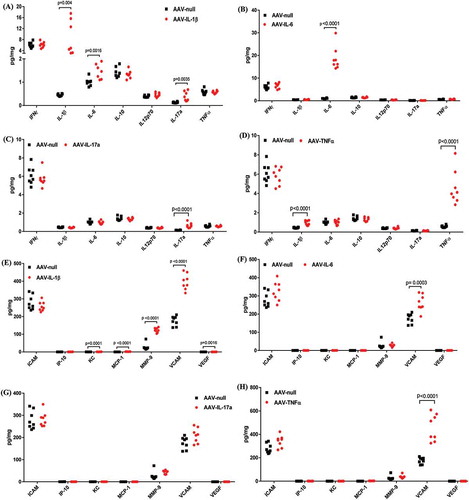
Overall, continuous overexpression of IL-1β or TNF induced additional inflammatory mediators that could amplify an inflammatory response leading to tissue damage and ocular pathology. To our surprise, a continuous overexpression of known proinflammatory cytokines IL-6 or IL-17A had minimal or no effects on the level of other cytokines and inflammatory mediators in the eye. It is possible that the overexpression of IL-6 or IL-17A alone is not sufficient to trigger an inflammation cascade; however, in the presence of additional proinflammatory mediators, these cytokines can then contribute to the amplification of the response.
Intraocular overexpression of IL-1Β led to severe eye pathology while only modest effects were seen with TNF overexpression
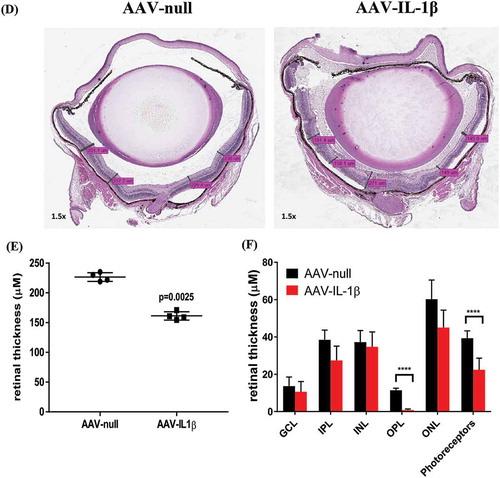
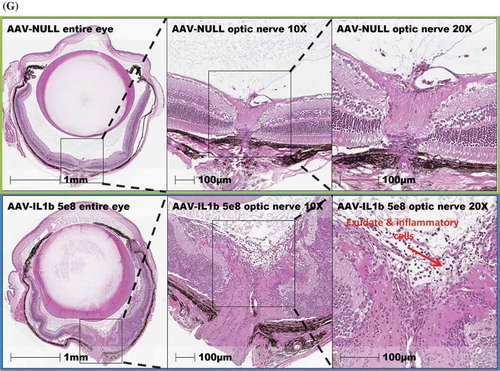
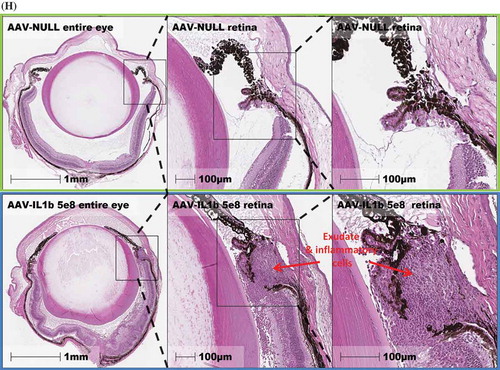
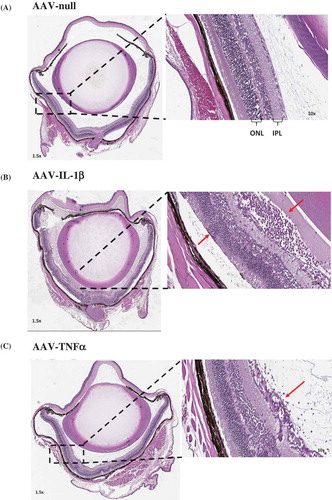
To examine the effect of continuous overexpression of proinflammatory cytokines on ocular tissues, we used H&E staining to review the whole eye. We first observed a marked number of cellular infiltrates in the AAV–IL-1β-injected eyes ()) as compared to AAV-null injected control eyes ()). AAV–TNF-injected eyes also had cellular infiltrates albeit much less than AAV–IL-1β-injected eyes ()). We did not observe a difference in cellular infiltrates between eyes that received AAV–IL-6 or AAV–IL-17A injection and AAV-null injected control eyes (data not shown). To measure the effect on the retina, we focused on AAV–IL-1β-injected eyes as they showed the highest level of cellular infiltrates. We first examined the thickness of the retina using the H&E images. We measured the thickness of the retina in multiple areas as shown in ) and found that AAV–IL-1β significantly reduced the thickness of the retina compared to AAV-null injected controls ()). We also measured the thickness of each individual layer in the retina and found that AAV–IL-1β significantly reduced the thickness of outer plexiform layer (OPL) and photoreceptor but not the other layers ()). To further examine the effect of AAV–IL-1β in the back of the eye, we focused on the optic nerve head. We found that overexpression of IL-1β in the eye resulted in a marked increase in the number of cellular infiltrates around the optic nerve head ()). This was only seen in eyes that received AAV–IL-1β and not the other cytokines (data not shown). We also examined the front of the eye and found that AAV–IL-1β injection led to a disorganized ciliary body with a marked increase in the number of cellular infiltrates ()). Again, this was only observed in the eyes that received AAV–IL-1β and not the other cytokines (data not shown). Thus, overexpressing IL-1β in the eye can affect the structural integrity of the eye probably due to its strong ability to initiate a cascade of proinflammatory reactions. Although the overexpression of TNF did result in cellular infiltrates in the eye, this was not strong enough of a response to cause structural damage to the back and the front of the eye (data not shown).
Figure 3a. Eye histopathology following AAV–IL-1β and AAV–TNF-α IVT injection. H&E staining of the whole eye showing no cellular infiltrates in (A) the AAV-null injected eye and a markedly increased number of cellular infiltrates (red arrows) in (B) AAV–IL-1β-injected eye and in (C) AAV–TNF-α-injected eye albeit to a lesser extent. (D) The thickness of the mouse retina was measured in 4–5 sites as shown in the picture. (E) Each symbol represents the average thickness of one retina in each experimental group (n = 4). (F) The average thickness of the different retinal layers in the same eyes. Cellular infiltrates in (G) retina close to the optic nerve head and in (H) areas around the ciliary body in AAV–IL-1β-injected eye (red arrows) and not in the AAV-null injected eye. Mice were injected IVT with 5 × 108 vg/eye, and 5 weeks later eyes were collected for analysis. Histology pictures are representatives of four animals per experimental group.
Intraocular overexpression of IL-1Β or TNF induced retinal leakage and cellular infiltrates
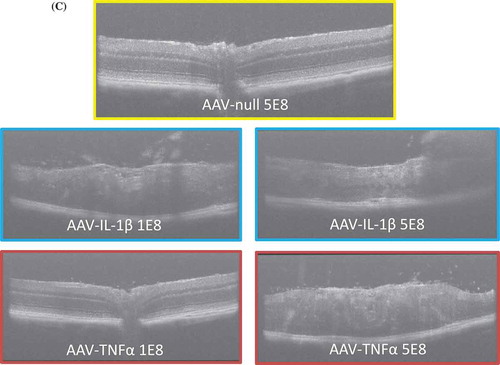
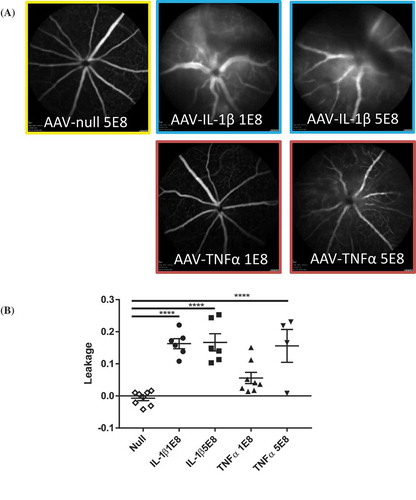
To examine the functional consequence of a continuous overexpression of proinflammatory cytokines, we injected fluorescein in mice and used SLO imaging to evaluate the amount of vascular leakage in the eye. To examine a cytokine dose effect on vascular leakage, we tested two doses (1E8 or 5E8 vg/eye) of AAV cytokines in the eye. ) shows that while both doses of AAV–IL-1β led to a significant increase in leakage, there was not an AAV-IL-1β dose-dependent effect. In contrast, AAV–TNF induced leakage in a dose-dependent manner. The low-dose (1E8) AAV–TNF did not significantly increase leakage compared to AAV-null controls even though the trend is higher ()). We did not find any difference in leakage between the AAV-null controls and AAV–IL-6- or AAV–IL-17A-injected animals (data not shown). We used OCT imaging to examine the retina. We found that there was a significant increase in cellular infiltrates in the retina of AAV–IL-1β- or AAV–TNF-injected eyes compared to AAV-null controls. Similar to vascular leakage, a low or a high dose of AAV–IL-1β induced similar number of cellular infiltrates in the retina, whereas with AAV–TNF, there was a dose-dependent increase of cellular infiltrates in the retina ()). Our results demonstrate that overexpression of IL-1β in the eye is highly pathogenic and can compromise retinal barrier function. Furthermore, this can cause cellular infiltration to the retina and damage retinal layers. Indeed, only a low dose of AAV–IL-1β is needed to induce severe pathology in the eye, and this agrees with the finding of a greater increase in the number of additional proinflammatory mediators with AAV–IL-β compared to AAV–TNF as shown earlier.
Figure 4a. AAV–IL-1β or AAV–TNF-α compromised retinal barrier function and damaged retinal layers. (A) SLO images of eyes injected with AAV-null (5E8 vg/eye), AAV–IL-1β, or AAV–TNF-α (1E8 and 5E8 vg/eye) following fluorescein injection. (B) Quantification of retinal leakage. Each symbol represents an eye sample. (C) OCT images of AAV-null- (5E8), AAV–IL-1β-, or AAV–TNF-α (1E8 and 5E8)-injected eyes. Images are representatives of each experimental group (n = 4 to 8 mice).
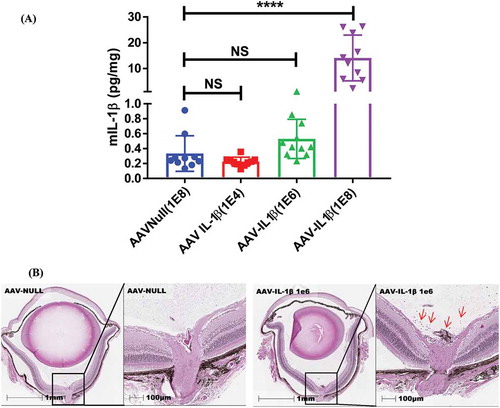
A continuous exposure to even an ultralow level of IL-1Β results in cellular infiltration to the eye
To further investigate the level of IL-1β required to induce ocular inflammation, we carried out an in vivo AAV–IL-1β dose–response study. We injected mice with 1E4, 1E6, and 1E8 vg/eye of AAV–IL-1β and examined the eyes by histology for signs of inflammation. We found that a dose of 1E4 or a dose of 1E6 AAV–IL-1β did not significantly increase the level of IL-1β in the eye, whereas a dose of 1E8 AAV–IL-1β did ()). However, even with an ultralow level of IL-1β induced by 1E6, AAV–IL-1β resulted in cellular infiltrates in the vitreous and in areas around the optic nerve ()). This effect was lost when we lowered the dose of AAV–IL-1β to 1E4 (data not shown). However, we do not know the effect of the 1E4 dose beyond 5 weeks of expression, and it is possible that a longer expression can still result in ocular inflammation even with this dose of AAV–IL-1β. Collectively, our results suggest that a continuous intraocular exposure to even an insignificant amount of IL-1β can lead to ocular inflammation over time.
Figure 5. Even an ultralow dose of AAV–IL-1β (1E6) induces cellular infiltrates in the vitreous. (A) Mice were injected IVT with 1E4, 1E6, or 1E8 vg/eye of AAV–IL-1β, and 5 weeks later whole eye lysates were used in ELISA to detect the amount of IL-1β in the eye. Each symbol represents an eye sample. (B) H&E staining of the whole eye showing no cellular infiltrates in the AAV-null-injected eye and the presence of cellular infiltrates (red arrows) in 1E6 AAV–IL-1β-injected eye. Histology pictures are representatives of 4 eyes per experimental group.
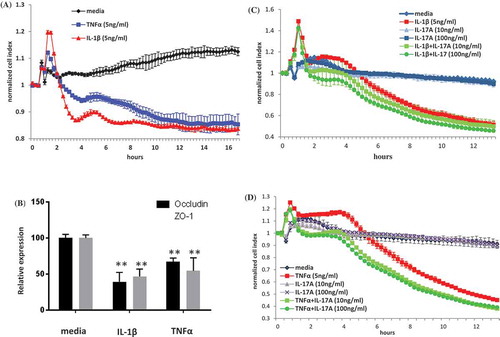
In vitro challenge with IL-1Β alone or TNF alone but not IL-6 alone or IL-17A alone decreased HRMEC impedance
We used an impedance assay with primary HRMECs to examine the functional effect of the proinflammatory cytokines on human cells. We first examined the individual contribution of cytokines to cell impedance. We found that whilst IL-1β alone or TNF alone ()) markedly decreased the impedance of HRMECs, IL-6 alone (data not shown) or IL-17A alone () and )) did not affect HRMEC impedance. The downregulatory effect of IL-1β and TNF on HRMEC impedance was associated with a significant decrease in mRNA expression of occludin and ZO-1 which are critical to endothelial cell tight junction formation and barrier functionCitation22 ()). We have shown that overexpressing IL-6 or IL-17A in vivo did not lead to an increase in inflammatory mediators in the eye (), ), ), and )). We hypothesized that IL-6 alone or IL-17A alone was not sufficient to trigger an inflammation cascade. However, in the presence of additional proinflammatory cytokines, IL-6 or IL-17A could amplify the response. To test this hypothesis, we added exogenous IL-6 or IL-17A alone or together with IL-1β or with TNF in the impedance assay. We did not see any effect on impedance by IL-6 alone (data not shown). We also did not observe an additive effect by IL-6 together with IL-1β or with TNF compared to IL-1β alone or TNF alone (data not shown). However, we did observe a trend for an additive effect of IL-17A together with IL-1β ()) or with TNF ()) on HRMEC impedance. In summary, our results demonstrate that not all proinflammatory cytokines can independently induce HRMEC impedance. IL-17A acts collaboratively with IL-1β or with TNF to further compromise barrier function.
Figure 6. HRMEC impedance. (A) HRMECs were cultured in the absence (media alone) or in the presence of IL-1β or TNF-α (5 ng/ml), and impedance values were automatically recorded at 15-min intervals. (B) HRMECs were harvested at 16-hours post in vitro stimulation with or without IL-1β or TNF-α and mRNA expression was measured by quantitative polymerase chain reaction (QPCR). HRMECs were cultured in the absence (media alone) or in the presence of (C) IL-1β with or without IL-17A at 10 or 100 ng/ml or in the presence of (D) TNF-α with or without IL-17A at 10 or 100 ng/ml and impedance was recorded over 13 hours. Data for cell impedance were normalized from the time of cytokine addition. Normalized CI is shown.
Discussion
Proinflammatory cytokines IL-1β, IL-6, IL-17A, and TNF are known to be critically involved in the progression of ophthalmic diseases. Here, we used AAV vectors as a delivery vehicle to overexpress the cytokines in the eye for 5 weeks in order to compare and contrast the pathogenicity of each cytokine in wildtype mice without other factors contributing to disease. We found that the overexpression of IL-1β caused an increase in IL-6, IL-17A, KC (CXCL1), MCP-1, MMP-9, VCAM-1, and VEGFA levels in the eye, while the overexpression of TNF increased IL-1β, MCP-1, and VCAM-1 levels in the eye. To our surprise, overexpressing IL-6 or IL-17A had minimal or no effects on the level of other cytokines and inflammatory mediators in the eye. The induction of additional proinflammatory mediators by the overexpression of IL-1β or TNF led to an influx of cellular infiltrates to the retina and resulted in retinal pathology. Using SLO and OCT imaging techniques, we found that the overexpression of IL-1β or TNF induced significant retinal vascular leakage as well as retinal degeneration. In a dose–response study, we demonstrated that even a marginal increase of IL-1β in the eye can result in ocular inflammation over time. Using impedance assay, we demonstrated that IL-1β alone or TNF alone was sufficient to decrease HRMEC impedance in vitro. IL-17A but not IL-6 together with IL-1β or TNF tended to further decrease HRMEC impedance, suggesting an additive proinflammatory effect of IL-17A. Interestingly, throughout our studies, we consistently found that the lowest cytokine expression level needed to induce ocular inflammation was with IL-1β, suggesting that IL-1β was the most pathogenic among the proinflammatory cytokines tested. Thus, our results suggest that there is a hierarchy of proinflammatory cytokines in the eye, and among the cytokines tested IL-1β has the highest potential to cause ocular inflammation.
Cytokines are key modulators of inflammation, participating in acute and chronic inflammation via complex and sometimes synergistic functions. IL-1β, IL-6, IL-17A, and TNF are some of the key proinflammatory cytokines. In response to stimulation by these cytokines, target cells release chemokines like MCP-1, IP-10, and KC to regulate the recruitment of inflammatory cells to the eye. It has long been known that in vitreoretinal disorders such as proliferative vitreoretinopathy (PVR), the vitreous levels of cellular mediators, including cytokines, chemokines, and growth factors, are often upregulated. Banerjee et al.Citation23 have shown that the predominant mediators detected in the vitreous were IL-6, IL-8, and MCP-1. The level of IL-1β was not examined in this study. Our results show that the overexpression of IL-1β augments IL-6 and MCP-1 in the mouse eye. Similar to PVR patient eyes, we found that AAV–IL-1β-injected mouse eyes had a high level of cellular infiltrates. This suggests that IL-1β may be the trigger for an inflammatory cascade that leads to eye pathology. To this end, we observed that both low and high expression of IL-1β resulted in retinal thinning, inflammation of the optic nerve head, disorganization of the ciliary body, and compromised retinal barrier function.
TNF takes part in the inflammatory process by inducing extracellular matrix protein synthesis, stimulating monocyte/fibroblast chemotaxis, and triggering the release of soluble adhesion molecules in retinal blood vessels.Citation24 Experimental studies showed that IVT injection of recombinant TNF caused an increase of aqueous humor proteins, corneal neovascularization, and posterior synechia.Citation25 Furthermore, TNF is an independent serum marker for proliferative retinopathy in type 1 diabetic patients.Citation26 Although we did not examine extracellular matrix proteins in our mouse eyes injected with AAV–TNF, we did observe a significant increase in VCAM-1 adhesion molecule. This was accompanied by an increase in cellular infiltrates in the vitreous as well as the in the retina. Interestingly, unlike AAV–IL-1β, we found that a higher dose of AAV–TNF was needed to induce retinal pathology, suggesting that IL-1β may be more pathogenic in the eye. TNF and IL-1β are cytokines with multiple, overlapping biologic activities. For example, IL-1β together with TNF induces the release of IL-6,Citation27 IL-8,Citation28 and MCP-1Citation29 in vitro. Co-injection of recombinant IL-1β and TNF proteins intravitreally in rabbits leads to the development of posterior as well as anterior uveitis that shows an increase of leukocyte infiltration in the aqueous humor. Treated animals also showed disruption of blood retinal barrier (BRB), resulting in elevated protein concentration in the anterior and posterior segments of the eye.Citation30 We did not co-inject AAV–IL-1β and AAV–TNF in mice due to technical constrains; however, we would expect similar if not more severe ocular phenotype than that was shown in the previous study because of a longer duration of cytokine expression with AAV transduction. Nonetheless, we did observe an upregulation of IL-1β with AAV–TNF injection, suggesting that these two cytokines can indeed regulate each other’s expression. Other studies have also reported on the pathogenicity of IL-1β and TNF in the eye. For instance, IVT injection of recombinant IL-1β and not PBS or bovine serum albumin in rats led to a significant increase in capillary cell death and acellular capillaries in the retinal microvessels.Citation31 Injection of recombinant IL-1β in the rat eye rapidly increased the permeability of the BRB (peaking between 4 and 8 hours postinjection), and this coincided with the appearance of both polymorphonuclear and mononuclear leukocytes within the retina.Citation32 In a similar manner, IVT injection of TNF in rats also led to BRB permeability by decreasing the level of tight junction proteins ZO-1 and claudin-5.Citation33 Our findings that IL-1β and TNF decreased the mRNA expression of ZO-1 and occludin in primary HRMECs further support this mechanism in humans.
In a previous study aimed at elucidating the mechanism underlying the induction of ocular inflammation in the rat model of endotoxin-induced uveitis (EIU) it was found that IVT injection of endotoxin-free human recombinant IL-6 resulted in uveitis.Citation34 More recently, a study showed that IVT injection of IL-17A aggravated ganglion cell apoptosis in Akita mice with established hyperglycemia.Citation35 Based on these previous observations, although the studies were carried out in rats or Akita mice with established disease, respectively, we had hypothesized that a continuous intraocular exposure to IL-6 or IL-17A in naïve wildtype mice could lead to ocular inflammation. Contrary to our expectation, overexpressing IL-6 or IL-17A in the mouse eye did not lead to observable pathogenic effects. This could be due to the fact that the expression level of these cytokines did not reach the required level to induce a pathogenic effect or these cytokines are pathogenic only in the presence of other proinflammatory mediators. In support of the latter hypothesis, using an in vitro system, we showed that IL-17A together with IL-1β or with TNF resulted in a trend for a further decrease in HMRVC impedance compared to IL-1β alone or TNF alone. This suggests that in the presence of additional cytokines, IL-17A may provide an additive proinflammatory effect.
Our study demonstrates the existence of a functional hierarchy of proinflammatory cytokine in the eye. Amongst the cytokines tested IL-1β has the highest potential to initiate ocular inflammation. Compared to IL-1β, a higher level of TNF is needed to trigger ocular inflammation. Our data question the direct role of IL-6 and IL-17A in the induction of ocular inflammation. Our findings provide new insight into the relative pathogenicity of proinflammatory cytokines in the eye and may guide the design of pathway selective therapies for ocular diseases.
Declaration of interest
All authors are employees of Novartis Institutes for Biomedical Research, Cambridge, USA.
Acknowledgments
A.P. Cunha, Q. Zhang, M. Prentiss, X.Q. Wu, and Y.Y. Xu performed and analyzed experiments and helped in writing the manuscript. V. Kainz, J. Vrouvlianis, H. Li, N. Rangaswamy, B. Leehy, and T. McGee performed and analyzed experiments. C. Bell, C. Bigelow, V. Kansara, and Q. Medley analyzed data and gave scientific input. Q. Huang and H.Y. Wu conceived the project, designed experiments, analyzed data, and wrote the manuscript.
References
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi:10.1056/NEJMra1004965.
- Probert L, Eugster HP, Akassoglou K, Bauer J, Frei K, Lassmann H, Fontana A, et al. TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 2000;123(Pt 10):2005–19. doi:10.1093/brain/123.10.2005.
- Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterol. 2004;126(4):989–96. doi:10.1053/j.gastro.2004.01.012.
- Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–83. doi:10.1172/JCI57158.
- Federici S, Martini A, Gattorno M. The central role of anti-IL-1 blockade in the treatment of monogenic and multi-factorial autoinflammatory diseases. Front Immunol. 2013;4:351. doi:10.3389/fimmu.2013.00351.
- Ooi KG, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: is there a correlation with clinical phenotype? Clin Med Res. 2006;4(4):294–309. doi:10.3121/cmr.4.4.294.
- Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37(5):416–20. doi:10.3109/02713683.2012.661114.
- Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H, Sonoda Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina. 2014;34(4):741–48. doi:10.1097/IAE.0b013e3182a48917.
- Nagata K, Maruyama K, Uno K, Shinomiya K, Yoneda K, Hamuro J, et al. Simultaneous analysis of multiple cytokines in the vitreous of patients with sarcoid uveitis. Invest Ophthalmol Vis Sci. 2012;53(7):3827–33. doi:10.1167/iovs.11-9244.
- Yuuki T, Kanda T, Kimura Y, Kotajima N, Tamura J, Kobayashi I, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001;15(5):257–59. doi:10.1016/S1056-8727(01)00155-6.
- Liu X, Ye F, Xiong H, Hu DN, Limb GA, Xie T, et al. IL-1beta induces IL-6 production in retinal Muller cells predominantly through the activation of p38 MAPK/NF-kappaB signaling pathway. Exp Cell Res. 2015;331(1):223–31. doi:10.1016/j.yexcr.2014.08.040.
- Shams NB, Sigel MM, Davis JF, Ferguson JG. Corneal epithelial cells produce thromboxane in response to interleukin 1 (IL-1). Invest Ophthalmol Vis Sci. 1986;27(10):1543–45.
- Strieter RM, Standiford TJ, Huffnagle GB, Colletti LM, Lukacs NW, Kunkel SL. “The good, the bad, and the ugly.” The role of chemokines in models of human disease. J Immunol. 1996;156(10):3583–86.
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82(5):765–70. doi:10.1002/(ISSN)1097-0215.
- Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705–14. doi:10.1016/S1074-7613(01)00151-0.
- Van Der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176(2):439–44. doi:10.1086/jid.1997.176.issue-2.
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19(6):353–61. doi:10.1016/j.smim.2007.10.008.
- Limb GA, Webster L, Soomro H, Janikoun S, Shilling J. Platelet expression of tumour necrosis factor-alpha (TNF-alpha), TNF receptors and intercellular adhesion molecule-1 (ICAM-1) in patients with proliferative diabetic retinopathy. Clin Exp Immunol. 1999;118(2):213–18. doi:10.1046/j.1365-2249.1999.01067.x.
- Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46(11):4251–59. doi:10.1167/iovs.05-0444.
- Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, et al. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS One. 2015;10(9):e0137358. doi:10.1371/journal.pone.0137358.
- Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29(4):351–61. doi:10.1016/S1046-2023(02)00359-6.
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273(45):29745–53. doi:10.1074/jbc.273.45.29745.
- Banerjee S, Savant V, Scott RA, Curnow SJ, Wallace GR, Murray PI. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2007;48(5):2203–07. doi:10.1167/iovs.06-1358.
- Limb GA, Soomro H, Janikoun S, Hollifield RD, Shilling J. Evidence for control of tumour necrosis factor-alpha (TNF-alpha) activity by TNF receptors in patients with proliferative diabetic retinopathy. Clin Exp Immunol. 1999;115(3):409–14. doi:10.1046/j.1365-2249.1999.00839.x.
- Fleisher LN, Ferrell JB, McGahan MC. Ocular inflammatory effects of intravitreally injected tumor necrosis factor-alpha and endotoxin. Inflammation. 1990;14(3):325–35. doi:10.1007/BF00915816.
- Gustavsson C, Agardh E, Bengtsson B, Agardh CD. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J Diabetes Complications. 2008;22(5):309–16. doi:10.1016/j.jdiacomp.2007.03.001.
- Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, et al. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142(2):549–53.
- Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunol. 1989;68(1):31–36.
- Larsen CG, Zachariae CO, Oppenheim JJ, Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989;160(3):1403–08. doi:10.1016/S0006-291X(89)80160-3.
- Fleisher LN, Ferrell JB, McGahan MC. Synergistic uveitic effects of tumor necrosis factor-alpha and interleukin-1 beta. Invest Ophthalmol Vis Sci. 1992;33(7):2120–27.
- Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45(11):4161–66. doi:10.1167/iovs.04-0633.
- Bamforth SD, Lightman SL, Greenwood J. Interleukin-1 beta-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. Am J Pathol. 1997;150(1):329–40.
- Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59(11):2872–82. doi:10.2337/db09-1606.
- Hoekzema R, Murray PI, Van Haren MA, Helle M, Kijlstra A. Analysis of interleukin-6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1991;32(1):88–95.
- Qiu AW, Bian Z, Mao PA, Liu QH. IL-17A exacerbates diabetic retinopathy by impairing Muller cell function via Act1 signaling. Exp Mol Med. 2016;48(12):e280. doi:10.1038/emm.2016.117.