ABSTRACT
Purpose: To analyze the visual and refractive outcomes of combined accelerated cross-linking with femtosecond laser intracorneal ring segment implantation for the treatment of pediatric keratoconus.
Materials and Methods: This retrospective multicenter noncomparative clinical study included 63 eyes of 37 patients (age, 9–17 years) who underwent between August and September 2016 combined cross-linking with intracorneal ring segment implantation for keratoconus. Preoperative and postoperative (6, 12, and 18 months) uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA), subjective refractions, keratometry (K), and pachymetry measurements were compared.
Results: The postoperative spherical equivalent refraction was within ±1 D, ±2 D, and ±3 D in 19 (30.2%), 27 (42.9%), and 37 (58.8%) eyes, respectively. Only 27 eyes achieved the attempted preoperative spherical equivalent refraction. The mean spherical equivalent refraction significantly improved from −6.01 ± 2.97 to −3.13 ± 2.78 D postoperatively (P < 0.0001). The mean K average reading significantly decreased from 48.75 ± 4.25 to 46.65 ± 3.89 D postoperatively (P < 0.0001). The mean postoperative myopic, astigmatic, and spherical equivalent corrections were −2.17 ± 2.19, −1.52 ± 2.03, and −2.93 ± 2.35 D, respectively. The mean UDVA and CDVA showed significant improvements (0.89 ± 0.33 to 0.40 ± 0.28, P < 0.0001; 0.35 ± 0.31 to 0.25 ± 0.24, P = 0.004; respectively) at 18 months postoperatively. Keratoconus progression, segment migration, and segment extrusion were seen in four (6.4%), one (1.6%), and three (4.7%) eyes, respectively, probably contributing to the lower mean postoperative CDVA.
Conclusion: Cross-linking plus is only partially effective for pediatric keratoconus. Despite some improvements in vision and keratometry measures, it resulted in complications such as keratoconus progression, segment extrusion, and segment migration that affected the vision in some patients. These findings suggest an assessment of standard epithelium-off collagen cross-linking as a sole procedure to treat pediatric keratoconus in future studies.
Introduction
Advances in corneal topographic analysis have greatly facilitated the early diagnosis of keratoconus (KC), an ectatic noninflammatory disease, and helped in documenting KC progression. Higher rates of corneal thinning at the thinnest location, increasing keratometry (K) readings, and higher central back elevation are the risk factors for KC progression.Citation1 Most patients with KC experience progressive thinning and weakening of the corneal stromal tissue, leading to the development of a conical cornea. Furthermore, KC is usually associated with progressive myopia and irregular astigmatism, which lead to progressive visual deterioration.Citation2 However, appropriate management of KC remains an unresolved issue to date.
Pediatric KC includes all cases of KC in patients aged under 18 years. In general, many studies have documented that pediatric KC has a more aggressive and progressive course than adult KC.Citation3–Citation6 Other studies have reported an inverse relationship between the age at KC onset and its severity.Citation7,Citation8 In addition, they demonstrate that the presence of keratoconic corneas in children is associated with a sevenfold higher chance of requiring a keratoplasty in comparison to that in older patients. Vernal keratoconjunctivitis (VKC) is a primary cause of treatment failure in pediatric KC, which may negatively influence the education and lifestyle of these young patients.Citation9 There are many challenges in treating pediatric KC including difficulties in obtaining accurate corneal topography measurements, tolerating contact lenses, and preventing eye rubbing. The long-term nature of VKC is frequently associated with behavioral eye rubbing as well as limbal stem cell dysfunction and deficiency with an increase in inflammatory markers of the tear film.Citation3,Citation7,Citation9
Currently, the only available therapeutic approach for KC is corneal collagen cross-linking (CXL), which halts the progression of KC pathology and, thus, prevents further visual deterioration. Although other known treatment modalities, such as intracorneal ring segment (ICRS) implantation and wavefront-guided photorefractive keratectomy (WFG PRK), can improve the refractive status of the keratoconic eye and help to reduce anterior corneal surface irregularities and higher-order aberrations, they cannot prevent KC progression.Citation10,Citation11 Cross-linking plus (CXL-Plus) uses a combination of CXL to halt KC progression and another refractive procedure, such as ICRS and WFG PRK, to improve the postoperative visual outcomes.Citation11,Citation12 Many studies have reported the outcomes of using CXL-Plus in adults with KC and concluded that CXL-Plus is an effective and safe procedure to treat KC in adults.Citation11–Citation13 Other studies have also reported good results with CXL and ICRS implantation in pediatric KC.Citation14–Citation16 To determine whether CXL-Plus could provide similarly good results in the treatment of pediatric KC, our study aimed to analyze the visual and refractive outcomes with respect to the stability and effectiveness of CXL combined with femtosecond laser ICRS implantation in pediatric KC.
Materials and methods
Our study employed a retrospective multicenter design. All surgeries were performed by five experienced surgeons at the Sohag University and Alexandria University Hospitals. The authors obtained the approval of the Medical Ethics Committee at Sohag University Hospital for a study from August 2016 to April 2018. This study followed the principles of the Declaration of Helsinki. All data were collected from the patients’ medical files, and informed consent was obtained from the parents of the pediatric KC patients.
Our study enrolled 63 eyes of 37 pediatric KC patients (age, 9–17 years) who underwent accelerated epithelium-on CXL along with femtosecond laser Keraring (Mediphacos Inc., Belo Horizonte, Brazil) implantation (i.e. CXL-Plus) for treatment of KC. The devices used in our study included the CSO SIRIUS Topographer (CSO, Firenze, Italy), the advanced femtosecond laser (iFS; Abbott Laboratories, Abbott Park, IL, USA), the KXL System (Avedro Inc., MA, USA), and the Opto XLink CXL system (Opto Global Pty Ltd., Adelaide, Australia).
All study eyes fulfilled the following criteria: age < 18 years; grade 1, 2, or 3 KC (Amsler-Krumeich classification); documented KC progression (on the basis of the presence of two or more of the following criteria: increase of >1 D in the maximum simulated keratometry [Kmax] value, decrease of >2% in the corneal thickness, increase of >0.5 D in the spherical equivalent [SE] value, and an increase in the K readings on the posterior corneal surface or in the back surface elevation), Keraring selection from the Standard Keraring Nomogram, CXL-Plus performed using the KXL System and advanced femtosecond laser, and 18-month follow-up data available. The patient information included the results of uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) measurements, slit-lamp examinations, subjective refraction assessments, and corneal topography assessments performed preoperatively and at 6, 12, and 18 months postoperatively.
Surgical procedure
All five surgeons followed similar procedures. The SI-5 Keraring segment had a 5.0-mm optical zone with a triangular cross-sectional design. The decision to use a single or two Keraring segments was made on the basis of the Standard Keraring Nomogram (Mediphacos Nomogram for Keraring calculation guidelines 2009 version 5.2).
The first step in the CXL-Plus procedure was the implantation of the Keraring segments using the femtosecond laser, which created a corneal tunnel with the following parameters: inner diameter, 5 mm; outer diameter, 5.9 mm; tunnel depth, 80% of the corneal thickness at the thinnest location; and incision site created according to the direction of the steepest corneal meridian.
Topical benoxinate hydrochloride 0.4% anesthetic eye drops (Benox, Sterile Ophthalmic Solution, Pharmaceutical Industries Company, E.I.P.I.CO., Egypt) were instilled into the eye 10 min before the surgery. In children who did not tolerate topical anesthesia, the general anesthetic Ketalar (Ketamine HCl 50 mg/mL Injection Vial 10 mL CIV; JHP Pharmaceuticals, NY, USA) was used. The corneal center was marked by asking the patient to fixate the eye at a flashing light. The suction ring was applied onto the eye, which was followed by corneal tunnel formation using the femtosecond laser device with 5-mJ energy. Tunnel patency was checked by using a spatula to be passed through the tunnel limbs. Implantation of the nasal and temporal Keraring segments or a single segment was performed through the corneal tunnel.
Accelerated epithelium-on CXL was performed using the Avedro KXL system. According to the Avedro nomogram, two types of riboflavin had to be administered to the cornea. The first type was ParaCel riboflavin, which was instilled three times at 90-s intervals over a 4.5-min period. The second type was Vibex Xtra riboflavin, which was instilled four times at 90-s intervals over a 6-min period. The total soaking time was 10.5 min. A total energy dose of 7.2 J/cm2 was delivered at 45-mW/cm2 intensity. The pulsed mode was used (1 s on and 1 s off) to achieve a total ultraviolet (UV) duration of 160 s, with the total treatment duration being 320 s. At the end of the surgery, a bandage contact lens (CooperVision; The Cooper Companies, Inc. California, USA) was placed onto the cornea, followed by administration of gatifloxacin 0.3% eye drops (Zymar; Allergan, Inc., Jersey City, USA).
Postoperative medication, care, and follow-up
Topical therapy was prescribed for all patients in the first three postoperative weeks. The topical therapy included three types of eye drops: gatifloxacin 0.3% eye drops, prednisolone acetate 1% eye drops (Econopred Plus; Alcon Laboratories, Inc, Texas, USA), and sodium hyaluronate 0.15% eye drops (Hyabak; THEA Laboratories, Clermont-Ferrand, France). The patients were instructed to take the three topical medications five times daily in the first postoperative week, three times daily in the second postoperative week, and twice daily in the third postoperative week.
All patients were followed up for 18 months with data collection at the postoperative 6-, 12-, and 18-month visits. All eyes underwent postoperative UDVA and CDVA measurements, subjective refraction assessments, slit-lamp examinations, and corneal topography analyses at each follow-up visit.
Statistical analysis
The social sciences software (SPSS version 22 for Windows - SPSS 22, IBM, Armonk, NY, USA) with its statistical package were used for data analysis. Range, median, mean, and standard deviation (SD) were used to describe the quantitative data. Number and percentage were used to describe qualitative data. When data were normally distributed according to the Kolmogorov–Smirnov test, a paired sample t-test was used, whereas the Wilcoxon paired test was applied for nonparametric data. Comparisons were made between preoperative and postoperative follow-up data at 6, 12, and 18 months using an RMANOVA for repeated measures with the Bonferroni post hoc test to examine the difference at each time point. The different time points were used as within-subject factors, while the surgeons were used as between-subject factors. P values less than 0.05 were considered statistically significant.
Results
Eight eyes (12.7%) underwent accelerated CXL with implantation of one Keraring segment, while 55 eyes (87.3%) underwent accelerated CXL with implantation of two Keraring segments.
Visual and topographic outcomes
At the 18-month follow-up examination, the mean UDVA had improved significantly from 0.89 ± 0.33 logMAR preoperatively to 0.40 ± 0.28 logMAR (mean ± SD; P < 0.0001). Similarly, the mean CDVA had improved slightly from 0.35 ± 0.31 logMAR preoperatively to 0.25 ± 0.24 logMAR (P = 0.004), which is a less pronounced improvement compared to that observed in UDVA. shows the cumulative logMAR visual acuity of both postoperative UDVA and CDVA.
Figure 1. The graphical presentation of the refractive and visual outcomes: (A) the cumulative logMAR visual acuity; (B) the postoperative UDVA versus the preoperative CDVA; (C) the postoperative SE refraction; (D) the postoperative refractive cylinder; (E) the postoperative refractive sphere; (F) the scatterplot of the attempted preoperative SE refraction versus the achieved postoperative SE refraction; (G) the stability of the postoperative SE refraction; and (H) the stability of the postoperative refractive cylinder.
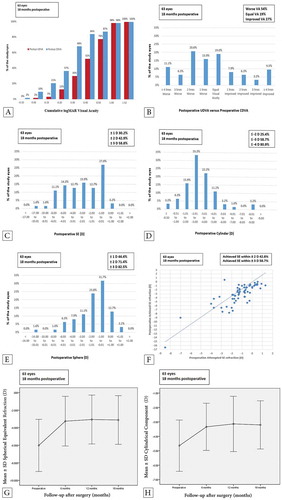
In a comparison of the postoperative UDVA with the preoperative CDVA, both components were similar in 12 eyes (19%). At the end of the 18-month follow-up period, the postoperative UDVA was better than the preoperative CDVA in 17 eyes (27%), whereas it was worse in 34 eyes (54%) ().
The mean K1, K2, and K average decreased from 46.51 ± 4.11 D, 51.03 ± 4.65 D, and 48.75 ± 4.25 D preoperatively to 44.56 ± 3.53 D, 48.74 ± 4.53 D, and 46.65 ± 3.89 D, respectively (all P < 0.0001). The mean corneal thickness at the thinnest location showed a significant reduction from 447.76 ± 31.12 to 442.33 ± 36.79 µm (P = 0.02) at the postoperative 18-month follow-up. shows the summary of the preoperative and postoperative visual and topographic outcomes.
Table 1. CXL-Plus preoperative and postoperative data summary of the results during the follow-up period.
Refractive outcomes
In general, all refractive components of sphere (mean difference, 95% confidence interval [CI]: 2.17 ± 2.19 [1.62–2.72]), cylinders (1.44 ± 2.07 [0.92–1.96]), and SE (2.89 ± 2.32 [2.31–3.47]) refractions showed marked improvements at 18 months postoperatively. The mean preoperative SE significantly improved from −6.01 ± 2.97 D preoperatively to −3.13 ± 2.78 D postoperatively (P < 0.0001; ). In addition, the mean cylindrical component showed a significant improvement from −4.65 ± 1.78 D preoperatively to −3.20 ± 1.67 D postoperatively (P < 0.0001; ). The mean spherical component showed a marked improvement from −3.69 ± 3.07 D preoperatively to −1.52 ± 2.41 D postoperatively (P < 0.0001; ).
shows the attempted refraction in relation to the achieved refraction. The SE refraction target set before the operation was a postoperative SE refraction within ±2 D. At the end of the follow-up period, 27 eyes (42.9%) met this SE refraction target. In addition, 37 eyes (58.8%) showed a postoperative SE refraction within ±3 D.
Furthermore, all refractive components showed a stable development in the follow-up evaluations at the 6th, 12th, and 18th month with statistically insignificant differences between time points (, ). The mean SE refraction was −3.25 ± 2.83 D, −3.07 ± 2.79, and −3.13 ± 2.78 (P4 = 0.052, P5 = 0.24, and P6 = 1.00, respectively; see ) at the 6th, 12th, and 18th follow-up month, respectively. summarizes the preoperative and postoperative visual, topographic, and refractive outcomes using ANOVAs with repeated measures and post hoc analysis, including P values and 95% CIs.
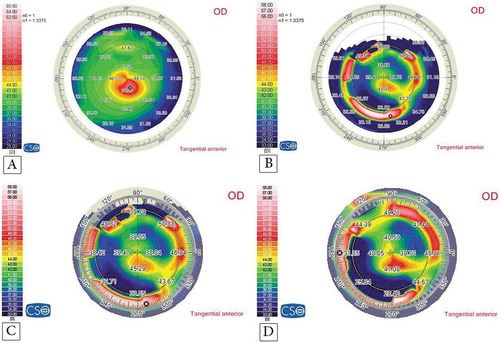
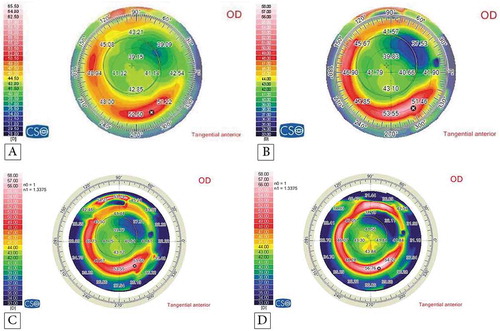
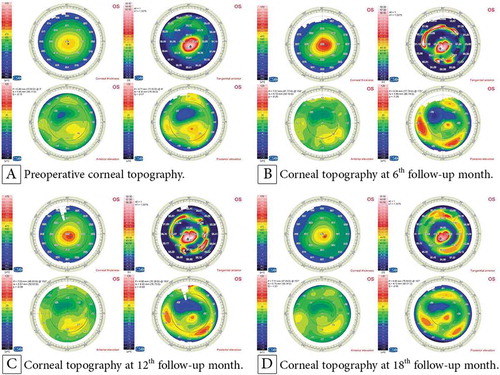
shows the preoperative and postoperative corneal topographies of the left eye of a 14-year-old female patient who underwent accelerated CXL along with femtosecond laser-based implantation of two Keraring segments. It demonstrates the good stability of the K readings and refractive outcomes at the 6th, 12th, and 18th month follow-up. shows the visual, topographic, and refractive outcomes of this case. and show the topographies of the youngest and the oldest patient.
Table 2. Summary of the preoperative and postoperative data of a 14-year-old female patient.
Figure 2. Corneal topography of a 14-year-old female patient with KC: (A) preoperatively; (B) at the 6th follow-up month postoperatively with an improvement in the K readings; and at the 12th (C) and 18th (D) follow-up month postoperatively with stabile K readings.
Complications
Complications were observed in eight eyes (12.7%). One eye (1.6%) showed migration of the Keraring toward the end of the incision site. The patient was an 11-year-old boy with a history of eye rubbing and redness in the first postoperative week. The patient was followed up during the first postoperative month, but the ring edge continued to migrate and reached the incision site. The two segments were explanted. The patient’s parents refused an ICRS reimplantation procedure, and spectacles were prescribed for the patient, who was continually monitored over the 18-month follow-up period. shows the postoperative refractive follow-up pathway of this case.
Table 3. Visual, topographic, and refractive pathways of the eight eyes with complications preoperatively and after 18 months.
Keraring segment extrusion due to intolerance was recorded in three eyes (4.7%) of two patients aged 9 and 11 years who had a history of chronic seasonal allergic conjunctivitis. Chronic eye rubbing was most probably the main risk factor for KC progression in these patients. The three eyes showed signs of frequent eye rubbing, such as marked irritation, redness, and lacrimation. The patients were treated for allergic conjunctivitis, but the three eyes showed an exposure of the Keraring segments’ edges, necessitating explantation of the Keraring segments within the first three postoperative months. However, we consider the idea of postponing a Keraring reimplantation until these patients have grown up and the allergic reaction has alleviated, after which the possibility of ICRS reimplantation will be reevaluated. Spectacles were prescribed for visual rehabilitation to both patients, and both of them completed the 18-month follow-up period.
KC progression was recorded in four eyes (6.4%) of three patients. KC progression was diagnosed on the basis of an increase of >1 D in the K reading average in comparison with the preoperative value along with greater corneal thinning and higher back surface elevations. KC progression in the four eyes was diagnosed from the third to sixth postoperative month. All four eyes were subjected to additional procedures that are explained below. shows the postoperative visual and refractive outcomes of the eight eyes that presented complications. Over the remaining course of the 18-month follow-up period, all eight eyes showed lower postoperative visual acuity but good stability after management with segment explantations or standard CXL retreatment.
Additional procedures performed in the four patients with complications
These four eyes (6.4%) of three patients, with KC progression, were subjected to the standard 30-min CXL using the Opto XLink CXL system. The corneal epithelium was removed from the central 4- to 4.5-mm corneal zone, thus avoiding removal of the corneal epithelium that overlies the Keraring segments to avoid postoperative delayed epithelial healing or persistent epithelial defects. Removal of the central corneal epithelium was performed using a hockey knife. Direct administration of riboflavin 0.1% with Dextran T500:20 g eye drops (Ricrolin Sooft SpA, Montegiorgio, Italy) onto the de-epithelialized central corneal area was performed at 2-min intervals for 30 min. Corneal irradiation with UVA was performed for another 30 min with continuation of riboflavin administration at 2-min intervals during UVA corneal irradiation. The parameters that were used to treat these cases included 1.50-mW power and 2.984-mW/cm2 intensity to deliver a total dose of 5.371 J/cm2 within 30 min of UVA corneal irradiation. At the end of the surgery, a bandage contact lens (CooperVision) was placed onto the de-epithelialized cornea.
All patients received the same postoperative treatment, which included Ibuprofen tablets (Brufen 400 mg, Abbott Pharmaceuticals, Illinois, USA) twice daily. The topical therapy included antibiotic eye drops (Zymar; Gatifloxacin 0.3%, Allergan, Inc, Jersey City, USA), prednisolone acetate 1% eye drops (Pred Forte; Allergan, Inc), and carboxymethylcellulose sodium 0.5% (Refresh Tears; Allergan, Inc). All eye drops were prescribed five times daily during the first postoperative week and tapered gradually over the next three postoperative weeks. Close follow-ups with careful slit-lamp examinations were performed until complete re-epithelialization was recorded and the contact lens was removed. The three patients continued to use spectacles during the follow-up period.
Discussion
This study found significant improvements in the refractive components after Keraring implementation supplemented with accelerated CXL. The mean SE improved from −6.01 ± 2.97 to −3.13 ± 2.78 D, while the mean myopic and astigmatic components improved from −3.69 ± 3.07 and −4.65 ± 1.78 to −1.52 ± 2.41 and −3.20 ± 1.67, respectively (all P < 0.0001). Twenty-seven eyes (42.9%) achieved the target of a postoperative SE refraction of ±2 D. The mean preoperative UDVA and CDVA improved significantly from 0.89 ± 0.33 and 0.35 ± 0.31 to 0.40 ± 0.28 (P < 0.0001) and 0.25 ± 0.24 (P = 0.004) 18 months postoperatively.
The good refractive and visual outcomes observed in this study could be attributed to the effect of the Keraring implantation, which flattened the cornea and reduced the myopic, astigmatic, and SE components of the KC.Citation10,Citation17–Citation20 The high rate of Keraring explantation in the present study (6.4%) could be attributed to eye rubbing associated with VKC in these eyes.Citation6,Citation7,Citation9,Citation12,Citation13,Citation21–Citation23 In addition, a high rate of KC progression was noted (6.4%), which could be attributed to the incomplete effectiveness of the accelerated epithelium-on CXL.Citation24,Citation25
As mentioned before, all investigated visual acuity parameters improved postoperatively. Abdelmassih et al. studied retrospectively in pediatric patients the 4-year outcomes of ICRS implantation followed by CXL 1 month later.Citation19 They report a significantly improved mean CDVA after 6 months. Abreu et al. describe a lesser postoperative mean UDVA and CDVA improvement 5 years after ICRS implantation.Citation20 The main difference between their study and our study was that they implanted Intacs and Intacs SK using the mechanical dissection without CXL while we implanted Kerarings with accelerated CXL using the femtosecond laser for tunnel creation. In addition, Henriquez et al. demonstrate a lesser improvement in both UDVA and CDVA at 12 months postoperatively comparing epithelium-on and epithelium-off CXL groups.Citation4 In comparison, the greater improvement in visual outcomes in our study could be attributed to the effect of Keraring implantation which is absent in their study. Mazzotta et al. present evidence for a similar significant improvement in both UDVA and CDVA 10 years after standard CXL.Citation26 Furthermore, Buzzonetti and Petrocelli report a lesser statistically significant improvement in CDVA, which is only recorded at 18 months postoperatively following transepithelial CXL unlike our study that records this improvement at 6 months postoperatively thanks to the implanted Kerarings.Citation24 Zotta et al. evaluated the long-term outcomes of standard CXL in pediatric KC patients and observed a similar significant improvement in the CDVA from 0.28 ± 0.17 to 0.14 ± 0.16 logMAR.Citation17
According to our data, the K readings all decreased significantly postoperative and remained stable over time. For instance, the mean K2 was 49.05 ± 4.57, 49.10 ± 4.72, and 48.74 ± 4.53 at 6, 12, and 18 months postoperatively. Two studies, Henriquez et al. and Abreu et al., report similar improvements in the K readings 12 and 60 months after the surgical procedures.Citation4,Citation20 The mean Kmax improved in Badawi from 49.12 ± 3.7D to 47.9 ± 3.7 D, which is close to the values presented in this study.Citation27 In their prospective study, Mazzotta et al. demonstrate a stable improvement of the Kmax in the first eight years after standard CXL, after which they record a 24% KC progression with increase in the Kmax during the 9th and 10th follow-up years in pediatric patients aged 15 years or younger.Citation26 In contrast to our study, Buzzonetti and Petrocelli determined a statistically significant deterioration of K readings with KC progression.Citation24
All refractive components of KC displayed in our study a stable improvement over the whole follow-up period. Similar to the results in our study, the mean SE in Abdelmassih et al. showed a similar significant reduction from −4.00 to −1.56 D.Citation19 Badawi describes a statistically significant decrease in the mean refractive cylinder from 2.4 ± 1.01 D to 2.01 ± 0.8 D, which is lesser than the improvement in the refractive cylinder in our study as she performed only accelerated epithelium-off CXL without ICRS implantation.Citation27 By contrast, Buzzonetti and Petrocelli found no statistically significant changes in SE, myopic component, astigmatic component, and corneal thickness at the thinnest location.Citation24 Their findings are supported by Mazzotta et al. with no statistically significant deviation from the cylinder baseline of 2.90 ± 1.74 D in a 10-year follow-up period.Citation26
In the present study, eight eyes (12.7%) experienced severe postoperative complications. This includes Keraring segment extrusion in three eyes (4.7%) and segment migration in one eye (1.6%) so that the Keraring had to be explanted in these four eyes (6.4%). Another four eyes (6.4%) showed KC progression and presented a declining postoperative CDVA. In accordance with our data, Abreu et al. report one eye (7.1%) complicated with Intacs extrusion at the seventh postoperative month.Citation20 In comparison to our results, Godefrooij et al. report a higher percentage of complications. They evaluated standard CXL and recorded 12 eyes (22% of the study eyes) showing KC progression.Citation18 They attribute this high percentage of disease progression to the decentralization of the cones in these eyes. Furthermore, Mazzotta et al. also had a higher percentage of KC progression within a 10-year follow-up after standard CXL in 13 eyes (24% of the study eyes).Citation26 By contrast, Tian et al.,Citation14 Sarac et al.,Citation15 Baenninger et al.,Citation16 and BadawiCitation27 report that no complications occurred in their studies of pediatric keratoconus treatments. Tian et al. conclude that accelerated transepithelial CXL is a safe and effective procedure to treat progressive KC in pediatric patients.Citation14 Sarac et al. and Philipp et al. compare standard CXL with accelerated CXL and conclude that both procedures are safe and effective with no statistically significant differences.Citation15,Citation16 Badawi evaluates accelerated epithelium-off CXL as a safe and effective procedure.Citation27 Their results do not match our results demonstrating that accelerated epithelium-on CXL is only partially effective in stabilizing the condition as we observed KC progression in four eyes (6.4%).
In accordance with our data, Piñero and Alio reported that ICRS might be not the ideal method for visual rehabilitation in pediatric KC patients because of the chronic eye rubbing due to ICRS intolerance and noncompliance as well as the aggressive and progressive nature of pediatric KC.Citation21 Many authors have stressed that VKC must be treated aggressively in pediatric KC patients before cross-linking can be initiated.Citation7,Citation9,Citation12,Citation13,Citation22,Citation23,Citation28,Citation29 Their statements are supported by the results in our study as four eyes (6.4%) had VKC and eventually required segment migration or extrusion. Yet, Shetty et al. had a higher complication rate as they record three eyes (10%) with KC progression resulting from VKC within a 2-year follow-up period after accelerated epithelium-off CXL.Citation28 Furthermore, VKC not only accelerates the progression of KC but it is also associated with limbal stem cell dysfunction and deficiency and, thus, protecting the limbal stem cells during CXL is important.Citation28,Citation29
In conclusion, our study proves that CXL-Plus is only partially effective for the treatment of pediatric KC. The high rate of KC progression after accelerated epithelium-on CXL indicates the aggressive nature of pediatric KC and strongly supports the need for a shift to standard CXL in the treatment of pediatric KC. In addition, despite the apparently excellent results of Keraring implantation, the high segment explantation rate over a very short follow-up period leads us to the recommendation to postpone in pediatric patients the implantation of any type of ICRS. We strongly recommend considering VKC and chronic eye rubbing as contraindications to ICRS implantation in these patients. Our findings also suggest an assessment of standard epithelium-off collagen cross-linking as a sole procedure to treat pediatric keratoconus in future studies.
Acknowledgments
We are grateful for the help and support provided by Dr. Ahmed Reda, Dr. Islam Awny, Engineer Ahmed Saber, Mr. El Khominy Omar, Mr. Mohammed Mahmoud, Mr. Amr Abou El-Hamd, and Mr. Waleed Hatour, and all the facilities they provided to the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hamilton A, Wong S, Carley F, Chaudhry N, Biswas S. Tomographic indices as possible risk factors for progression in pediatric keratoconus. J AAPOS. 2016;20(6):523–26. doi:10.1016/j.jaapos.2016.08.006.
- Spoerl E, Hafezi F, Bradley J. Corneal collagen cross-linking. Thorofare (NJ): SLACK Incorporated; 2013. p. 139–42.
- McAnena L, Doyle F, O’Keefe M. Cross-linking in children with keratoconus: A systematic review and meta-analysis. Acta Ophthalmol. 2017;95(3):229–39. doi:10.1111/aos.13224.
- Henriquez MA, Rodríguez AM, Izquierdo LJ. Accelerated epi-on versus standard epi-off corneal collagen cross-linking for progressive keratoconus in pediatric patients. Cornea. 2017;12(36):1503–08. doi:10.1097/ICO.0000000000001366.
- Panos GD, Kozeis N, Balidis M, Marilita MM, Hafezi F. Collagen cross-linking for paediatric keratoconus. Open Ophthalmol J. 2017;11:211–16. doi:10.2174/1874364101711010211.
- El-Khoury S, Abdelmassih Y, Hamade A, Slim E, Cherfan CG, Chelala E, Bleik J, Jarade EF. Pediatric keratoconus in a tertiary referral center: incidence, presentation, risk factors, and treatment. J Refract Surg. 2016;32(8):534–41. doi:10.3928/1081597X-20160513-01.
- Léoni-Mesplié S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplié N, Colin J. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154(1):56–62.e1. doi:10.1016/j.ajo.2012.01.025.
- Wiechers EG, Aguilar JL, Payne AG, Ramirez-Miranda AJ, Navas A, Jimenez-Corona A, Graue EO. Keratoconus in children: long term follow-up. Invest Ophthalmol Vis Sci. 2014;55:1583.
- Sangwan VS, Jain V, Vemuganti GK, Murthy SI. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 2011;30(5):491–96. doi:10.1097/ICO.0b013e3181cbf9d3.
- Mukhtar S, Ambati B. Pediatric keratoconus: a review of the literature. Int Ophthalmol. 2018;38(5):2257–66. doi:10.1007/s10792-017-0699-8.
- Saleem MIH, Elzembely HAI, AboZaid MA, Elagouz M, Saeed AM, Mohammed OA, Kamel AG. Three-year outcomes of cross-linking PLUS (combined cross-linking with femtosecond laser intracorneal ring segments implantation) for management of keratoconus. J Ophthalmol. 2018;17:6907573.
- Ibrahim O, Elmassry A, Said A, Abdalla M, El Hennawi H, Osman I. Combined femtosecond laser-assisted intracorneal ring segment implantation and corneal collagen cross-linking for correction of keratoconus. Clin Ophthalmol. 2016;10:521–26.
- Kymionis GD, Grentzelos MA, Portaliou DM, Kankariya VP, Randleman JB. Corneal collagen cross-linking (CXL) combined with refractive procedures for the treatment of corneal ectatic disorders: CXL plus. J Refract Surg. 2014;30(8):566–76. doi:10.3928/1081597X-20140711-10.
- Tian M, Jian W, Sun L, Shen Y, Zhang X, Zhou X. One-year follow-up of accelerated transepithelial corneal collagen cross-linking for progressive pediatric keratoconus. BMC Ophthalmol. 2018;18(1):75. doi:10.1186/s12886-018-0739-9.
- Sarac O, Caglayan M, Uysal B, Uzel AGT, Tanriverdi B, Cagil N. Accelerated versus standard corneal collagen cross-linking in pediatric keratoconus patients: 24 months follow-up results. Cont Lens Anterior Eye. 2018;41(5):442–47. doi:10.1016/j.clae.2018.06.001.
- Baenninger PB, Bachmann LM, Wienecke L, Thiel MA, Kaufmann C. Pediatric corneal cross-linking: comparison of visual and topographic outcomes between conventional and accelerated treatment. Am J Ophthalmol. 2017;183:11–16. doi:10.1016/j.ajo.2017.08.015.
- Zotta P, Diakonis V, Kymionis G, Grentzelos M, Moschou K. Long-term outcomes of corneal cross-linking for keratoconus in pediatric patients. J AAPOS. 2017;21(5):397–401. doi:10.1016/j.jaapos.2017.07.205.
- Godefrooij DA, Soeters N, Imhof SM, Wisse RP. Corneal cross-linking for pediatric keratoconus: long-term results. Cornea. 2016;35(7):954–58. doi:10.1097/ICO.0000000000000819.
- Abdelmassih Y, El-Khoury S, Dirani A, Antonios R, Fadlallah A, Cherfan G, Chelala E, Jarade F. Safety and efficacy of sequential intracorneal ring segment implantation and cross-linking in pediatric keratoconus. Am J Ophthalmol. 2017;178:51–57. doi:10.1016/j.ajo.2017.03.016.
- Abreu AC, Malheiro L, Coelho J, Neves MM, Gomes M, Oliveira L, Menéres P. Implantation of intracorneal ring segments in pediatric patients: long-term follow-up. Int Med Case Rep J. 2018;11:23–27. doi:10.2147/IMCRJ.S151383.
- Piñero DP, Alio JL. Intracorneal ring segments in ectatic corneal disease- a review. Clin Exp Ophthalmol. 2010;38(2):154–67. doi:10.1111/j.1442-9071.2010.02197.x.
- Soeters N, Van der Lelij A, van der Valk R, Tahzib NG. Corneal crosslinking for progressive keratoconus in four children. J Pediatr Ophthalmol Strabismus. 2011;48:E26–E29.
- Kocak I, Aydin A, Kaya F, Koc H. Comparison of transepithelial corneal collagen crosslinking with epithelium-off crosslinking in progressive keratoconus. J Fr Ophtalmol. 2014;37(5):371–76. doi:10.1016/j.jfo.2013.11.012.
- Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28(11):763–67. doi:10.3928/1081597X-20121011-03.
- Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28(11):753–58. doi:10.3928/1081597X-20121011-01.
- Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A, Caporossi A. Corneal collagen cross-linking with riboflavin and ultraviolet a light for pediatric keratoconus: ten-year results. Cornea. 2018;37(5):560–66. doi:10.1097/ICO.0000000000001505.
- Badawi AE. Accelerated corneal collagen cross-linking in pediatric keratoconus: one year study. Saudi J Ophthalmol. 2017;31(1):11–18. doi:10.1016/j.sjopt.2017.01.002.
- Shetty R, Nagaraja H, Jayadev C, Pahuja NK, Kummelil MK, Nuijts RM. Accelerated corneal collagen cross-linking in pediatric patients: two-year follow-up results. Biomed Res Int. 2014;894095. doi:10.1155/2014/894095.
- Gupta PC, Ram J. Corneal cross-linking for pediatric keratoconus: long-term results. Cornea. 2016;35(11):e36. doi:10.1097/ICO.0000000000000983.