ABSTRACT
The lacrimal glands produce the aqueous component of the pre-ocular tear film, which is essential for ocular health and optimal vision. This review explores its history, current understanding, and recent advances, and scope for future research. It traces the evolution of human knowledge about the source of tears across several millennia, with specific emphasis on the individuals who made seminal contributions to this field. It provides a detailed update on the morphology, microscopic structure, innervation, vascular supply, and imaging modalities of both the main and accessory lacrimal glands. The review also summarizes the recent advances in lacrimal gland regeneration and repair for the treatment of dry eye disease, particularly the role of mesenchymal stem cells. Lastly, the review gazes into the future of lacrimal gland research, which aims at translating the existing laboratory knowledge into clinical application, with the possibility of transplanting in vitro cultivated lacrimal constructs or the use of cell-based therapies for in situ repair of diseased human lacrimal glands.
Purpose
The lacrimal glands produce the aqueous component of the pre-ocular tear film, which is essential for ocular health and optimal vision. This review explores its history, current understanding, recent advances, and scope for future research.
Methods
The authors reviewed the major studies discussing the history of lacrimal glands and their anatomical description, including microscopic anatomy, innervation patterns, imaging, and ongoing translational research.
Results
The review traces the evolution of human knowledge about the source of tears across several millennia, with specific emphasis on the individuals who made seminal contributions to this field. It provides a detailed update on the morphology, microscopic structure, innervation, vascular supply, and imaging modalities of both the main and accessory lacrimal glands. The review also summarizes the recent advances in lacrimal gland regeneration and repair for the treatment of dry eye disease, particularly the role of mesenchymal stem cells. Lastly, the review gazes into the future of lacrimal gland research, which aims at translating the existing laboratory knowledge into clinical application, with the possibility of transplanting in vitro cultivated lacrimal constructs or the use of cell-based therapies for in situ repair of diseased human lacrimal glands.
Conclusions
Knowledge about the lacrimal glands in health and disease has improved tremendously since its discovery in the mid-eighteenth century. Today we stand at the cusp of exploring potential regenerative approaches for the treatment of lacrimal gland damage in dry eye disease.
Introduction
Blood, sweat, and tears are terms commonly used to describe the human experience. Of which tears are possibly the most romanticized, because of their powerful emotional connection. We shed tears in response to a range of emotions like anger, sadness, and happiness and also as a reaction to pain. Tears have profound religious connotations too. Exemplified by the statues of a weeping Mary Magdalene and numerous reports in popular culture of various figures miraculously weeping all kinds of different fluids. However, the genesis of tears in the eyes was a mystery that perplexed humankind for centuries.Citation1 Our knowledge of the tear-producing lacrimal glands has seen fascinating evolution over time, starting from amusing ideas to the detailed yet complex understanding of its structure and function, that exists today. In the digital age of smart devices, as the world stands on the brink of a possible dry eye disease epidemic, tears and their production are subjects of renewed interest.Citation2 Therefore, it might be relevant to retrace the steps of how our knowledge about the source of human tears has improved over the last three and a half millennia. This review provides a historical perspective, an update on our current understanding, recent advances and a gazing into the future research potential of the enigmatic fount of ocular tears, the human lacrimal glands.
Historical perspective: pre-renaissance era
The earliest references to the source of tears are in ancient Egyptian inscriptions, which describe the “metu” systems in the body. These were the channels through which blood and air supposedly traveled across the human body. A similar system was thought to be responsible for the supply of fluids into the eye.Citation3 Georg Moritz Ebers, a German Egyptologist, discovered one of the oldest medical papyri written around 1550 BCE.Citation4 The “Ebers Papyrus” mentions four vessels in the temple area of the face as the source of blood supply to the eyes as well as humors that lubricate the eyes. There are references in the Old Testament (ca. seventh century BCE) as well, which mentions the “cardiocentric view of tears” suggesting that breakdown of a firm substance in the heart led to tearing in the eye. In India, one of the oldest textbooks on ayurvedic medicine “Sushruta Samhita” (ca. sixth century BCE) written by Acharaya Sushruta described the ocular anatomy, role of the tear film, and its alteration in ocular diseases. In Ayurveda, tears are referred to as “Ashru” and constitute the “baahya patala” (outermost layer) of the eyeball.Citation5,Citation6 Sushruta mentions in his textbook that increased production of tears in acute inflammation of the eye is beneficial for cleansing of the ocular surface.Citation6 He states that “Rakta dhatu” (blood) and “Rasa dhatu” (fluid) are responsible for secreting the watery tears that protect and nourish the ocular surface;Citation7,Citation8 and that disturbances in this layer result in blurring of vision.Citation9 Hippocratic writings (ca. fifth century BCE) depict the eye anatomically as three membranes enclosing the ocular, aqueous, and vitreous humors. He postulated tears to have a neurogenic rather than a cardiogenic source.Citation3 At the time, the emotional connection of tears with crying led to the presumption that the brain or ventricles were producing tears, and the act of crying was a catharsis of harmful toxins from the brain.
Aristotle (384–322 BCE), the great philosopher and polymath of ancient Greece, like Sushruta proposed blood to be the source of tears.Citation1 The Greek physician Claudius Galen (129–210 CE), was the first to suggest the possible existence of tear-producing glands. He proposed the presence of these glands in the upper and lower eyelids as well as in conjunctiva.Citation10 He wrote, “To assure easy motility of the eyes, there are glands in the eye that secrete fluid similar to the saliva secreted by the glands at the root of tongue.” However, Galen stopped short of describing a major tear-producing gland, akin to the main lacrimal gland. His peers refuted his theory because of the small size of the glands he described. They were deemed insufficient for producing enough tears for crying. Rhazes (865–925 CE), a Persian physician, scholar, and philosopher of the medieval ages, noted that copious tear flow originated from the brain.Citation3 Avicenna (980–1037 CE), the Persian founder of medicine also propagated the theory of Hippocrates that tears originated from the brain and specific nerves were responsible for the transport of tears into the eyes.Citation3
Historical perspective: renaissance era
Both the cardiocentric and neurogenic theories were prevalent at the turn of the second millennium CE. Timothy Bright (1550–1615 CE) and Laurent Joubert (1529–1582 CE) supported the neurogenic source of human tears. Leonardo da Vinci (1452–1519 CE), however, proposed that a duct carried fluid from the heart to the eyes via the nasal cavity.Citation1 Descartes (1596–1650 CE) drew an interesting analogy between the tear production and rain. He stated that fluctuations in the temperature of the heart caused by emotions resulted in vapor condensation in colder areas like the eyes. Julius Casserius (1561–1616 CE) proposed that veins running through the lamina cribrosa transported the fluid onto the ocular surface.Citation10 He further stated that tears work like a purgative, draining off the impurities, and hence purifying the entire organism. The neurogenic theory suffered a setback when Thomas Wharton (1656 CE) revealed that the lamina cribrosa did not open into the orbit but opened into the nasal cavity.Citation10 He supported Galen’s theory and named the tear glands in the eye as glandula innominata of Galen in his treatise Adenographia. At around the same time Conrad Victor Schneider (1614–1680 CE) showed that mucosae and not the brain were responsible for secreting catarrhal fluids. He discovered the mucosal lining of the maxillary sinuses (the Schneiderian membrane) as the source of nasal secretions. Hence, both the cardiocentric and neurogenic theories had been discarded by the end of the renaissance era.
Historical perspective: modern era
Niels Stensen (Nicolas Steno, 1638–86 CE), a Danish scholar, neuroanatomist, and saint, made a pivotal discovery in 1660.Citation11 While dissecting a sheep’s head; he isolated the duct of the parotid gland (Stensen’s duct), which carries the saliva for the oral cavity.Citation11,Citation12 Stensen then started working on the other glands of the body. He hypothesized that membranes in the body are wet with the help of the fluid secreted by glands through ducts. He published these findings in “Observationes anatomicae” (1662 CE), where he described saliva, tears, and phlegm, as well as the vessels of the mouth, eyes, and nostrils and gave a clear explanation of the mechanism and function of the lacrimal apparatus. Stensen stated, “I suppose, that liquid, which facilitates the movement of eyelashes takes origin in a tear gland and is transported by its ducts. The formation of tears is influenced by external and internal conditions.” Samuel Thomas von Soemmerring’s (1775–1830 CE) illustrations display the lacrimal glands and meibomian glands like what we know today in his beautiful anatomical atlas. He also described the ligament that anchors the orbital surface of the lacrimal gland to the lacrimal fossa (Soemmerring ligament).Citation13 Samuel Ernst Whitnall (1876–1950 CE) elaborated on the orbital and palpebral lobes of lacrimal gland in his textbook on “The anatomy of Human Orbit”.Citation13 Sappey (1853–1872 CE) described 8–13 excretory ducts of the lacrimal gland that open into the conjunctiva, 3 to 5 from the orbital lobe and 2 to 5 from the palpebral lobe.Citation14 Whitnall also supported the findings of Sappey and Gosselin.Citation13 He observed that the number of ducts were always two or more; that the largest duct opened behind the lateral commissure; and one or two ducts from the palpebral lobe drained into the lower fornix. The anatomical description of the lacrimal gland divided into the orbital and palpebral lobes along with its excretory ducts has been mentioned in many textbooks around that time. Accessory lacrimal glands of Krause were first identified in 1854 CE at the lateral halves of the superior (8–20) and inferior fornix (2–5).Citation15,Citation16 Similar accessory glands though less in number were identified along the superior border of tarsus by Wolfring (1872 CE) and Cicaccio (1874 CE).Citation17,Citation18
Two scientists who made remarkable contributions to modern lacrimal gland science are Otto Schirmer, and Henrik Samuel Conrad Sjögren.Citation19 Otto Schirmer (1864–1918 CE), a German ophthalmologist, introduced a significant innovation in the form of a simple test that estimated the amount of lacrimation (Schirmer’s test). He also elaborated on the microscopic anatomy and physiology in the second edition of the Graefe-Saemisch Handbuch in 1904 CE.Citation20 His father Rudolf Schirmer had written the first edition of this handbook in 1877 CE. His other contributions include: lacrimal secretion and excretion post-dacryocystectomy (1902 CE); physiology and pathology of lacrimal flow (1903 CE); the influence of blinking on tear drainage (1904 CE); histology and physiology of the lacrimal apparatus (1904 CE); composition of lacrimal secretions (1904 CE) and innervation of the lacrimal gland (1909 CE).Citation20–Citation24 He initially tested the function of trigeminal and sympathetic nerves by evaluating the tear secretion with 1 × 1 cm square blotting paper in rabbits, dogs, and cats. Later he simplified the technique for clinical use in humans by placing a blotting paper measuring 41 × 5 mm and described three variations of this test, namely without anesthesia, and with nasal and photic stimulation after topical anesthesia. Jones (1966 CE) introduced a variant of the Schirmer test I to evaluate basal secretion without any nasal or light stimuli.Citation25 Schmidt introduced the term “dacryoadenitis” to mean inflammation of the lacrimal gland and “dacryops” to mean cystic dilatation of the gland in 1803 CE. While Todd published the first detailed case report in 1822 CE further describing the signs and treatment of dacryoadenitis.Citation10
Henrik Sjögren (1899–1986 CE), a Swedish ophthalmologist, worked on keratitis sicca associated with atrophy of the lacrimal gland. Although, W.B. Hadden and J.W. Hutchinson had earlier (1871 CE) described the coexisting symptoms of dry eyes and dry mouth; Sjögren identified a group of women with the triad of dry eyes, xerostomia and polyarthritis (Sjögren’s syndrome).Citation26 Sjögren published his first case report in 1930 CE, and his monograph of 19 patients with dry eyes and arthritis in his doctoral thesis titled “On knowledge of keratoconjunctivitis” at the Karolinska Institutet in 1933 CE.
Over the last three millennia, philosophers and researchers like Shushruta, Hippocrates, Galen, Wharton, Stensen, Schirmer, and Sjögren (), have made seminal incremental contributions to advancing our knowledge on this topic. Having revisited the intriguing history of how the human understanding of tearing and its relationship with the lacrimal gland has evolved over many centuries, it’s now appropriate to discuss the current concepts on the subject. In the following sections, we describe the current understanding of the structure of main and accessory lacrimal glands, their regenerative potential, and future perspective.
Current understanding: main lacrimal gland
The main lacrimal gland is situated in the superotemporal orbit and is divided into two lobes by the aponeurosis of levator palpebrae superioris muscle (LPS) across its frontal surface ().Citation13,Citation27-Citation29 The gland is placed obliquely sandwiched between orbital bones and soft tissue of the orbit.Citation30 The lacrimal gland spans the superotemporal aspect of the globe extending from the lateral edge of superior rectus muscle to the frontozygomatic suture below and reaches upto the posterior aspect of the globe in antero-posterior direction, more apparent on radiological imaging.Citation31 It has two surfaces (orbital and ocular), and four edges (superior, inferior, medial, and lateral).
The gland is kept in position by four ligaments, as stated by Whitnall.Citation13 Although, the Sommering’s ligament, technically is not an actual defined ligamentous structure. The orbital surface of the gland lies in direct contact with the periosteum of the lacrimal fossa via loose strands of connective tissues that can be easily separated with blunt dissection. The posterior pole of the gland abuts the orbital fat, and even fat lobules are seen intermingling with lacrimal gland lobules. The nerves and vessels enter the gland at the hilum (on the ocular surface) where it’s strongly adherent to the periorbita via their sheath. The toughest ligament is the “inferior ligament of Schwalbe” that anchors the lower part of the gland between the lateral orbital wall and superior rectus’ sheath expansion. Jones stated the presence of a lacrimal foramen formed by the inferior ligament of Schwalbe and the upper free border of LPS for passage of ducts from main lacrimal gland into the palpebral lobe and conjunctival surface.Citation25
The orbital and palpebral lobes are continuous with each other along the lateral edge of the LPS aponeurosis but are dissimilar in size, shape, and lobular distribution ().Citation27,Citation31 The orbital lobe is larger, constitutes 60–70% of the whole lacrimal gland, and has its lobules packed more tightly. The medial surface of the orbital lobe rests on the LPS aponeurosis. Its size varies from 20 to 25 mm along its long axis, 10–15 mm transversely and 3–6 mm in thickness, and weighs 670–720 mg.Citation32–Citation37 The palpebral lobe is smaller, relatively flat and varies in dimensions from 9 to 15 mm along its long axis, 8 mm transversely and 2 mm in thickness.Citation32–Citation37 The gland is non-uniform in thickness; the anterior pole is well defined but thin, while the central part is the thickest. The palpebral lobe measures roughly 1/3rd to 1/4th of the orbital lobe and weighs 220 mg.Citation32–Citation37 The shape of the orbital lobe has been considered similar to an almond or walnut. However, many believe that it doesn’t conform to any of these described shapes.Citation13,Citation27,Citation31 We agree with Noyes’ description of it having a concavo-convex shape, the medial surface being concave.Citation38
The organized ductal system consists of intercalated ducts draining into intralobular ducts, which merge into interlobular ducts, and finally terminating into wide excretory ducts that open onto the ocular surface. The number of excretory ducts varies in the lacrimal gland ranging from 6 to 14 from the orbital lobe, and 5 to12 from the palpebral lobe.Citation13,Citation27 The excretory ducts are very closely adherent to the conjunctiva and it is difficult to isolate the ductular openings macroscopically. The excretory ducts of the orbital and palpebral lobe are separate and usually follow a wavy course (). Adolf Alt in his anatomical descriptions of the lacrimal gland stated that he never found the ducts to be as numerous (6–12) as stated in previous reports.Citation39 Kim et al. demonstrated an average of 3 to 5 visible ductal openings on the palpebral lobe in healthy individuals, which varies considerably from textbooks.Citation40 We have also noted only about 3 to 5 of ductular openings on performing dynamic assessment of tear secretion using a fluorescein strip (). The number of ductules and their exact location is still controversial. The majority of these ducts open into the superior fornix 4–5 mm above the superior border of tarsus with one to two ducts opening beyond lateral canthus into the inferior fornix.
Current understanding: neural and vascular supply
The lacrimal gland is supplied by the lacrimal artery, a branch of the ophthalmic artery that enters the posteromedial surface of the orbital lobe. The palpebral lobe receives its blood supply from the branches entering the orbital lobe.Citation41 The lacrimal vein drains the lacrimal gland, which is a tributary of the ophthalmic vein. The lacrimal nerve and artery course along the superior edge of the lateral rectus muscle and pierce the orbital fat to enter the lacrimal gland. The lacrimal nerve carries sensory fibres and receives parasympathetic fibres of the facial nerve via anastomosis with the zygomatic-temporal branch of the zygomatic branch of the maxillary nerve.Citation13 There are different concepts regarding the relation of the zygomaticotemporal nerve with the lacrimal nerve. Some state that a branch from zygomaticotemporal nerve carries parasympathetic fibres and joins the lacrimal nerve before it enters the gland. However, Scott et al. found this communication to be present in only 3% of cases while in 60% of the cases (n = 20/34) the zygomaticotemporal nerve entered the gland directly.Citation42 Postganglionic sympathetic fibres from superior cervical ganglion reach the gland via the zygomatic nerve. The accessory lacrimal glands receive blood supply from the dense plexus along the superior tarsal border and the vessels supplying the forniceal conjunctiva.
Jones postulated that the main lacrimal gland is a reflex secretor based on its efferent nerve supply.Citation25 The main lacrimal gland receives both sensory and autonomic nerve fibres.Citation43,Citation44 Demonstration of neuropeptides like substance P, calcitonin gene-relatedd peptide (CGRP), and S-100 positive fibres in the intertubular stroma of the human main and accessory lacrimal glands, point towards neural regulation of secretion in both the glands.Citation44–Citation46 Naked axons containing dense core vesicles have been shown in the periglandular interstitium of accessory lacrimal glands strongly supporting the possibility of autonomic innervation.Citation47 Sensory nerve fibres positive for Sub P and CGRP, stimulated secretion with VIP, pilocarpine, melanocyte-stimulating hormone, and glucagon suggest neural as well as hormonal regulation of the accessory lacrimal gland secretion.Citation48,Citation49
Current understanding: microscopic anatomy
The lacrimal gland is a seromucinous, acinotubular gland of merocrine variety.Citation50 Microscopically, the acinar epithelium is composed of tubular columnar cells surrounded by a basal layer of myoepithelial cells ().Citation13,Citation28 Acinar cells have tight desmosomes and junctional complexes along the lateral and luminal surfaces while the basal surfaces of these cells rest on a thick basement membrane (0.5 microns).Citation51,Citation52 The luminal surfaces of the acinar epithelial cells have several irregular microvilli. Distinct polarity is present in the acinar epithelial cells with nuclei located in the basal portion, endoplasmic reticulum, and golgi apparatus in the middle and granules scattered in the cytoplasm towards the luminal side.Citation52 Different sized electron-dense granules are present within the cells varying from 1 to 7 microns.Citation52 Myoepithelial cells surround the basal portion of acini with finger-shaped cytoplasmic outgrowths surrounding many acinar epithelial cells. The ductal epithelium is cuboidal and 2–3 cell layers thick (). Intralobular and interlobular ducts lack myoepithelial cells, unlike acinar cells.Citation27 However, Ito and Shibazaki reported the presence of myoepithelial cells in the terminal portions and in small interlobular and intralobular secretory ducts on electron microscopic study.Citation53
Histologically, there are no differences between the two lobes (). However, there is a gradual change in the epithelia and secretory function across intralobular, interlobular, and terminal secretory ducts. Intra- and interlobular ductules are two cell layers thick and the cells are cuboidal in shape. The terminal ducts have a larger lumen surrounded by 2–3 layers of tall columnar epithelial cells that contribute to the secretion as well.Citation54 The ductal cells secrete electrolytes and water and have less dense secretory granules. In the normal lacrimal gland, acinar and luminal duct epithelial cells stain positive for cytokeratin 5 (CK5), lactoferrin, lysozyme, aquaporin 5, and S-100 antibodies whereas myoepithelial cells are positive for actin and CK 5 antibodies.Citation54,Citation55
Interstitial connective tissue contains lymphocytes, plasma cells, fibroblasts, blood vessels, and nerve fibres.Citation56 Plasma cells secrete IgA antibody necessary for mucosal immunity on the ocular surface.Citation57 Gender-related differences in the morphology of the lacrimal gland are present in many species. Males tend to have a larger acinar area.Citation58,Citation59 The gland undergoes age-related changes and shows a functional decrease in its secretory activity with time.Citation60 In a study of 80 lacrimal glands from Japanese cadavers, age-related changes displayed sexual dimorphism. Diffuse lobular fibrosis, atrophy, and periductular fibrosis were more common in females (mean age 56 years).Citation29 Orbital and palpebral lobes revealed differences in terms of lobular architecture with aging, more in the former. More interlobular ductal dilatation without any periductular fibrosis was present in the palpebral lobes.Citation29
Current understanding: accessory lacrimal glands
There are two types of accessory lacrimal glands. The glands of Wolfring (along the tarsal border) and Krause (in the substantia propria of fornices). The glands of Krause vary in number from 4 to 35 and are more in the upper eyelid.Citation61 The glands of Wolfring are larger, range from 2 to 5 in number in the upper eyelid and are rarely seen in the lower eyelid.Citation13 The main and the accessory lacrimal glands are similar in terms of their tubuloacinar structure (), distribution of secretory proteins, and plasma cells.Citation60 The accessory lacrimal glands aren’t arranged into lobules like the main lacrimal gland. Structurally, they have an excretory duct that branches into intralobular ducts, which further branches into secretory tubules terminating finally into secretory end pieces.
These glands are serous in nature and of the eccrine variety. The ductular epithelium is composed of one to two layers of cells having a basally located nuclei and sparse, less dense secretory granules in the apical portion. The epithelium of intralobular ducts shows a gradual transition into secretory epithelium near the secretory tubules. Secretory tubules and end piece epithelium are polar and have dense granules in the apical portion, abundant mitochondria, and golgi apparatus in middle segment and nuclei in the basal portion. There is a rich network of capillaries around secretory tubules; and the connective tissue has collagen, fibroblasts, plasma cells, and lymphocytes. Myoepithelial cells are present in the basement membrane with extended spindle-shaped ends enclosing epithelial cells, however less in number compared to the main lacrimal gland. The accessory lacrimal glands also display similar immunoreactivity for CK 5, lactoferrin, lysozyme, aquaporin 5, S-100, and actin antibodies as the main lacrimal gland.Citation61 Parasympathetic nerves and hormones stimulate the accessory lacrimal glands. Therefore they also contribute to both reflex as well as basal secretion.Citation62 The adrenergic receptors have been located in the acinar epithelium of glands of Wolfring with a predominance of beta-1 positivity whereas myoepithelial cells showed alpha-adrenergic positivity.Citation48
Current understanding: embryology and recent advances in lacrimal gland regeneration
Embryologically, the lacrimal gland derives from the conjunctival epithelium and its surrounding mesenchyme. There occurs a thickening of the epithelium at the temporal conjunctival fornix at 22 to 25 mm embryonic stage. This thickening outgrows as solid branching cords into the adjacent mesenchyme.Citation63 The canalization of the cords happen by apoptosis of the central cells at 55–60 mm stage.Citation10 At the fifth month, the developing levator aponeurosis divides the gland into two parts.
The main lacrimal gland has stem cells, which undergo proliferation during repair and restore the function of acinar cells.Citation64–Citation66 Accessory lacrimal glands also express precursor cell markers such as nestin, ABCG2, and CD90 within the acinar cells.Citation67 The location of these stem cells within the human lacrimal gland is not well established. Keratin 15, expressed in the basal cells surrounding the intercalated duct of the lacrimal gland in mice, is also expressed in the lacrimal gland epithelium during embryonic stage.Citation68 Meneray and Rismondo were the first to culture the lacrimal acinar cells in 1994.Citation69,Citation70 successfully This development led to in vitro cultivation of cells in three-dimensional lacrispheres, and on various matrices like matrigel, denuded human amniotic membrane, and collagen-1 for a minimum period of 21 days.Citation64,Citation69-Citation71 A three-dimensional (3D) lacrimal gland model was regenerated using the mouse and human lacrimal gland progenitor cells in the organ germ method.Citation72 Also, an actively secreting 3D lacrimal gland was constructed using a decellularized 3D porcine lacrimal gland scaffold that provided a physiological niche for the harvested epithelial cells to form acinar, ductal, and myoepithelial cells.Citation73 The three types of progenitor cells observed within the lacrimal cultures are the epithelial progenitor cells, mesenchymal stem cells (MSCs), and progenitor cells of myoepithelial origin. Injury model of the rabbit lacrimal gland showed induction of proliferative cells expressing the stem cell markers nestin, Ki67, and ABCG2 at 1 weekk.Citation74 In normal rabbit and murine lacrimal glands, cells expressing nestin, nanog, SOX2, CD133 markers show upregulation following experimentally induced injury and duct ligation.Citation75,Citation76 Similar findings were seen in interleukin-1 damaged murine and human lacrimal glands.Citation76,Citation77
Experimental studies using direct injection of MSCs into the murine and rabbit model of dry eye disease have shown a reduction in glandular inflammation and improved function of the lacrimal gland that lasted for 6 monthss.Citation78–Citation82 Addition of MSCs improved the viability of epithelial cells in the ethanol-damaged lacrimal acinar cell cultures.Citation83 Only one clinical trial involving MSC transplantation in patients with graft-versus-host disease showed improved lacrimal gland function.Citation84 The ongoing research is focusing upon creating a bioengineered lacrimal gland for clinical transplantation, administration of lyophilized cell extract, and direct cell-based therapies for in situ repair and regeneration of the lacrimal gland.
Current understanding: imaging
Traditional imaging modalities for the lacrimal gland are computed tomography (CT) and magnetic resonance imaging (MRI).Citation85,Citation86 CT is preferred for diagnosing the pathologies of the lacrimal gland where bony involvement is suspected. MRI provides better soft tissue details, and microimaging using diffusion weighted MRIs has been shown useful for assessing the abnormalities of the lacrimal glands in Sjögren´s syndrome ().Citation86 In vivo confocal microscopy (IVCM) provides useful microscopic features of the lacrimal gland parenchyma.Citation87 Imaging of the lacrimal gland using IVCM can delineate its normal lobular architecture, acini, intralobular, and interlobular ducts and pathological findings such as acinar dilatation, interstitial fibrosis, and presence of inflammatory cells in Sjögren’s Syndrome. The excretory ducts, and lobules can be visualized on optical coherence tomography (OCT) scanning (using enhanced depth imaging) of the exposed palpebral lobe.Citation88,Citation89 However, the limitations of OCT imaging include less depth of penetration (200–400 microns) and low resolution (4 microns).
Future directions: research possibilities
The presence of tear-producing glands in the eye was firmly established by the mid-eighteenth century. Our understanding of the lacrimal gland in health and disease has improved tremendously since that discovery. Today we stand at the cusp of exploring potential regenerative approaches for the treatment of lacrimal gland damage in dry eye disease. However, there are still certain areas that need deeper exploration. With respect to the anatomy, we need better delineation of the exact ductular structure, specific localization of stem cells within the lacrimal glands, and a clearer understanding of the mechanism of secretion in the normal versus diseased glands. Another question that remains unanswered is the exact role of lacrimal glands in basal versus reflex tear secretion.Citation90–Citation94 The relative contributions of main versus accessory lacrimal glands to the basal tear secretion are still unclear.Citation85 Furthermore, better imaging modalities that can visualize the dynamic flow of the secretions along with the parenchymal details of the gland would help in improving our understanding of the pathological involvement of the lacrimal gland in dry eye disease.
In the current millennium, dry eye disease is emerging as an epidemic and is estimated to involve 40–50% of urban residents by 2030.Citation2,Citation95 In United States, 16 million adults have dry eye disease as per the 2017 National Health and Wellness survey. However, no definitive therapy for this condition, particularly for aqueous deficiency, currently exists. Efforts are currently ongoing towards developing regenerative approaches for conditions like Sjögren´s syndrome, where the lacrimal gland undergoes progressive inflammatory damage and atrophy.Citation96 In other specific situations, like Stevens-Johnson syndrome, where there is preservation of the gland architecture but dryness resulting from peri-ductal fibrosis, alternative therapeutic strategies like use of antifibrotics or anti-inflammatory agents may prove to be useful.Citation90 As our understanding of the lacrimal gland in both health and disease evolves further, future research is likely to focus not only on regeneration, but also repair and rejuvenation of the lacrimal gland.
Figure 1. A historical timeline illustration of the important figures who contributed to improving the knowledge of tear production and lacrimal glands in human eyes.
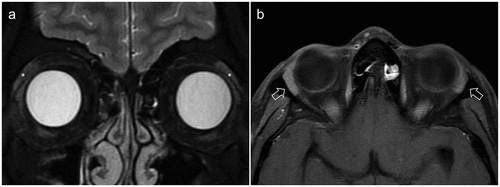
Figure 2. A. Three-dimensional MRI image of 65-year-old female showing the highlighted lacrimal glands situated in the lacrimal fossa. B. Smooth convex orbital surface of excised orbital lobe of lacrimal gland from a 60 years old cadaver can be appreciated with visible lobular arrangement and intervening whitish color septae. C. Orbital lobe is contiguous with the palpebral lobe posteriorly along the edge of levator aponeurosis (black star denotes orbital lobe; Inset shows the illustration depicting the relationship of lacrimal gland (in pink) and levator aponeurosis; Paper has been used to mark levator). D. Concave medial surface of the lacrimal gland (with palpebral lobe; arrow denotes duct) showing excretory ducts passing from the orbital to palpebral lobe. Note the wavy course along the lobules.
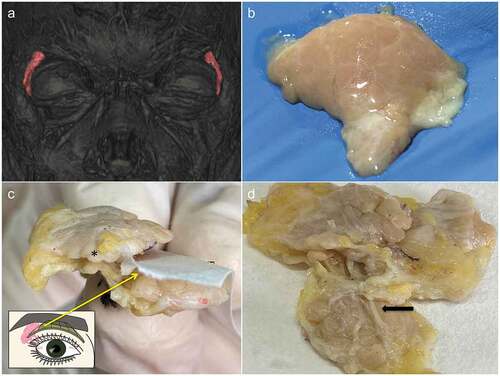
Figure 3. A. Slit lamp photograph of the left palpebral lobe of the main lacrimal gland in a 26-year-old male demonstrates three excretory openings with surrounding fluorescein washout area (marked with arrow, on dynamic assessment of tear secretion), seen under cobalt blue light; B. whereas a 56-year-old male patient with Sjogren’s syndrome demonstrates reduction in the size of the gland with no visible secretory openings.
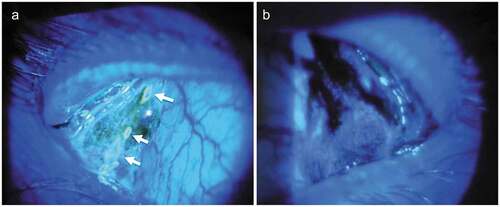
Figure 4. A. Schematic illustration showing the three main components of the lacrimal gland: acini, ductal units and myo-epithelial cells. B. Acini composed of epithelial cells of pyramidal shape and basally located nuclei (hematoxylin and eosin stained X140 photomicrograph). C. Ductal units composed of two-three layered cellular tubular structure draining the secretions from the acini (hematoxylin and eosin stained X200 photomicrograph). D. Myoepithelial cells with epithelial and smooth muscle phenotype located in the basal layer of acinar epithelial cells (stained with alpha-smooth muscle actin antibody; X200 photomicrograph).
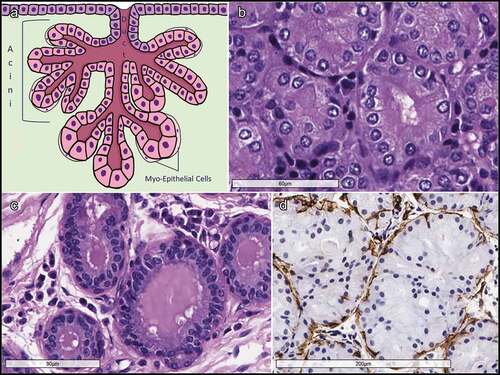
Figure 5. A & B. Photomicrograph (X20) of hematoxylin and eosin-stainedd orbital lobe of lacrimal gland reflecting the lobular arrangement of gland (marked with arrow) and tall columnar epithelial cells forming acini around a central lumen (marked with dashed circle; B at X200). C. Intra and interlobular ducts with 2–3 cell layers of cuboidal ductal epithelial cells and flat cells in the peripheral most layer (intralobular duct marked with arrowhead; lumen of interlobular marked with asterisk). D. Photomicrograph (X40) of hematoxylin and esoin stained palpebral lobe of the main lacrimal gland showing more loosely packed lobules and a large excretory duct (marked with an asterisk).
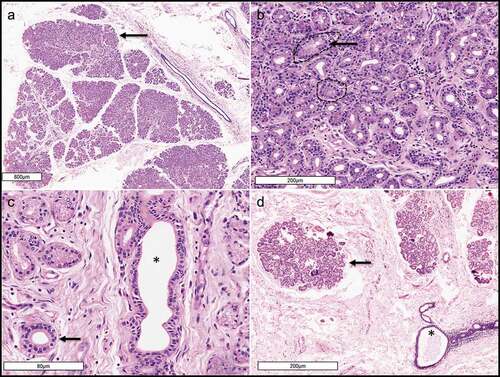
Figure 6. A. Photomicrograph (X20) of hematoxylin and esoin stained cadaveric posterior lamella of the upper eyelid showing the glands of Wolfring located along the inferior tarsal border (T for tarsus). B. Photomicrograph of the accessory lacrimal glands of Krause (X200) showing fine cytoplasmic granules within the secretory epithelial cells forming acini.
Sources of support
Hyderabad Eye Research Foundation (HERF).
Conflicts of interest
None of the authors have any conflicts of interest.
Acknowledgments
The authors wish to acknowledge Mr Sridhar and Mr Naidu, Technicians in the Ophthalmic Pathology Laboratory, Brien Holden Eye Research Centre (BHERC) for their help with image processing.
Additional information
Funding
References
- Vingerhoets A. Why only humans weep: unravelling the mysteries of tears. 1st. Oxford: Oxford University Press; 2013.
- Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India: electronic medical records driven big data analytics report I. Ocul Surf. 2019;17(2):250–56. doi:10.1016/j.jtos.2019.02.007.
- Hirschberg J. The renaissance of ophthalmology in 18th century. In: Hirschberg J editor. The history of ophthalmology. Vol. 1. Amsterdam: Wayenborg publications; 1984.
- Ebers G, Ebers P, HermetischeBuch D URL link: https://digi.ub.uni-heidelberg.de/diglit/ebers1875bd1/0001
- Susrutha I. Susrutha Samhita Uttaratantra 1/21.
- Susrutha I. Susrutha Samhita Uttaratantra, 6/6-7, 22–23.
- Dalhana. Susrutha Samhita Chowkhambha Orientalia Varanasi, 2002; Uttaratantra 1/18.
- Susrutha I. Susruta samhita Sutrasthana 14/3.
- Sushruta. Sushruta samhita dalhana comm.nibandhasangraha, chowkhambha orientalia varanasi, 2002, Uttaratantra 1/16.
- Duke-Elder S, Wybar KC. The lacrimal glands. In: Duke-Elder S editor. System of ophthalmology. vol. ii. the anatomy of the visual system. Vols. 526–7. London: Henry Kimpton; 1961. p. 562–68.
- Holomanova A, Ivanova A, Brucknerova I. Niels Stensen–prestigious scholar of the 17th century. Bratisl Lek Listy. 2002;103:90–93.
- Tubbs RS, Mortazavi MM, Shoja MM, Loukas M, Cohen-Gadol AA. The bishop and anatomist Niels Stensen (1638-1686) and his contributions to our early understanding of the brain. Childs Nerv Syst. 2011;27(1):1–6. doi:10.1007/s00381-010-1236-5.
- Whitnall SE. Anatomy of the human orbit and accessory organs of vision. 2 ed. London: Oxford Univ Press; 1932. p. 209.
- Sappey. CR. Soc Biol Mem (Paris). 1853;5:13.
- Krause C. Z Rat Med. 1854;4:337.
- Krause F. Die Neuralgie D Trigeminus, Leipzig. 1896.
- Wolfring. Zbl Med Wiss. 1872;10:852.
- Ciaccio Moleschott’s. Untersuch Naturlehre D Mensch. 1876;11:420.
- Murube J. The Schirmer test: celebration of its first centenary. Ocul Surf. 2003;1(4):157–59. doi:10.1016/S1542-0124(12)70011-3.
- Schirmer O. Sur la sécrétion et l’excrètion lacrymale aprés extirpation du sac. com. to the deutsch ophthalmol ges (heidelberg 3 aug 1901). Abstract in Ann Ocul (Paris). 1902;128:218–19.
- Schirmer O. Principios y métodos de vdeterminación funcional de lasv glándulas lagrimales. Arch Oftalmol Hisp-Amer. 1903;3:366–67.
- Schirmer O. Über lidschlaglähmung und lidschlusslähmung zugleich ein beitrag zur lehre von der tränenabfuhr. Zeitsch Augenheilk. 1904;11:97–105.
- Schirmer O. Über den einfluss des sympathicus auf die funktion der tr.nendrüse. Pflu¨ger’s Arch Ges Physiol. 1909;126(5–8):351–70. doi:10.1007/BF01677796.
- Schirmer O. Studien zur physiologie und pathologie der tränenabsonderung und tränenabfuhr. Graefe’s Arch Ophthalmol. 1903;56(2):197–291. doi:10.1007/BF01946264.
- Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62(1):47–60. doi:10.1016/0002-9394(66)91676-X.
- Ghafoor M. Sjögren’s before sjögren: did henrik sjögren (1899-1986) really discover sjögren’s disease? J Maxillofac Oral Surg. 2012;11(3):373–74. doi:10.1007/s12663-011-0303-0.
- Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea. 2006;25(10 Suppl 1):S82–9. Review. doi:10.1097/01.ico.0000247220.18295.d3.
- Jakobiec FA, Iwamoto T. The ocular adnexa: lids, conjunctiva, and orbit. In: Fine BS, Yanoff M, Hagerstown MD, Row H editors. Ocular histology: a text and atlas.. 2 ed. New York: Harper and Row; 1979. p. 318
- Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmol. 1995;102(4):678–86. doi:10.1016/S0161-6420(95)30971-2.
- Takahashi Y, Watanabe A, Matsuda H, Nakamura Y, Nakano T, Asamoto K, Ikeda H, Kakizaki H. Anatomy of secretory glands in the eyelid and conjunctiva: a photographic review. Ophthalmic Plast Reconstr Surg. 2013;29(3):215–19. doi:10.1097/IOP.0b013ex3182833dee.
- Lorber M. Gross characteristics of normal human lacrimal glands. Ocul Surf. 2007;5(1):13–22. Review. doi:10.1016/S1542-0124(12)70049-6.
- Eisler P. Die anatomie des menschlichen auges. in: schieck f, bruchner a editors. kurzes handbuch ophthalmol. Vol. 1. Berlin: Julius Springer; 1930. p. 237–38.
- Prager A. Macroscopic and microscopic investigations on senile atrophy of the lacrimal gland. Bibl Ophthalmol. 1966;69:146–58.
- Forrester JV, Dick AD, McMenamin PG, Lee WR. The eye. basic sciences in practice. 2 ed. Edinburgh: WB Saunders; 2002. p. 84.
- Werb A. The anatomy of the lacrimal system. In: Milder B, Weil BA, editors. The lacrimal system. Norwalk, CT: Appleton-Century-Crofts; 1983. p. 23–32.
- Tripathi RC, Tripathi BJ. Anatomy of the human eye, orbit, and adnexa. In: Davson H editor. The eye. Vol. 1A. 3. Orlando, FL: Academic Press; 1984. p. 79.
- Murray AN. Anatomy (gross) of the human eye and of its appendages. In: Wood CA editor. The American encyclopedia and dictionary of ophthalmology. Vol. 1. Chicago, IL: Cleveland Press; 1913. p. p 350.
- Noyes HD. A textbook of diseases of the eye. New York, NY: Wm Wood Co; 1894. p 292.
- Alt A. Original contributions concerning the glandular structures appertaining to the human eye and its appendages. Trans Acad Sci St Louis. 1900;10:185–207.
- Kim EC, Doh SH, Chung SY, Yoon SY, Kim MS, Chung SK, Shin MC, Hwang HS. Direct visualization of aqueous tear secretion from lacrimal gland. Acta Ophthalmol. 2017;95(4):e314–e322. doi:10.1111/aos.13335.
- Tucker SM, Lambert RW. Vascular anatomy of the lacrimal gland. Ophthalmic Plast Reconstr Surg. 1998;14(4):235–38. doi:10.1097/00002341-199807000-00002.
- Scott G, Balsiger H, Kluckman M, Fan J, Gest T. Patterns of innervation of the lacrimal gland with clinical application. Clin Anat. 2014;27(8):1174–77. doi:10.1002/ca.22447.
- Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–77. doi:10.1016/j.preteyeres.2009.04.003.
- Seifert P, Stuppi S, Spitznas M, Weihe E. Differential distribution of neuronal markers and neuropeptides in the human lacrimal gland. Graefe’s Arch Clin Exp Ophthalmol. 1996;234(4):232–40. doi:10.1007/BF00430415.
- Seifert P, Stuppi S, Spitznas M. Distribution pattern of nervous tissue and peptidergic nerve fibers in accessory lacrimal glands. Curr Eye Res. 1997;16(4):298–302. doi:10.1076/ceyr.16.4.298.10698.
- Seifert P, Spitznas M. Demonstration of nerve fibers in human accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol. 1994;232(2):107–14. doi:10.1007/BF00171672.
- Ruskell GL. Nerve terminals and epithelial cell variety in the human lacrimal gland. Cell Tissue Res. 1975;158(1):121. doi:10.1007/BF00219955.
- Esmaeli-Gutstein B, Hewlett BR, Harvey JT. Characterization of adrenergic receptors in the accessory lacrimal glands of the upper eyelid. Ophthalmic Plast Reconstr Surg. 1999;15(4):245–51. doi:10.1097/00002341-199907000-00005.
- Gilbard JP, Rossi SR, Heyda KG, Dartt DA. Stimulation of tear secretion by topical agents that increase cyclic nucleotide levels. Invest Ophthalmol Vis Sci. 1990;31:1381–88.
- Paulsen F, Langer G, Hoffmann W, Berry M. Human lacrimal gland mucins. Cell Tissue Res. 2004;316(2):167–77. doi:10.1007/s00441-004-0877-7.
- Kobayashi M. Studies on lacrimal glands by use of electron microscope. Acta SOC Ophthal Jap. 1958;62:230–41.
- Egeberg J, Jensen QA. The ultrastructure of the acini of the human lacrimal gland. Acta Ophthalmol (Copenh). 1969;47(2):400–10. doi:10.1111/j.1755-3768.1969.tb02897.x.
- Ito T, Shibasaki S. Lichtmikroskopische untersuchungen iiber die glandula lacrimalis des menschen. Arch Histol Jap. 1964;25(2):117–44. doi:10.1679/aohc1950.25.117.
- Vigneswaran N, Wilk CM, Heese A, Hornstein OP, Naumann GO. Immunohistochemical characterization of epithelial cells in human lacrimal glands. I. Normal major and accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol. 1990;228(1):58–64. doi:10.1007/BF02764293.
- Franklin RM, Kenyon KR, Tomasi TB. [r.: immunohistologic studies of human lacrimal gland. Localization of immunoglobulins, secretory component and lactoferrin. J Immunol. 1973;110:984.
- Kivelä T. Antigenic profile of the human lacrimal gland. J Histochem Cytochem. 1992;40(5):629–42. doi:10.1177/40.5.1374091.
- Allansmith MR, Kajiyama G, Abelson MB, Simon MA. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976;82(6):819. doi:10.1016/0002-9394(76)90056-8.
- Sullivan DA, Hann LE, Yee L, Allansmith MR. Age and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol (Copenh). 1990;68(2):188–94. doi:10.1111/j.1755-3768.1990.tb01902.x.
- Cornell-Bell AH, Sullivan DA, Allansmith MR. Gender-related differences in the morphology of the lacrimal gland. Invest Ophthalmol Vis Sci. 1985;26:1170–75.
- Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6(4):162–74. doi:10.1016/S1542-0124(12)70177-5.
- Gillette TE, Allansmith MR, Greiner JV, Janusz M. Histologic and immunohistologic comparison of main and accessory lacrimal tissue. Am J Ophthalmol. 1980;89(5):724–30. doi:10.1016/0002-9394(80)90295-0.
- Seifert P, Spitznas M, Koch F, Cusumano A. The architecture of human accessory lacrimal glands. Ger J Ophthalmol. 1993;2:444–54.
- De La Cuadra-Blanco C, Peces-Pena MD, Merida-Velasco JR. Morphogenesis of the human lacrimal gland. J Anat. 2003;203(5):531–36. doi:10.1046/j.1469-7580.2003.00233.x.
- Tiwari S, Ali MJ, Vemuganti GK. Human lacrimal gland regeneration: perspectives and review of literature. Saudi J Ophthalmol. 2014;28(1):12–18. doi:10.1016/j.sjopt.2013.09.004.
- Dietrich J, Schrader S. Towards lacrimal gland regeneration: current concepts and experimental approaches. Curr Eye Res. 2020;45(3):230–40. doi:10.1080/02713683.2019.1637438.
- Gromova A, Voronov DA, Yoshida M, Thotakura S, Meech R, Dartt DA, Makarenkova HP. Lacrimal gland repair using progenitor cells. Stem Cells Transl Med. 2017;6(1):88–98. doi:10.5966/sctm.2016-0191.
- Ali M, Shah D, Pasha Z, Jassim SH, Jassim Jaboori A, Setabutr P, Aakalu VK. Evaluation of accessory lacrimal gland in muller’s muscle conjunctival resection specimens for precursor cell markers and biological markers of dry eye disease. Curr Eye Res. 2017;42(4):491–97. doi:10.1080/02713683.2016.1214966.
- Hirayama M, Liu Y, Kawakita T, Shimmura S, Tsubota K. Cytokeratin expression in mouse lacrimal gland germ epithelium. Exp Eye Res. 2016;146:54–59. doi:10.1016/j.exer.2015.11.020.
- Meneray MA, Fields TY, Moses RL, Bromberg BB, Moses RL, Fields TY, Moses RL, Bromberg BB, Moses RL, Fields TY, et al. Morphology and physiologic responsiveness of cultured rabbit lacrimal acini. Invest Ophthalmol Vis Sci. 1994;35:4144–58.
- Rismondo V, Gierow JP, Lambert RW, Golchini K, Feldon SE, Mircheff AK. Rabbit lacrimal acinar cells in primary culture: morphology and acute responses to cholinergic stimulation. Invest Ophthalmol Vis Sci. 1994;35:1176–83.
- Garg A, Zhang X. Lacrimal gland development: from signaling interactions to regenerative medicine. Dev Dyn. 2017;246(12):970–80. doi:10.1002/dvdy.24551.
- Shatos MA, Haugaard-Kedstrom L, Hodges RR, Dartt DA. Isolation and characterization of progenitor cells in uninjured, adult rat lacrimal gland. Invest Ophthalmol Vis Sci. 2012;53(6):2749–59. doi:10.1167/iovs.11-9025.
- Spaniol K, Metzger M, Roth M, Greve B, Mertsch S, Geerling G, Schrader S. Engineering of a secretory active three-dimensional lacrimal gland construct on the basis of decellularized lacrimal gland tissue. Tissue Eng Part A. 2015;21(19–20):2605–17. doi:10.1089/ten.tea.2014.0694.
- Lin H, Liu Y, He H, Botsford B, Yiu S. Lacrimal gland repair after short-term obstruction of excretory duct in rabbits. Sci Rep. 2017;7(1):8290. doi:10.1038/s41598-017-08197-2.
- Liu Y, Hirayama M, Kawakita T, Tsubota K. A ligation of the lacrimal excretory duct in mouse induces lacrimal gland inflammation with proliferative cells. Stem Cells Int. 2017;2017:4923426.
- Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49(10):4399. doi:10.1167/iovs.08-1730.
- Dietrich J, Schlegel C, Roth M, Witt J, Geerling G, Mertsch S, Schrader S. Comparative analysis on the dynamic of lacrimal gland damage and regeneration after Interleukin-1α or duct ligation induced dry eye disease in mice. Exp Eye Res. 2018;172:66–77. doi:10.1016/j.exer.2018.03.026.
- Villatoro AJ, Fernández V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed Res Int. 2015;2015:1–10. doi:10.1155/2015/527926.
- Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015;23(1):139–46. doi:10.1038/mt.2014.159.
- Bittencourt MK, Barros MA, Martins JFP, Vasconcellos JPC, Morais BP, Pompeia C, Bittencourt MD, KdS E, Kerkis I, Wenceslau CV, et al. Allogeneic mesenchymal stem cell transplantation in dogs with keratoconjunctivitis sicca. Cell Med. 2016;8(3):63–77. doi:10.3727/215517916X693366.
- Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, Meng Z, Sendra VG, Hamrah P, Kublin CL. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of sjögren’s syndrome. Stem Cells Int. 2017;2017:1–10. doi:10.1155/2017/3134543.
- Shi B, Qi J, Yao G, Feng R, Zhang Z, Wang D, Chen C, Tang X, Lu L, Chen W, et al. Mesenchymal stem cell transplantation ameliorates sjögren’s syndrome via suppressing il-12 production by dendritic cells. Stem Cell Res Ther. 2018;9(1):308. doi:10.1186/s13287-018-1023-x.
- Dietrich J, Roth M, König S, Geerling G, Mertsch S, Schrader S. Analysis of lacrimal gland derived mesenchymal stem cell secretome and its impact on epithelial cell survival. Stem Cell Res. 2019;38:101477. doi:10.1016/j.scr.2019.101477.
- Weng J, He C, Lai P, Luo C, Guo R, Wu S, Geng S, Xiangpeng A, Liu X, Du X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus- host disease. Mol Ther. 2012;20(12):2347–54. doi:10.1038/mt.2012.208.
- Mafee MF, Haik BG. Lacrimal gland and fossa lesions: role of computed tomography. Radiol Clin North Am. 1987;25:767–79.
- Kawai Y, Sumi M, Kitamori H, Takagi Y, Nakamura T. Diffusion weighted MR microimaging of the lacrimal glands in patients with Sjogren’s syndrome. AJR Am J Roentgenol. 2005;184(4):1320–25. doi:10.2214/ajr.184.4.01841320.
- Sato EA, Matsumoto Y, Dogru M, Kaido M, Wakamatsu T, Ibrahim OMA, Obata H, Tsubota K. Lacrimal gland in sjögren’s syndrome. Ophthalmol. 2010;117(5):1055–1055.e3. doi:10.1016/j.ophtha.2009.11.034.
- Doh SH, Kim EC, Chung SY, Kim MS, Chung SK, Shin MC, Hwang HS. Optical coherence tomography imaging of human lacrimal glands: an in vivo study. Ophthalmol. 2015;122(11):2364–66. doi:10.1016/j.ophtha.2015.05.020.
- Singh S, Basu S. Secretory ductules of the lacrimal gland. Ophthalmic Plast Reconst Surg. 2020; 1. [In press]. doi:10.1097/IOP.0000000000001687.
- Jordan A, Baum J. Basal tear flow: does it exist? Ophthalmol (Rochester). 1980;87(9):920–30. doi:10.1016/S0161-6420(80)35143-9.
- Scherz W, Dohlman CH. Is the lacrimal gland dispensable? Keratoconjunctivitis sicca after lacrimal gland removal. Arch Ophthalmol. 1975;93(4):281–83. doi:10.1001/archopht.1975.01010020291009.
- Lamberts P, Foster CS, Perry HD. The Schirmer test after topical anesthesia, and the tear meniscus height in normal eyes. Arch Ophthalmol. 1979;97(6):1082–85. doi:10.1001/archopht.1979.01020010536004.
- Abusharha AA, AlShehri TM, Hakami AY, Alsaqr AM, Fagehi RA, Alanazi SA, Masmali AM. Analysis of basal and reflex human tear osmolarity in normal subjects: assessment of tear osmolarity. Ther Adv Ophthalmol. 2018;10:2515841418794886.
- Singh S, Basu S. Effect of topical anesthesia on the secretory activity of the main lacrimal gland. Cornea.2020; [In press].
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–65. doi:10.1016/j.jtos.2017.05.003.
- Singh S, Narang P, Vashist U, Mittal V. Histomorphological changes in lachrymal glands of patients with chronic stevens-johnson syndrome. Cornea. 2019;38(9):e39–e40. doi:10.1097/ICO.0000000000002037.