ABSTRACT
Purpose: To evaluate the morphologic and hemodynamic changes of bulbar conjunctival vessels in thyroid-associated ophthalmopathy (TAO) patients and the correlations with the activity.
Methods: Patients diagnosed as TAO with different clinical activity scores (CAS) and healthy participants were recruited. All subjects underwent a complete ophthalmic examination and functional slit-lamp biomicroscope. Vascular variables including the vessel density, vessel complexity, average diameter, blood flow velocity and blood flow rate in microvascular networks were measured. The correlations among microvascular parameters, CAS and exophthalmos were analyzed. Areas under the receiver operating characteristic curves (AUROCs) were applied to evaluate the diagnostic accuracy of microvascular alterations for active TAO.
Results: A total of 46 eyes were enrolled in our study. The vessel complexity and blood flow velocity increased in the active TAO group significantly compared with the inactive group and healthy controls (P < .05). Meanwhile, the vessel complexity and blood flow rate were positively correlated with CAS (r = 0.641 and r = 0.526). Bulbar conjunctival microvascular parameters performed a good ability in distinguishing the active stage of TAO (AUROC = 0.793).
Conclusions: Increasing bulbar conjunctival vessel complexity and blood flow were evident in TAO with severe inflammation. The measurements of bulbar conjunctival microvasculature could be a reference to evaluate activity in TAO.
Introduction
Thyroid-associated ophthalmopathy (TAO) is the most common immune orbital disorders. The clinical features are multiple, including upper eyelid retraction, erythema, edema, proptosis, strabismus cornea exposure, and impaired vision.Citation1, Citation2 Among these, the conjunctival symptoms like injection and chemosis display the activity of TAO and are valuable in evaluating the progress of the disease. At present, the grade of activity in TAO patients is classified based on the clinical activity score (CAS) system proposed by Mourits in 1983. The high score of CAS indicates the stage of acute inflammation. The autoimmunity plays an important role in the acute stage, glucocorticoid and radiotherapy can make a difference in this progress.
The different stages usually accompany the ocular surface changes in TAO. There are increased Langerhans cell density, increased conjunctival epithelium, and reduced goblet cell density in bulbar conjunctiva of TAO patients.Citation3 On account of the corneal exposure and corneal hypoesthesia, many patients have microbial keratitis and scarring.Citation4,Citation5 About two-thirds of moderate and severe TAO patients suffer from the dry eye symptom,Citation6 their tear film is hyperosmosis and stimulates high expression of inflammatory cytokines such as IL-1β and TNF-α.Citation5,Citation7 The orbital inflammation can also impact the meibomian gland and lacrimal gland, induce their inappropriate secretions and dry eye disease.Citation8,Citation9
Functional slit-lamp biomicroscope (FSLB) is a reliable and accurate system to measure the morphometric and dynamic characteristics of the bulbar conjunctival vessels. It can capture the movement of red blood cells to measure the blood flow velocity (BFV). Moreover, the fractal analysis is performed to determine the microvascular network density (Dbox) and microvascular network complexity (D0).Citation10,Citation11 It is widely applied in ocular surface disorders associated with inflammation, such as dry eye disease and habitual contact lens wearers.Citation12–16 In this study, with the application of FSLB, we focus on the alteration of bulbar conjunctival microvasculature in TAO patients and aim to explore a new thought to evaluate the activity of TAO.
Methods
Study population
This study was approved by the Ethics Committee of the Zhongshan Ophthalmic Center and conducted adhering to the Declaration of Helsinki. TAO patients were recruited from Zhongshan Ophthalmic Center from July 1, 2019, to October 1, 2019. Age- and sex-matched healthy subjects were enrolled simultaneously. Written informed consent was obtained from each subject.
The diagnosis of TAO is following the consensus statement of the European Group on Graves’ Orbitopathy criteria. All TAO patients meeting the following criteria were included in the study: (1) age of >18 years and <60 years, (2) normal thyroid function, free T3, free T4, and thyroid-stimulating hormone are within the normal range, (3) no treatment with systemic glucocorticoids for at least three months before the study, (4) spherical equivalent of <6.00 diopters (D). The exclusion criteria for all subjects were as follows: (1) systemic disease such as hypertension and diabetes, (2) history of any ophthalmopathy (e.g., glaucoma, diabetic retinopathy, and uveitis), (3) history of any ocular surgery or ocular trauma, (4) history of contact lens wearing.
The activity of TAO was evaluated by the CAS system, which includes seven reference items: chemosis, redness of conjunctiva and eyelid, swelling of the eyelid, swelling of the caruncle, spontaneous ache behind the eyeball, ache on attempted upgaze. One symptom added one point, and the CAS score≥ 3 was classified as an active stage.Citation17
Ophthalmic and systemic examination
All subjects underwent a comprehensive ocular examination, including BCVA, refraction, Goldmann applanation tonometry, slit-lamp biomicroscopy, fundoscopy, ocular motility assessment, and proptosis measurements. Blood pressure was measured after at least 5 minutes of resting. Pulse blood pressure was computed as systolic blood pressure minus diastolic blood pressure.
Functional slit lamp biomicroscopy imaging
Before the FSLB examination, all patients were requested to take a rest at least 15 minutes. The examination was carried out from 3:00 PM to 5:00 PM in the same research laboratory with constant temperature and humidity. Physical interference with the microcirculation, such as pressing on the eyelids or eyeballs, was avoided.
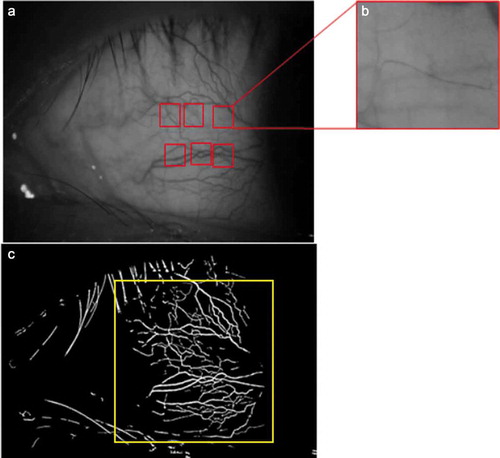
The FSLB imaging system consists of a traditional slit-lamp adapted and a high-speed digital camera,Citation18 enabling a magnification into the viewfinder up to 175 ×, and the image size is 640 × 480 pixels. We set 16× magnification of the slit-lamp to acquire a view area of 15.24 × 10.16 mm2 (). Six different paranasal regions of view of 1.20 × 0.90 mm2 of the bulbar conjunctiva were adopted, distributed ~1 mm radially from the limbus ring (). For each region, the magnification was 175 ×, and image resolution was 60 feet per second, and the aggregate movements of red blood cells could be observed clearly. A built-in green filter with 16 × magnification and 3456 × 2034 pixels was used in the slit-lamp to obtain the microvascular network image. Then the images were exported and processed through a series of fractal analysis programs ().
Figure 1. (a) Images of paranasal bulbar conjunctival microvascular network with 16× magnification. (b) One of the six regions of view of 1.20 × 0.90 mm2 to capture the movements of red blood cell clusters. (c) The fractal analysis, including D0 and Dbox, was obtained in the blue area, approximately 15.24 × 10.16 mm2
A custom semi-automatically software was used to analyze the parameters of microvascular morphology and hemodynamics in all measurable venules.Citation12,Citation13,Citation18 The fractal dimension (Dbox, representing vessel density), multifractal dimension (D0, representing vessel complexity) and average vessel diameter (AD) were analyzed by the box-counting method. The red blood cells tracing images were input to a software based on MATLAB and the BFV was calculated automatically. The blood flow rate (BFR) was then determined using a standard flow rate equation: BFR = BFV*Π*AD2/4.Citation19
Statistical analysis
Statistical analysis was performed using statistical software (SPSS version 20; IBM Corp., Armonk, NY, USA). A P value of <0.05 was considered to be statistically significant. Continuous variables were described as means ± standard deviation (SD). Differences of bullbar conjunctival microvascular parameters among the three groups were analyzed by 1-way ANOVA, and Bonferroni correction was applied to post hoc multiple comparisons between groups. The correlations among bullbar conjunctival microvascular parameters, the CAS scores and exophthalmos were tested with Pearson’s correlation analysis. The receiver operating characteristic (ROC) curves were drawn to evaluate the sensitivity and specificity of bullbar conjunctival microvascular parameters in diagnosing active TAO.
Results
There were 30 subjects and 46 eyes enrolled in this study, including 10 healthy controls, 18 eyes with active TAO (CAS > 3) and 18 eyes with inactive TAO (CAS < 3). There were no differences in sex distribution (P = .488), age (P = .260), systolic blood pressure (P = .716), diastolic blood pressure (P = .550), pulse blood pressure (P = .431) and heart rate (P = .190) among the three groups ().
Table 1. Demographic characteristics of all enrolled subjects
Bulbar conjunctival microvascular parameters
In 1-way ANOVA analysis, statistically significant differences were found among the groups for all bullbar conjunctival microvascular parameters (all P < .001) ().
Table 2. Microvascular parameters in bulbar conjunctivas
Active TAO group had larger vessel density and more complex microvascular morphology: Dbox was 1.45 ± 0.07 and D0 was 1.45 ± 0.06 in active TAO group, Dbox was 1.41 ± 0.04 and D0 was 1.41 ± 0.04 in inactive group, Dbox was 1.32 ± 0.04 and D0 was 1.35 ± 0.03 in healthy controls. Also, the AD increased in TAO groups, especially in active TAO group. In the post hoc pairwise analysis, the differences of D0 and Dbox between the inactive TAO group and the healthy control group were statistically significant (all P < .05). However, between active and inactive groups, only the difference of D0 was statistically significant (P = .047).
For hemodynamic parameters, they were similar to the tendencies in morphology parameters. The BFV was 567.84 ± 48.16 μm/s and BFR was 255.61 ± 75.46 pl/s, they increased in active TAO group compared with the inactive group and healthy controls. Through multiple pairwise comparisons with Bonferroni correction, only the differences of BFV between active TAO group and inactive TAO group were statistically significant (P = .038).
Correlations between AD and BFV
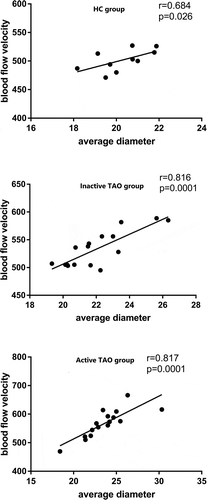
In all three groups, the BFV increased as the enlargement of AD, the disturbutions in three groups were shown in . Moderate correlations existed between BFV and AD (r = 0.684 and P = .026 in healthy group, r = 0.816 and P = .001 in inactive TAO group, r = 0.817 and P = .001 in active group).
Correlations among bulbar conjunctival microvascular parameters and CAS and exophthalmos
Correlations between bulbar microvascular parameters and CAS and exophthalmos were listed in . All bulbar conjunctival microvascular parameters were positively correlated with CAS (all P < .05). Especially, the correlation between D0 and CAS was strongest (r = 0.641). Moreover, all the bulbar conjunctival microvascular parameters seemed to be no correlation with exophthalmos (all r < 0.3).
Table 3. Correlations among bulbar conjunctival parameters and CAS and exophthalmos
Sensitivity and specificity of bulbar conjunctival microvascular parameters
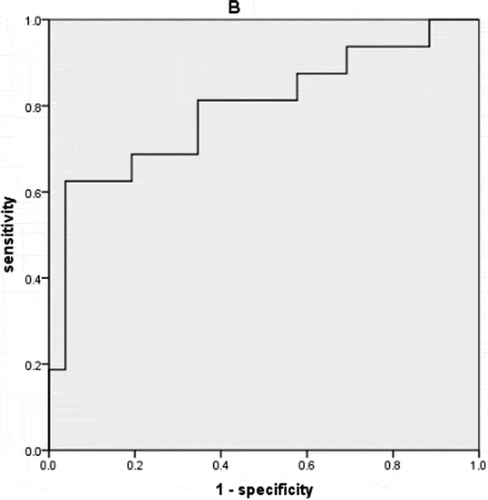
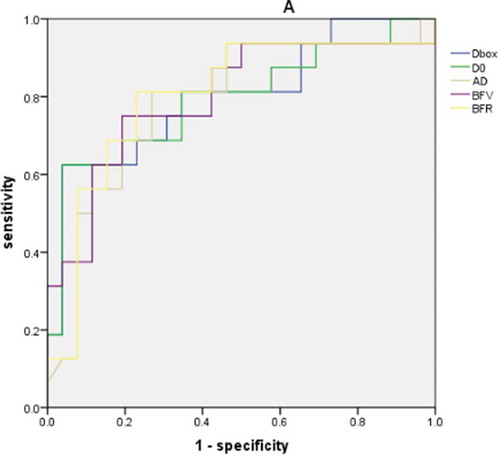
The areas under the receiver operating characteristic curves (AUROCs) of bulbar conjunctival microvascular parameters for differentiating active and inactive TAO groups were shown in , representing the sensitivity and specificity in diagnosing active TAO. BFR and Dbox were highest (0.800), followed by BFV (0.798), D0 (0.793), and average diameter (0.787). The multiple Logistic regression model was used to analyze the five bulbar conjunctival microvascular parameters (), AUROC was 0.793 and the cut-off value was 0.575.
Figure 3. The area under the receiver operator characteristic curves (AUROCs) for differentiating active TAO eyes from inactive TAO eyes of morphologic and hemodynamic microvascular parameters: Dbox (0.800), D0 (0.793), average diameter (0.787), blood flow velocity (0.798) and blood flow rate (0.800)
Discussion
As a superficial organ, bulbar conjunctiva can be affected by many systemic diseases, such as ischemic stroke, cancer, and diabetes. It is an ideal reference for evaluating pathologic conditions and circulations.Citation20–23 Usually accompanied by thyroid functional disorders, the conjunctival alterations are prevalent in TAO patients, and the immune inflammatory plays an important role. Previous studies have found many ocular surface changes in TAO patients, such as keratitis, the increase of tear inflammatory cytokines, hypersecretions of lacrimal glands, and the meibomian dysfunction gland.Citation8,Citation9,Citation24 And the ocular surface changes are proven to be associated with the activity of TAO.Citation24 In this study, we found the morphology and hemodynamics in bulbar conjunctival microvasculature significantly differed between TAO and healthy eyes.
To our knowledge, the alteration of BFV is usually a response to the vessel diameter in human conjunctival capillaries due to the change of wall shear stress in the vessel.Citation19,Citation25 In the TAO groups, the BFV showed strong correlations with AD, but the moderate correlation in the control group, which might result from the small sample size in the current study. Some evidences imply that the increase of BFV represents the acute stage of inflammatory in the ocular surface, and the change of vessel diameter is progress in chronic inflammation like long-term dry eye disease and habitual daily contact lens wearers.Citation12,Citation15 In this study, the BFV differed between the active TAO group and the inactive TAO group statistically significantly, but the increase of AD seemed not to be statistical. This indicated the hemodynamic alterations were before morphologic alterations in the active TAO patients, which were mainly with short-term inflammation.
The evaluation of TAO activity makes a difference in the judgment of disease duration and progression and making therapeutic schedules. The CAS is widely used in quickly evaluating the activity of TAO. However, the symptoms and appearances, such as hyperemia and edema, are subjective and hard to quantify and observers can not distinguish the subtle bulbar changes by the traditional approaches such as slit lamp microscopy. At present, many auxiliary examinations help the evaluation of TAO activity. For example, the change of molecules like thyrotropin receptor antibodies and glycosaminoglycans in peripheral blood seems to be related to the acute stage of TAO,Citation26,Citation27 imagings like muscle edema on T2-weighted or Short T1 Inversion Recovery-sequenced in MRI scans hint the soft tissue inflammation. Meanwhile, the increased vascular growth factors in acute orbital tissues suggest the imaging of ocular and orbital vessel parameters also plays important roles in evaluating the activity of TAO.Citation28 Color Doppler imaging shows the decreased BFV of the superior ophthalmic vein and increased BFV in the ophthalmic artery is closely associated with the active stage.Citation29 Angio-OCT finding suggests lower peripapillary and macular vessel density values in the active TAO.Citation30,Citation31 In this present study, we quantified the bullbar conjunctiva in TAO patients with different clinical activity scores, and this was FSLB applied in TAO patients first time. The measurements of the conjunctival microvascular morphology and hemodynamics by FSLB were noninvasive, repeatable and various.Citation25,Citation32 Compared with the retinal function imager, which is used in the assessment of human conjunctival microvasculature,Citation33 fractal and multifractal dimensions in FSLB can analyze the characteristics of wide-field microvascular networks in conjunctiva based on box-counting method.Citation10 In our study, microvascular changes including microcirculation and morphology were found in active and inactive TAO patients. Especially, the conjunctival vessel density and vessel complexity showed a significant correlation with the CAS and they were acquired from the whole paranasal region, therefore the morphologic parameters could reflect the acute inflammation of the ocular surface and correspond to the conjunctival hyperemia. The sensitivity and specificity of microvascular morphologic and hemodynamic measurements performed well in diagnosing active TAO. All AUROCs were above 0.75, which presents microvascular measurements obtained by FSLB are effective references in evaluating the activity of TAO.
There were several limitations to the present study. First, the sample size was small and the severely active TAO patients were little. The sample size of the three groups needs to be enlarged in the next study. Both eyes of TAO patients were enrolled except four eyes with ophthalmopathy but only right eyes were enrolled in healthy controls, not all observations are independent. Second, we didn’t detect the inflammatory cytokines in the ocular surface or peripheral blood in TAO patients, so there was no direct laboratory evidence to support the inflammatory reaction. Third, the eyelid retraction may be another factor influencing the conjunctival microvascular besides inflammation, we didn’t measure this to eliminate the interference. We will combine more ocular surface indexes with microvasculature in the future study. Additionally, the treatment efficacy could be evaluated by an intervention and a follow-up study, which would guide the clinical decisions.
In conclusion, it was the first study in morphologic and hemodynamic bulbar conjunctiva microvascular changes of TAO patients. Increased vessel complexity and BFV were associated with the activity of TAO, which indicates an underlying acute process in the active stage. Meanwhile, the measurements of FLSB could also be a new assessment system to evaluate the activity of TAO.
Author contributions
TZ and ML contributed equally to the work presented here and should therefore be regarded as equivalent authors.
Disclosure of interest
T. Zhang, None; M. Li, None; W. Xiao, None; H. Ye, None; R. Chen, None; J. Yuan, None; H. Yang, None
Additional information
Funding
References
- Dutton JJ. Anatomic considerations in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34:S7–S12.
- Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Visual Sci. 2014;55(3):1735. doi:10.1167/iovs.14-14002.
- Wei YH, Chen WL, Hu FR, Liao SL. In vivo confocal microscopy of bulbar conjunctiva in patients with graves’ ophthalmopathy. J Formos Med Assoc. 2015;114(10):965–72. doi:10.1016/j.jfma.2013.10.003.
- Naik MN, Vasanthapuram VH, Joseph J, Murthy SI. Microbial keratitis in thyroid eye disease: clinical features, microbiological profile, and treatment outcome. Ophthalmic Plast Reconstr Surg. 2019;35:543–48.
- Achtsidis V, Tentolouris N, Theodoropoulou S, Panagiotidis D, Vaikoussis E, Saldana M, Gouws P, Theodossiadis PG. Dry eye in graves ophthalmopathy: correlation with corneal hypoesthesia. Eur J Ophthalmol. 2013;23(4):473–79. doi:10.5301/ejo.5000259.
- Gupta A, Sadeghi PB, Akpek EK. Occult thyroid eye disease in patients presenting with dry eye symptoms. Am J Ophthalmol. 2009;147(5):919–23. doi:10.1016/j.ajo.2008.12.007.
- Jeffrey PGMD, Farris RL, Gilbard JP. Ocular surface drying and tear film osmolarity in thyroid eye disease. J Acta Ophthalmologica. 1983;61:108–16.
- Park J, Kim J, Lee H, Park M, Baek S. Functional and structural evaluation of the meibomian gland using a lipiview interferometer in thyroid eye disease. Can J Ophthalmol. 2018;53(4):373–79. doi:10.1016/j.jcjo.2017.11.006.
- Danping H, Quan L, Huasheng Y, Yuxiang M. Changes of lacrimal gland and tear inflammatory cytokines in thyroid-associated ophthalmopathy. J Invest Ophthalmol Vis Sci. 2014;55(8):4935–43. doi:10.1167/iovs.13-13704.
- Shu X, Wang J, Hu L. A review of functional slit lamp biomicroscopy. Eye Vis (Lond). 2019 May 21;6:15.
- Liu Z, Wang H, Jiang H, Gameiro GR, Wang J. Quantitative analysis of conjunctival microvasculature imaged using optical coherence tomography angiography. Eye Vis (Lond). 2019 Feb 2;6:5.
- Chen W, Deng Y, Jiang H, Wang J, Zhong J, Li S, Peng L, Wang B, Yang R, Zhang H, et al. Microvascular abnormalities in dry eye patients. Microvasc Res. 2018;118(155):155–61. doi:10.1016/j.mvr.2018.03.015.
- Wang J, Hu L, Shi C, Jiang H. Inter-visit measurement variability of conjunctival vasculature and circulation in habitual contact lens wearers and non-lens wearers. Eye Vis (Lond). 2019 Apr 1;6:10.
- Hu L, Shi C, Jiang H, Shi Y, Sethi Z, Wang J. Factors affecting microvascular responses in the bulbar conjunctiva in habitual contact lens wearers. Invest Ophthalmol Vis Sci. 2018;59(10):4108–14. doi:10.1167/iovs.18-24216.
- Shi Y, Hu L, Chen W, Qu D, Jiang H, Wang J. Evaluated conjunctival blood flow velocity in daily contact lens wearers. Eye Contact Lens. 2018;44(Suppl 1):S238–S243. doi:10.1097/ICL.0000000000000389.
- Chen W, Batawi HI, Alava JR, Galor A, Yuan J, Sarantopoulos CD, McClellan AL, Feuer WJ, Levitt RC, Wang J. Bulbar conjunctival microvascular responses in dry eye. Ocul Surf. 2017;15(2):193–201. doi:10.1016/j.jtos.2016.12.002.
- Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A. van der Gaag R. Clinical criteria for the assessment of disease activity in graves ophthalmopathy: A novel approach. Br J Ophthalmol. 1989;73(8):639–44. doi:10.1136/bjo.73.8.639.
- Jiang H, Zhong J, DeBuc DC, Tao A, Xu Z, Lam BL, Liu C, Wang J. Functional slit lamp biomicroscopy for imaging bulbar conjunctival microvasculature in contact lens wearers. Microvasc Res. 2014;92:62–71. doi:10.1016/j.mvr.2014.01.005.
- Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44(5–6):375–86.
- Kord Valeshabad A, Wanek J, Zelkha R, Lim JI, Camardo N, Gaynes B, Shahidi M. Conjunctival microvascular haemodynamics in sickle cell retinopathy. Acta Ophthalmol. 2015;93:e275–280.
- Kord Valeshabad A, Wanek J, Mukarram F, Zelkha R, Testai FD, Shahidi M. Feasibility of assessment of conjunctival microvascular hemodynamics in unilateral ischemic stroke. Microvasc Res. 2015 Jul;100:4–8.
- Khansari MM, Wanek J, Tan M, Joslin CE, Kresovich JK, Camardo N, Blair NP, Shahidi M. Assessment of conjunctival microvascular hemodynamics in stages of diabetic microvasculopathy. Sci Rep. 2017 Apr 7;7:45916.
- Startseva J, Sulimova N, Cherkassov V, Kon K, Lysov A. The diagnosis of transcapillary flow disturbances in the lungs of lung cancer. J Clin Hemorheol Microcircul. 2006;35:305–06.
- Gagliardo C, Radellini S, Morreale Bubella R, Falanga G, Richiusa P, Vadala M, Ciresi A, Midiri M, Giordano C. Lacrimal gland herniation in graves ophthalmopathy: A simple and useful MRI biomarker of disease activity. Eur Radiol. 2020;30(4):2138–41. doi:10.1007/s00330-019-06570-5.
- Xu Z, Jiang H, Tao A, Wu S, Yan W, Yuan J, Liu C, DeBuc DC, Wang J. Measurement variability of the bulbar conjunctival microvasculature in healthy subjects using functional slit lamp biomicroscopy (fslb). Microvasc Res. 2015;101(15):15–19. doi:10.1016/j.mvr.2015.05.003.
- Martins JR, Furlanetto RP, Oliveira LM, Mendes A, Passerotti CC, Chiamolera MI, Rocha AJ, Manso PG, Nader HB, Dietrich CP, et al. Comparison of practical methods for urinary glycosaminoglycans and serum hyaluronan with clinical activity scores in patients with graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2004;60(6):726–33. doi:10.1111/j.1365-2265.2004.02044.x.
- Gilbert JA, Gianoukakis AG, Salehi S, Moorhead J, Rao PV, Khan MZ, McGregor AM, Smith TJ, Banga J. Monoclonal pathogenic antibodies to the thyroid-stimulating hormone receptor in graves” disease with potent thyroid-stimulating activity but differential blocking activity activate multiple signaling pathways. J Immunol. 2006;176(8):5084–92. doi:10.4049/jimmunol.176.8.5084.
- Wong LL, Lee NG, Amarnani D, Choi CJ, Bielenberg DR, Freitag SK, D’Amore PA, Kim LA. Orbital angiogenesis and lymphangiogenesis in thyroid eye disease: an analysis of vascular growth factors with clinical correlation. Ophthalmology. 2016;123(9):2028–36. doi:10.1016/j.ophtha.2016.05.052.
- Walasik-Szemplinska D, Pauk-Domanska M, Sanocka U, Sudol-Szopinska I. Doppler imaging of orbital vessels in the assessment of the activity and severity of thyroid-associated orbitopathy. J Ultrason. 2015;15:388–97.
- Jamshidian Tehrani M, Mahdizad Z, Kasaei A, Fard MA. Early macular and peripapillary vasculature dropout in active thyroid eye disease. Graefes Arch Clin Exp Ophthalmol. 2019;257:2533–40.
- Wu Y, Tu Y, Bao L, Wu C, Zheng J, Wang J, Lu F, Shen M, Chen Q. Reduced retinal microvascular density related to activity status and serum antibodies in patients with graves’ ophthalmopathy. Curr Eye Res. 2020 May;45(5):576–584.
- Wang L, Yuan J, Jiang H, Yan W, Cintron-Colon HR, Perez VL, DeBuc DC, Feuer WJ, Wang J. Vessel sampling and blood flow velocity distribution with vessel diameter for characterizing the human bulbar conjunctival microvasculature. Eye Contact Lens. 2016;42(2):135–40. doi:10.1097/ICL.0000000000000146.
- Jiang H, Ye Y, DeBuc DC, Lam BL, Rundek T, Tao A, Shao Y, Wang J. Human conjunctival microvasculature assessed with a retinal function imager (RFI). Microvasc Res. 2013;85:134–37. doi:10.1016/j.mvr.2012.10.003.