ABSTRACT
Purpose: To evaluate the efficacy of a preservative free sodium hyaluronate/chondroitin sulfate ophthalmic solution (SH/CS-PF) in patients with dry eye disease (DED).
Methods: This was a randomized phase IV, multicentric, prospective, double-blind clinical trial. Intent-to-treat (ITT) and per-protocol (PP) analyses were performed. Patients were assigned to receive either SH/CS-PF, Systane® Ultra (PEG/PG) or Systane® Ultra PF (PEG/PG-PF) for 90 days. A total of 326 patients were included in the ITT, and 217 in the PP analysis. Efficacy endpoints were goblet cell density, Nelson’s grades (conjunctival impression cytology), tear break-up time (TBUT), Ocular Surface Disease Index (OSDI), and Schirmer’s test. Other parameters included were tolerability, measured by the ocular symptomatology; and safety, measured through corneal staining, intraocular pressure, visual acuity and adverse events.
Results: In the ITT, there was a significant increase in mean goblet cell density in all treatments compared with their baseline (28.4% vs 21.4% and 30.8%), without difference between arms (p = .159). Eyes exposed to SH/CS-PF, PEG/PG and PEG/PG-PF showed Grade 0-I squamous metaplasia (85.5%, 87.9% and 93.2%, respectively). Similar improvements were observed for TBUT (1.24 ± 2.3s vs 1.27 ± 2.4s and 1.39 ± 2.3s) and OSDI scores at day 90 (−8.81 ± 8.6 vs −7.95 ± 9.2 and −8.78 ± 9.8), although no significant intergroup difference was found. Schirmer’s test also presented improvement compared to baseline (1.38 ± 4.9 vs 1.50 ± 4.7 and 2.63 ± 5.9), with a significantly higher variation for PEG/PG-PF. There were no significant differences between treatments for any tolerability and safety parameter, nor between ITT and PP analyses for any outcome.
Conclusions: The topical application of SH/CS-PF is as effective, safe and well tolerated as that of PEG/PG or PEG/PG-PF. The results suggest that SH/CS-PF may lead to normalization of clinical parameters and symptom alleviation in patients treated for DED.
Introduction
Dry eye disease (DED) is a common and complex disease of the ocular surface eye that significantly reduces the quality of life and affects 6–34% of the word’s adult population.Citation1–3 DED is characterized by instability of the tear film that can be due to insufficient amount of tear production or to poor quality of tear film.Citation1,Citation2 The lacrimal functional unit (LFU) is an integrated system, consisting of ocular surface components (cornea, conjunctiva, accessory lacrimal glands and meibomian glands), the main lacrimal gland, and interconnecting innervation, all of them acting as a functional unit.Citation4 Defending the ocular surface from environmental exposure is one of the main functions of the tear film. One of DED’s main symptoms of is ocular dryness sensation. Other symptoms include burning, foreign body sensation (FBS), tearing, pain, redness and, photophobia.Citation5,Citation6 Symptoms and signs of DED are common among the elderly, but signs show a greater increase per decade compared to symptoms.Citation1,Citation3 Women have a higher prevalence of DED than men, although this difference becomes significant only with advanced age.Citation1
Diagnosing, staging, and determining the efficacy of therapy in DED is often challenging due to low of correlation between signs and symptoms.Citation7
Impression cytology serves as a minimally invasive alternative to ocular surface biopsy. The fluid moving from the conjunctival tissue over of the perimeter of the goblet cell may facilitate mucin hydration at time of secretion from the goblet cell. Some of the functions of mucins are lubrication, maintenance of surface wetting, maintenance of tear film across the epithelium, and prevention of infection.Citation8 The diagnosis of DED commonly includes tests such as tear film break-up time (TBUT), epithelial staining, ocular surface disease index (OSDI), and evaluation of tear production with Schirmer’s test. Each of these tests provides specific information regarding DED.Citation5,Citation9 The aim of the management of DED is to address tear film instability.
The clinical development of new DED treatments is slow since its pathogenesis is multiple, and its signs and symptoms are variable. The different stages of DED treatment have as a common denominator the use of ocular lubricants. There are many different formulations of ocular lubricants such as those containing sodium hyaluronate, cellulose-based derivatives, hypo-osmolar formulations, lipid-based emulsions, glycerin-containing drops, polysaccharides, and polyethylene glycol/propylene glycol (PEG/PG) with HP-guar.Citation9,Citation10
Systane® Ultra and Systane® Ultra preservative-free (Alcon Research. Ltd., Fort Worth, Texas, USA) are artificial tears containing PEG and PG with demonstrated efficacy in the management of DED.Citation11,Citation12 PEG/PG agents protect the ocular surface epithelium and offer extended periods of ocular comfort.Citation13
Sodium hyaluronate (SH) is a disaccharide belonging to the glycosaminoglycan biopolymers family, it has a high water-retaining capacity, and its use could play an important role in the management of ocular surface diseases like DED.Citation14 The SH 0.1% and chondroitin sulfate 0.18% (CS), constituents of the extra-cellular matrix, provide Humylub® Ofteno PF (Laboratorios Sophia, SA de CV, Zapopan, Jalisco, Mexico) with viscoelastic and water retention properties, working as an effective lubricant that protects the surface of the eye and reconstitutes the tear film. In patients suffering from DED, preservatives frequently aggravate the already existing symptoms. Chronic exposure of benzalkonium chloride (BAK) included in many ophthalmic drugs has been associated with inflammation, conjunctival and corneal damage, tear film abnormalities and signs and symptoms of ocular surface disease (OSD).Citation15,Citation16 Thus, SH/CS-PF is a preservative-free formulation that can offer a therapeutic option without the injurious effects of BAK.
The present study was designed to evaluate the efficacy, tolerability and safety of SH/CS-PF ophthalmic solution in the treatment of mild to moderate DED by normalizing the parameters of the ocular surface, reduction of symptoms and downgrading of squamous metaplasia as evaluated by conjunctival impression cytology (CIC).
Materials and methods
Study design
This was a randomized phase IV, parallel, prospective, double-blind, multicentric clinical trial. The study was registered in COFEPRIS before recruiting the first participant as 153301CT190645/2015 (Mexico), in clinicaltrials.gov as NCT03223909, and in INVIMA as 2017010882 (Colombia). It was conducted in nine centers in Mexico and one center in Colombia. An ethics committee in each center reviewed and approved the study’s protocol and informed consent (see Acknowledgments section). The research was conducted in compliance with the Declaration of Helsinki and in accordance with Good Clinical Practices Standards. All patients that participated in this study provided written and signed informed consent. Patients were recruited between October 2016 and October 2018 (FPFV: October 14, 2016 and LPLV: October 10, 2018).
Participants
Inclusion criteria included patients, men or women (aged ≥18 years), with a mild or moderate DED diagnosis (OSDI score < 30, TBUT > 5s and < 10s, Schirmer’s test ≥ 5 mm/5 min and, OSS < III in Oxford scale). Exclusion criteria included use of topical ocular drops and systemic medication that may have affected the study’s results (systemic steroids, β-blockers or any other drug with anticholinergic side effects, artificial tears with BAK, etc.), patients with active or chronic systemic diseases and/or currently taking immunomodulators, patients with proliferative diseases of the ocular surface, presence of any type of corneal ulcers or eye infections, history of uveitis, presence of any illness that could interfere with study parameters, history of penetrating keratoplasty, use of contact lenses, subjection to ocular surgery within 3 months before baseline, subjects with a functional single eye, and patients that were pregnant, at risk for pregnancy without birth control treatment, or breastfeeding.
Treatment and evaluations
Three hundred and twenty six subjects were randomized 1:1:1 to receive SH/CS-PF (Humylub® Ofteno PF, Laboratorios Sophia, SA de CV, Zapopan, Jalisco, Mexico; n = 120), PEG/PG (Systane® Ultra, Alcon Laboratories, Inc., Fort Worth, TX, USA; n = 109), or PEG/PG-PF (Systane® Ultra PF, Alcon Laboratories, Inc., Fort Worth, TX, USA; n = 97). Randomization numbers were generated using Microsoft® Office Excel 2016. Patients instilled a drop of study drug topically in the inferior conjunctival sac of both eyes four times a day for 90 days. All the researchers and other sponsoring team members were blind to treatment assignment throughout the study. Follow-up visits were on days 7, 30, 60 and 90 after randomization. A safety call was carried out 2 weeks after the final visit (105th day). The study’s schedule at each visit included: eligibility criteria (only for baseline visit), pregnancy test (if applicable), OSDI, visual acuity (VA), CIC, fluorescein corneal staining, TBUT, Rose Bengal corneal staining, Schirmer test, anterior biomicroscopy, posterior ophthalmoscopy under mydriasis, intraocular pressure (IOP), and adverse events (AEs) evaluation.
The study drug was discontinued if either the principal investigator or patient judged that it was not in the latter’s best interest to continue or if a female patient became pregnant.
Study endpoints
Efficacy assessment
The primary endpoints of the study were the change from baseline for conjunctival goblet cell density and Nelson’s grades (determined by CIC), the TBUT, the OSDI score and the Schirmer’s test, over the 3-month treatment period. The CIC was performed on day 0 (baseline) and on day 90 of the study (final visit). CIC refers to the application of a cellulose acetate filter (Millipore, Merck) over the conjunctiva in order to remove the superficial layers of the epithelium. The specimens were stained and graded using the Nelson grading system (0, normal density; I, decreased density; II, absence of cells; III, absence of cell plus squamous metaplasia). All the specimens were analyzed by the same investigator who was masked to the treatment of the samples. The TBUT with fluorescein was performed at each follow-up visit. Subjective symptoms were graded through a numerical scale of 0 to 4 using the OSDI questionnaireCitation17 at each follow-up visit. Schirmer’s test was performed with topical anesthesia at each follow-up visit. Thin strips of filter paper (Whatman of 5 mm x 35 mm, Sigma Aldrich, now Merck) were placed (in both eyes at the same time for 5 minutes) over the junction of the middle and outer thirds of the palpebral rim, to prevent injury of the cornea.
Tolerability and safety assessment
The tolerability was measured through the ocular symptomatology determined by burning, FBS, tearing, chemosis and conjunctival hyperemia (Efron scale). The safety was measured through fluorescein and Rose Bengal corneal staining, IOP, VA and the incidence of AEs. Surface dye staining was classified with a scale from 0 to IV in accordance to the percentage of the affected area (Oxford scale). The IOP was measured using a calibrated Goldmann applanation tonometer. The visual acuity was determined with a Snellen chart and expressed in LogMAR values. For the analysis of the variables described during the ophthalmological exploration (CIC, TBUT, Schirmer’s test, ocular symptomatology, corneal staining, IOP and VA), each eye was considered an individual case.Citation7
Statistical analysis
Statistical analysis was carried out using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Since intent-to-treat (ITT) analysis population is the most clinically relevant and may be more reflective of real-world conditions, all randomized study patients in the groups to which they were randomized were included in the analyses.Citation18 Additionally, in compliance with CONSORT guidelines evaluations were made also in per-protocol population (PP), established as randomized patients with no major deviation from the protocol.Citation19 Sample size calculation was performed to test the increase in Schirmer’s test results, with an expected difference of at least 0.75 mm/5 min with an alpha of 0.05, power of 80%, and a standard deviation of 1.72 mm/5 min.Citation20 For the ITT analysis, the Last Observation Carried Forward (LOCF) was used.Citation18,Citation21 The effects of treatment on continuous variables were assessed using a linear mixed effects model with fixed effects for treatment, time, and treatment by time interaction and random effect for subjects. The dependence due to repeated measures over time was modeled using a correlation matrix for measurements taken over time. The 95% confidence interval (CI) of these differences was computed. Throughout these analyses, partial eta squares (ηCitation2p) were calculated to report effect sizes. Tukey’s comparisons were used when required for the post-hoc analyses. The model’s fit was assessed via graphical analysis of the residuals and a Kolmogorov-Smirnov test for normality. The ordinal variables were analyzed using 2 × 2 contingency tables and the differences were calculated with Chi-square test. All statistical analyses performed in this study were 2 sided (p ≤ 0.05).
Results
Characteristics of the participants
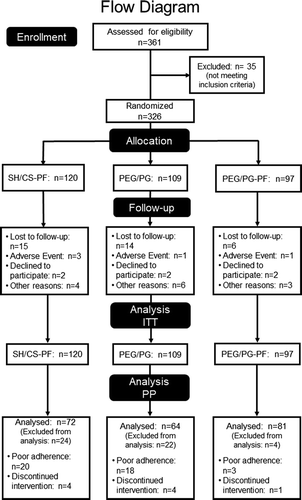
This study enrolled 326 patients (ITT population), from which 59 discontinued their participation because of AEs (5/59, 8.5%), patient’s decision unrelated to AE (6/59, 10.2%), follow-up loss (35/59, 59.3%), and protocol deviations (13/59, 22.0%). Exclusion reasons were similar between groups (p = .889). A total of 4.6% patients discontinued their participation prior to visit 1, 9.8% before visit 2, 15.9% before visit 3, and 18.1% before the final visit. Forty-one patients were excluded from PP analysis population due to poor adherence, determined as using <80% of indicated treatment. Of these 41 patients, 20 (21.7%) were in the SH/CS-PF group, 18 (21.9%) in the PEG/PG group and 3 (3.6%) in the PEG/PG-PF group, without differences (p = .238). Additionally, 9 patients were excluded after discontinuing their intervention (9.4%), see .
Demographic and baseline characteristics were similar between the three treatment groups and populations without significant differences, see . In the ITT, mean age ± standard deviation (SD) was 51.66 ± 16.5 years (range 19–88), and 74.2% of patients in both populations were female. In the PP, mean age ± SD was 52.34 ± 16.4 years (range 20–88). Clinical signs and symptoms were similar between groups and patients in each group were diagnosed with mild/moderate DED.
Table 1. Initial characteristics of each group
Efficacy
Conjunctival impression cytology
In the ITT population, after 3 months of treatment and compared to baseline, there was a significant increase in conjunctival goblet cell density in all groups (factor visit, F(1,637) = 197.52; p = .0001, 95% CI, [76.36, 101.17]). The SH/CS-PF group increased goblet cell density by 28.4% at day 90, PEG/PG group by 21.4% and, PEG/PG-PF group by 30.8%. No differences were observed between groups (p = .159), and between-factor interaction was not significant (visit x arm; p = .159). At baseline, 25.6% of patients in the SH/CS-PF group were classified as having abnormal CIC grade II–III (classification of Nelson), while for PEG/PG group it was 25.3% and 25.5% for PEG/PG-PF, without significant differences (p = .862). By day 90, 14.5% of SH/CS-PF, 12.1% of the PEG/PG and finally, 6.8% of PEG/PG-PF patients had grade II. The percentage of patients with grade 0 (normal density), increased in all groups by the end of 90-day protocol period. No significant differences were observed between groups (p = .054), see .
Table 2. Change from baseline at 3-month follow-up
Similar results were found in the PP analysis, at the final visit (F(1,429) = 143.49; p = .0001, 95% CI, [87.35, 115.21]), the density of goblet cells increased by 31.1% for SH/CS-PF, 22.1% by PEG/PG group and, PEG/PG-PF group by 34.9%, no differences were observed between groups or between-factor interaction (p values: 0.463 and 0.192 respectively). By day 90, 10.4% of SH/CS-PF, 14.8% of the PEG/PG and finally, 6.2% of PEG/PG-PF patients had abnormal CIC grade II. No significant differences were observed between groups (p = .146), see .
Tear break-up time (TBUT)
In the ITT analysis, after 3 months of treatment, the TBUT showed significant improvement compared to baseline in all groups (factor visit, F(3,647) = 17.354; p = .0001, 95% CI, [1.119, 1.478]). After 1 week of treatment, the mean change ± SD was 0.68 ± 2.3s for the SH/CS-PF group, 0.85 ± 1.9s for the PEG/PG and, 0.37 ± 1.6s for PEG/PG-PF group. Tukey’s test showed that PEG/PG had a significant improvement vs PEG/PG-PF group (p = .036, 95% CI, [0.02, 0.94]). After 1 month, it was 1.15 ± 2.5s for the SH/CS-PF, 0.89 ± 2.3s for the PEG-PG, and 0.87 ± 2.4s for the PEG/PG-PF group, no significant intergroup differences were found (p = .385). At 2 months, it was 1.11 ± 2.4s for the SH/CS-PF, 1.32 ± 2.3s for the PEG/PG, and 1.37 ± 2.4s for PEG/PG-PF group, without differences between treatments (p = .461). By day 90, the SH/CS-PF group had a mean change of 1.24 ± 2.3s, for the PEG/PG group it was 1.27 ± 2.4s and for PEG/PG-PF group 1.39 ± 2.3s, there was no statistically significant difference between groups (p = .795). Between-factor interaction was not significant (visit x arm; p = .055, η2p = .009), see .
Figure 2. TBUT with fluorescein, change from baseline in the ITT. Mean value ± SE. The increase in all groups was statistically significant after 3 months (p = .0001). The increase in PEG/PG was statistically significant at week 1 compared with PEG/PG-PF, *p < .05
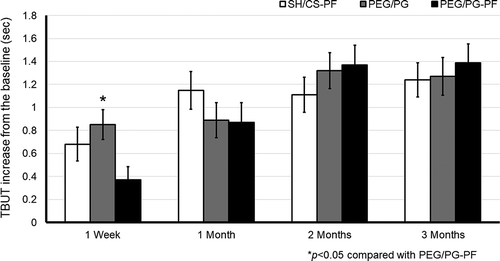
Overall, the results were similar for PP analysis, at the final visit the TBUT showed significant improvement compared to baseline in all groups (F(3,429) = 11.953; p = .0001, 95% CI, [0.985, 1.604]). The only difference (between groups) was after 1 week of treatment the Tukey’s test showed that SH/CS-PF had a significant improvement vs PEG/PG-PF group (p = .043, 95% CI, [0.01, 1.04]). There was no significant difference between all groups for TBUT at any remaining time points (p > .05). But in this case, between-factor interaction was significant (p = .003, ηCitation2p = .023), see .
Ocular surface disease index (OSDI)
There was a significant reduction in OSDI score after 3 months in all groups (factor visit, F(3,321) = 19.105; p = .0001, 95% IC, [−9.51, −7.51]) in ITT. This analysis showed that the three groups had a similar score reduction (i.e., no significant differences were found). The mean change ± SD from baseline to week 1 was −4.45 ± 7.2 for the SH/CS-PF group, −4.86 ± 9.4 for PEG/PG and, −4.54 ± 9.4 for PEG/PG-PF. No significant intergroup differences were found (p = .931). After 1 month, it was −5.88 ± 8.2 for SH/CS-PF, −5.78 ± 11.4 for PEG/PG and, −7.08 ± 7.9 for PEG/PG-PF, without differences between treatments (p = .539). At 2 months, it was −7.48 ± 9.5 for SH/CS-PF, −7.60 ± 9.1 for PEG/PG, and −7.68 ± 10.7 for PEG/PG-PF, without differences between treatments (p = .998). The mean change from baseline to day 90 was −8.81 ± 8.6 for SH/CS-PF, −7.95 ± 9.2 for PEG/PG and, −8.78 ± 9.8 for PEG/PG-PF, also no significant between-group differences were observed (p = .733). Between-factor interaction was not significant (visit x arm; p = .696), see .
Figure 3. OSDI score, change from baseline in the ITT. Mean value ± SE. The decrease in all groups was statistically significant at 1, 2 and 3 months (p = .0001). There was no significant difference in the increase in the OSDI score between groups
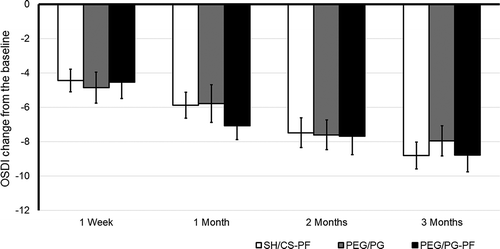
The findings of PP analysis identified comparable values, a significant reduction in OSDI score at day 90 in all groups (F(3,212) = 12.24; p = .0001, 95% IC, [−11.415, −7.909]). The mean change from baseline to day 90 was −9.90 ± 7.9 for SH/CS-PF, −9.27 ± 8.7 for PEG/PG and, −9.81 ± 10.2 for PEG/PG-PF, no differences between-group or between-factor interaction were observed (p values: 0.909 and 0.976 respectively), see .
Schirmer’s test
On day 90, in the ITT the linear mixed effects model found that factor visit was significant (factor visit, F(3,649) = 5.512; p = .001, 95% CI [1.440, 2.235]). The mean change ± SD from baseline to week 1 was 1.79 ± 4.8 mm/5 min for the SH/CS-PF group, 1.44 ± 5.0 mm/5 min for PEG/PG and, 2.14 ± 5.6 mm/5 min for PEG/PG-PF; no significant intergroup differences were found (p = .386). After 1 month, it was 2.26 ± 5.4 mm/5 min for SH/CS-PF, 1.64 ± 4.9 mm/5 min for PEG/PG and 2.19 ± 5.8 mm/5 min for PEG/PG-PF, no significant intergroup differences were found (p = .414). At 2 months, it was 2.82 ± 5.8 mm/5 min for SH/CS-PF, 2.38 ± 5.7 mm/5 min for PEG/PG and, 2.42 ± 6.7 mm/5 min for PEG/PG-PF, without differences between treatments (p = .688). After 3 months of treatment, it was 1.38 ± 4.9 mm/5 min for SH/CS-PF, 1.50 ± 4.7 mm/5 min for PEG/PG and, 2.63 ± 5.9 mm/5 min for PEG/PG-PF. The PEG/PG-PF group showed a significantly greater improvement compared to SH/CS-PF (p = .033, 95% CI [0.08, 2.41]). Between-factor interaction was not significant (p = .054, ηCitation2p = .006), see .
Figure 4. Schirmer I score, change from baseline in the ITT. Mean value ± SE. The increase in PEG/PG-PF was statistically significant after 3 months compared to SH/CS-PF, *p < .05
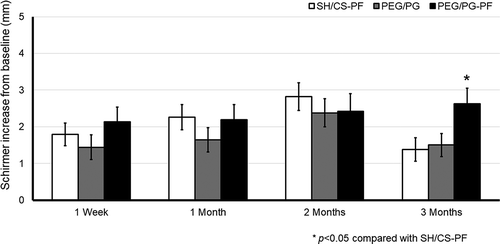
Comparable results were found in PP, at day 90 compared to baseline (F(3,429) = 4.826; p = .003, 95% IC, [0.908, 2.229]). The mean change from baseline at day 90 of treatment was 0.71 ± 4.2 mm/5 min for SH/CS-PF, 1.16 ± 4.8 mm/5 min for PEG/PG and, 2.91 ± 5.5 mm/5 min for PEG/PG-PF. The PEG/PG-PF group showed a significantly greater improvement compared to SH/CS-PF (p = .0001, 95% CI [0.88, 3.53]) and PEG/PG (p = .008, 95% CI [0.38, 3.12]). In this analysis, between-factor interaction was significant (p = .006, ηCitation2p = .021), see .
Tolerability and safety
Ocular symptomatology
Burning, FBS, tearing, chemosis and conjunctival hyperemia were considered the parameters of tolerability. All groups showed a significant improvement of ocular symptomatology over the duration of the study. In the ITT analysis population, the decrement in the presence of ocular burning was 45% in the SH/CS-PF group vs 27% and 36.1% in PEG/PG and PEG/PG-PF groups, respectively; 38.4% vs 31.7% and 43.8% decrement of FBS; 16.2% vs 17.9% and 27.8% decrement of tearing; 13.8% vs 12.4% and 11.4% decrement of presence of chemosis; and 21.3% vs 14.2% and 27.3% decrement of conjunctival hyperemia. Compared to baseline after 3 months of treatment, the ocular symptomatology showed more significant improvements in the SH/CS-PF and PEG/PG-PF groups (all p < .05) than in the PEG/PG group, see .
Table 3. Ocular symptomatology (presence)
Similar results were found for PP analysis, all groups showed a significant decrement of ocular burning (49.3%, 25.8%, and 36.4% in SH/CS-PF, PEG/PG and PEG/PG-PF groups, respectively), FBS (45.1%, 35.1%, and 46.3%), tearing (19.5%, 18%, and 29.7%), chemosis (13.2%, 18%, and 29.7%), and conjunctival hyperemia (18.1%, 12.5%, and 25.9%) compared to baseline, see .
Fluorescein and rose bengal staining
In the ITT, there was a statistically significant improvement in the fluorescein staining score in all groups after 3 months (p = .0001). There was a marked decrease in corneal staining from baseline (grades II–IV), the SH/CS-PF group had an increment of grade 0-I in 17.5% of patients at day 7, 18.8% at day 30, 22.5% at day 60 and 24.6% at the final visit. For PEG/PG group it was 17.9%, 20.2%, 17.9% and 23.4% respectively, and for the PEG/PG-PF group it was 9.8%, 16%, 19.1% and 17.6% respectively. There was no significant difference in all groups for change from baseline at any time point (p > .05), see .
There was a statistical improvement in the Rose Bengal staining score in all groups from baseline (p = .0001). The SH/CS-PF group had an increase of grade 0-I in 8.5% of patients at day 7, 12.1% at day 30, 11.7% at day 60 and 115.9% at the final visit. For PEG/PG group it was 7.8%, 12.0%, 12.9% and 14.3% respectively. Meanwhile for the PEG/PG-PF group it was 8.2%, 11.3%, 14.9% and 14.4% respectively. There was no significant difference in all groups for change from baseline at any time point (p > .05), see .
The increment in grade 0–I for fluorescein staining was similar in PP analysis, the SH/CS-PF group had and improvement of grade 0-I in 20.8% of patients, vs 22.6% in PEG/PG group, and for the PEG/PG group it was 16.6% at the final visit. Meanwhile, the improvement in RBS score at the final visit was 15.3%, 17.9%, and 15.4% for SH/CS-PF, PEG/PG, and PEG/PG groups respectively, see .
Intraocular pressure (IOP) and visual acuity
On day 90 in ITT, the ocular tonometry showed no significant differences in IOP between treatments (p = .995). The factor of days was significant (F(1,649) = 20.906; p = .0001, 95% CI, 13.046, 13.834]). Between-factor interaction was not significant (p = .409). After the intervention time, the VA did not increase from baseline to final visit, no significant differences between treatments were observed (p = .469). The factor of days was statistically significant (F(1,649) = 34.276, p = .0001, 95% CI, [0.022, 0.044]). Between-factor interaction was not significant (p = .090, ηCitation2p = .007), see .
Overall, the results were similar for PP analysis population, at the final visit, no significant differences in IOP between treatments were observed (p = .176). The factor of visits was significant (F(1,431) = 17.126; p = .0001, 95% CI, 12.946, 13.381]), but between-factor interaction was not significant (p = .263). The VA did not increase from baseline to final visit, no significant differences between treatments were observed (p = .774). The factor of days was statistically significant (F(1,431) = 6.430, p = .015, 95% CI, [0.031, 0.047]). Between-factor interaction was not significant (p = .534), see .
Adverse events (AE)
Data on safety were analyzed for the ITT population. A total of 88 AEs was reported by 20.2% (66/326) of the patients randomized during the protocol. There were no significant differences between treatments for the incidence of AEs (p = .930). 32 AEs were reported for SH/CS-PF (23 ocular AEs and 9 non-ocular), 31 AEs for PEG/PG group (22 ocular AEs and 9 non-ocular), and 25 for PEG/PG-PF group (15 ocular AEs and 10 non-ocular). There were no significant differences for the ocular or non-ocular AEs between treatments (p = .582). A total of 6 serious AEs occurred during the study (3 allergic conjunctivitis, 2 bacterial conjunctivitis and one migraine), see .
Table 4. Treatment-related adverse events (88 AEs/66 subjects)
Discussion
Establishing the main causes behind DED is critical for proper management, algorithms are structured to recommend a series of treatments per disease stage. The first step includes alteration of the local environment, patient education, and dietary modifications; in addition to treatment with ocular lubricants of various types.Citation1,Citation2 It is generally believed that rather than representing two distinct categories (aqueous-deficient DE; ADDE or meibomian gland related DE; MGD), most people with symptoms related to OSD suffer from variable combinations of both. Estimates of the degree of overlap between these two categories range from 30 to 70%.Citation22 Added to this consideration, there is the issue that, other than for Sjogren’s syndrome, there is no gold standard for ADDE classification, which could lead for investigator bias and misclassification. The aim of this study was to evaluate the efficacy, tolerability and safe of SH-0.1%/CS-0.18% PF ophthalmic solution (Humylub® Ofteno PF) in the treatment of mild to moderate DED by normalizing the parameters of the ocular surface, reducing the symptoms and the squamous metaplasia.
In randomized clinical trials, exclusion of participants, particularly if their number is large, may lead to a significant reduction in sample size and hence in study power.Citation19 In the current study, a total of 217 from 326 enrolled participants strictly adhered to the planned treatment, and completed the protocol, so the true efficacy of one intervention over the other can be assessed. However, approximately 33% of patients were excluded from PP analysis, and this can introduce bias. To address this issue, the Last Observation Carried Forward (LOCF) was used to minimize the dropped-out rate. The use of ITT analysis guarantees the maintenance of compatibility between groups as obtained through randomization, keeping sample size, and eliminating bias.Citation18 The results of this study showed that in this population of prospectively recruited patients with complete follow-up, SH/CS-PF eyedrops alleviate significantly DED symptoms after 3 months of treatment in a mixed population of patients with a combination of symptoms, in the ITT and PP population.
Goblet cells within the conjunctival epithelium are specialized cells that secrete mucins onto the ocular surface, patients with DED show a decrease of conjunctival goblet cell numbers.Citation8 The improvements from baseline in the change of goblet cell density and classification of Nelson were similar between both preservative-free eyedrops. SH/CS-PF and PEG/PG-PF as showed in ITT population with a 28.4% and 30.8% decrease in the squamous metaplasia score (an increase in goblet cell density) compared with baseline; vs 31.1% and 34.9%in PP analysis (p values: 0.497 and 0.510 respectively). Also, an improvement in Nelson’s impression cytology grade was present, having 79.5% and 73.5% vs 70.1% and 72.8% of SH/CS-PF and PEG/PG-PF patients in ITT and PP analysis, respectively, reach grade 0. A study performed by Aguilar et al,Citation12 evaluated the efficacy of PEG/PG in reducing squamous metaplasia in patients with DED, with the limitation that they did not use a control/comparator group. PEG/PG reduced squamous metaplasia, in addition to improving TBUT. In our study, the PEG/PG group was less effective than the preservative-free treatments in reducing the squamous metaplasia (determined by CIC).
Reduced TBUT is a global criterion of DED.Citation5,Citation9 This study revealed that the effects of SH/CS-PF versus PEG/PG and PEG/PG-PF, were comparable at the end of the study period. After 3 months of intervention, mean TBUT increased in all groups versus baseline, and compared to PEG/PG and PEG/PG-PF, SH/CS-PF showed statistically significant increase of TBUT after 1 week of treatment in both analyses. Increased tear residence time can help the microenvironment of the eye and extend ocular surface protection.Citation23 Similar improvements were found for Schirmer’s test. There were statistically significant improvements in Schirmer’s test in the PEG/PG-PF group at 1 week and 3 months. However, by the end of the treatment period, Schirmer’s test increased in all groups versus
baseline. These results are consistent with the results produced by Hwang et al,Citation24 who evaluated the efficacy of preservative-free SH 0.1% in DED for three months. The investigators concluded that SH-PF seems to be effective in decreasing the damage to the ocular surface in patients with DED.
The OSDI questionnaire is an instrument for measuring of the symptoms of ocular irritation related to DED and their impact on vision-related function.Citation17 The improvement from baseline in the OSDI score was similar between SH/CS-PF and PEG/PG-PF groups at the end of the treatment period. However, there were no differences between different treatment scores in either populations.
Studies have demonstrated the potential benefits of regular use of artificial tears, showing an improvement of ocular symptomatology and quality of life in patients with DED.Citation5,Citation9,Citation12,Citation13 In this study, all treatments caused a reduction in burning, FBS, tearing, chemosis and conjunctival hyperemia by the end of treatment period. However, once again, patients with DED better tolerated preservative-free lubricants over that with preservatives.
Diagnosing, staging, and determining the efficacy of therapy in DED is often challenging due to an irregular correlation between signs and symptoms.Citation7 SH has been widely used as a lubricant. Lubricants’ rheological profile usually translate as a higher viscosity under static conditions on the eye which decreases during blinking.Citation25 Epithelial staining with special dyes such as fluorescein and Rose Bengal are used to determine abnormalities of the eye surface, tear film’s quality, and dryness severity.Citation5 When one portion of the LFU is compromised, normal lacrimal production is impaired, yielding the ocular surface symptoms associated with DED.Citation4 This study also demonstrated that SH/CS-PF improved the corneal fluorescein and Rose Bengal staining score without differences between treatments, but mean ocular staining was significantly reduced in all groups after 3 months.
In other findings, IOP, VA and AEs were studied to evaluate the safety of the solutions No differences were observed between groups for these parameters. However, we hypothesize that preservative-free lubricants may be safer than eye drops with preservatives in patients with DED.Citation26–28 All AEs were resolved by the end of the study period and no safety issues were raised.
Summarizing, all the data analyzed in this study, from both ITT and PP populations, found that the topically applied SH/CS-PF is as effective, well tolerated and safe as PEG/PG and PEG/PG-PF drops. No significant differences were observed in any outcome between populations. QID dosing of either lubricant was not superior when compared to non-adherence (using <80% of indicated treatment) in terms of normalizing the parameters of the ocular surface, reduction of symptoms and downgrading of squamous metaplasia. These results are in agreement with previous studies, in which the clinical effects of using QID versus as-needed dosing of an artificial tear in DED were evaluated. QID dosing of an artificial tear was not superior to as-needed dosing in terms of ocular staining.Citation13
Finally, this study had some limitations, including the dropout rate. Randomized controlled trials often suffer from two major complications, noncompliance and missing outcomes. One solution to this problem is an ITT analysis by LOCF method.Citation21 The advantage of this approach is that it minimizes the number of patients who were eliminated from the analysis, however LOCF’s disregards the trajectory of the change prior to the final value; that is, it does not take into account that some dropouts may have shown no change up to and including the last evaluation, while others may have been improved, or worsened.Citation18 The incorrect application of ITT analysis with an evaluation of the outcomes using a PP analysis, may cause either an overestimation of the results or a reduced sample size with a subsequent diminished statistical power.Citation29 Despite this limitation, the overall strength of ITT and PP analyses and the data management allowed a mitigation of the risk of relatively high noncompliance rate in the groups.Citation19 Another limitation was that the study recruited a mixed population of ADDE and MGD patients.Citation22 Additionally, since preservative-free treatments have similar clinical effects, in our study, we could not claim noninferiority or equivalence over the clinical impact of the magnitude of the effects. Further studies will be required to investigate those outcomes a priori.
In conclusion, topically applied SH/CS-PF is as effective, well tolerated and safe as PEG/PG or PEG/PG-PF eye drops in patients with mild to moderate DED. It showed no difference in efficacy, safety or tolerability outcomes between ITT or PP analyses. The results suggest that Humylub® Ofteno PF may lead to normalize the ocular surface’s clinical parameters and reduce symptoms in the treatment of mild/moderate dry eye syndrome.
Declaration of interest
This study was endorsed by Laboratorios Sophia, SA de CV (Zapopan, Jalisco, México). The funder provided support in the form of salaries for authors [BDL, OMO and MVP], but this commercial affiliation did not have any additional role in the data collection. The rest of the authors declare that they have no personal, financial, commercial or academic interest.
All authors made substantial contributions to conception and design, data acquisition, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
In addition to summary statistics, the data points behind means, frequencies and variance measures are openly available in Open Science Framework (https://osf.io) as DOI 10.17605/OSF.IO/9S26U.
Abbreviations
| AE | = | Adverse events |
| BAK | = | Benzalkonium chloride |
| CIC | = | Conjunctival impression cytology |
| DED | = | Dry eye disease |
| FBS | = | Foreign body sensation |
| IOP | = | Intraocular pressure |
| ITT | = | intent-to-treat |
| OSDI | = | Ocular surface disease index |
| PP | = | per-protocol |
| TBUT | = | Tear break-up time |
| VA | = | Visual acuity |
Acknowledgments
The authors thank Graue E (Fundación de Asistencia Privada Conde de Valenciana, IAP), Hernández JC (Fundación Santos y de la Garza Evia, IBP), Gutierrez AL (Centro Oftalmológico San Ángel SA de CV) and Rodríguez F (Clínica de Especialidades Murano). The study protocol was approved by their respective Institutional Review Boards, as follows: Comité de Ética en Investigación Fundación Oftalmológica Nacional; Comité de Ética en Investigación de Asociación para Evitar la Ceguera en México IAP; Comité de Ética en Investigación del Centro Hospitalario Vicor, SA de CV, CHG Hospitales; Comité de Investigación Instituto Jalisciense de Investigación Clínica SA de CV; Comité de Ética en Investigación de Investigación Biomédica para el Desarrollo de Fármacos, SA de CV; Comité de Ética en Investigación de Escuela de Medicina del Instituto Tecnológico y de Estudios Superiores de Monterrey; Comité de Ética en Investigación de Accelerium s de RLCV; Comité de Ética en Investigación de Fundación de Asistencia Privada Conde de Valenciana, IAP.
The authors thank Alejandra Sánchez-Rios, MD for the medical writing support.
Additional information
Funding
References
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–65. doi:10.1016/j.jtos.2017.05.003.
- The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workshop (2007). Ocul Surf. 2007;5(2):93–107. doi:10.1016/S1542-0124(12)70082-4.
- Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalece of dry eye among the elderly. Am J Ophthalmo. 1997;124(6):723–28. doi:10.1016/S0002-9394(14)71688-5.
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–89. doi:10.1097/00003226-199811000-00002.
- Suvarna PP, Munira M, Premanand N, Sonali A, Kamalinder KS. A comprehensive review on dry eye disease: diagnosis, medical management, recent developments, and future changes. Adv Pharmac. 2015;2015, article ID 70494:12.
- Geerling G, Brewitt H, eds. Surgery for the dry eye. Dev Ophthalmol Basel Karger. 2008;41:36–53.
- Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, Geerling G, Figueiredo F, Lemp MA. Correlations between commonly used objetive signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161–66. doi:10.1111/aos.12012.
- Gipson IK. Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res. 2016;54:49–63. doi:10.1016/j.preteyeres.2016.04.005.
- Springs CL. Novel hydroxypropyl-guar gellable lubricant eye drops for treatment of dry eye. Adv Ther. 2010;27(10):681–90. doi:10.1007/s12325-010-0052-3.
- Pérez-Balbuena AL, Ochoa-Tabares JC, Belalcazar-Rey S, Urzúa-Salinas C, Saucedo-Rodríguez LR, Velasco-Ramos R, Suárez-Sánchez RG, Rodríguez-Carrizalez AD, Oregón-Miranda AA. Efficacy of a fixed combination of 0.09% xanthan gum/0.1% chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016;16:164.
- Llamas-Moreno JF, Baiza-Durán LM, Saucedo-Rodríguez LR, JF A-DLO. Efficacy and safety of chondroitin sulfate/xanthan gum versus polyethylene glycol/propylene glycol/hydroxypropyl guar in patients with dry eye. Clin Ophthalmol. 2013;7:995–99.
- Aguilar A, Berra M, Trédicce J, Berra A. Efficacy of polyethylene glycol-propylene glycol-based lubricant eye drops in reducing squamous metaplasia in patients with dry eye disease. Clin Ophthalmol. 2018;12:1237–43. doi:10.2147/OPTH.S164888.
- Asbell P, Vingrys AJ, Tan J, Ogundele A, Downie LE, Jerkins G, Shettle L. Clinical Outcomes of fixed versus as-needed use of artificial tears in dry eye disease: a 6-week, observer-masked phase 4 clinical trial. Invest Ophthalmol Vis Sci. 2018;59(6):2275–80. doi:10.1167/iovs.17-23733.
- Aragona P, Stefano GD, Ferreri F, Spinella R, Stilo A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjögren’s syndrome patients. Br J Ophthalmol. 2002;86(8):879–84. doi:10.1136/bjo.86.8.879.
- Coroi MC, Bungau S, Tit M. Preservatives from the eye drops and the ocular surface. Rom J Ophthalmol. 2015;59:2–5.
- Kaur IP, Lal S, Rana C, Kakkar S, Singh H. Ocular preservatives: associated risks and newer options. Cutan Ocul Toxicol. 2009;28(3):93–103. doi:10.1080/15569520902995834.
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–21. doi:10.1001/archopht.118.5.615.
- Streiner D, Geddes J. Intention to treat analysis in clinical trials when there missing. Data Evid Based Ment Health. 2001;4(3):70–71. doi:10.1136/ebmh.4.3.70.
- Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: intent-to-treat versus per-protocol analysis. Perspect Clin Res. 2016;7(3):144–46. doi:10.4103/2229-3485.184823.
- Moon JW, Lee H-J, Shin KC, Wee WR, Lee JH, Kim MK. Short term effects of topical cyclosporine and viscoelastic on the ocular surfaces in patients with dry eye. Korean J Ophthalmol. 2007;21(4):189–94. doi:10.3341/kjo.2007.21.4.189.
- Kenward MG, Molenberghs G. Last observation carried forward: a crystal ball? J Biopharm Stat. 2009;19(5):872–88. doi:10.1080/10543400903105406.
- Hom M, Kwan J. Prevalence of dry eye sub-types and severity of evaporative dry eye using objective tests. Invest Ophthalmol Vis Sci. 2013;54:4339.
- Ousler GW, Michaelson C, Christensen MT. An evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye drops. Cornea. 2007;26(8):949–52. doi:10.1097/ICO.0b013e3180de1c38.
- Hwang HO, Sung Y-M, Lee WS, Kim EC. Additive effect of preservative-free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea. 2014;33(9):935–41. doi:10.1097/ICO.0000000000000213.
- Fallarca A, Vertuani S, Panozzo G, Pecorelli A, Valacchi G, Manfredini S. Novel artificial tears containing cross-linked hyaluronic acid: an in vitro re-epithelization study. Molecules. 2017;22(12):2104. doi:10.3390/molecules22122104.
- Jun I, Choi S, Lee GY, Choi YJ, Lee HK, Kim EK, Seo KY, Kim TI. Effects of preservative-free 3% Diquafosol in patients with pre-existing dry eye disease after cataract surgery: a randomized clinical trial. Sci Rep. 2019;9(1):12659. doi:10.1038/s41598-019-49159-0.
- Walsh K, Jones L. The use of preservatives in dry eye drops. Clin Ophthalmol. 2019;13:1409–25. doi:10.2147/OPTH.S211611.
- Nasser L, Rozycka M, Gomez Rendon G, Navas A. A real-life results of switching from preservative-free artificial tears containing hyaluronate in patients with dry eye disease. Clin Ophthalmol. 2018;12:1519–25. doi:10.2147/OPTH.S160053.
- Bondemark L, Abdulraheem S. Intention to treat (ITT) analysis as reported in orthodontic randomized controlled trials evaluations of methodology and recommendations for the accurate use of ITT analysis and handling dropouts. Eur J Orthod. 2018;40(4):409–13. doi:10.1093/ejo/cjx084.