ABSTRACT
Purpose: Evaluate potential corneal biomechanical changes following corneal crosslinking (CXL) by paired differential tonometry intraocular pressure (IOP) measurements with a Goldmann tonometer (GAT) prism and corneal compensating, correcting applanation tonometry surface (CATS) prism.
Methods: IOP was measured prospectively on 23 unique eyes undergoing CXL for keratoconus with a GAT using a standard flat GAT prism and a curved corneal error correcting CATS prism before treatment and at 2 weeks, 2 months and 6 months after treatment. Concurrent measurements of central corneal thickness (CCT) and corneal hysteresis (CH) were completed.
Results: Paired IOP measurements with standard GAT and corneal correcting CATS prisms indicated a significant sustained relative increase in the differential IOP between the two prisms after CXL (p = .002,0.051,0.062). CH initially decreased at two weeks post-CXL then returned to sustained pre-op levels (p = .033,0.20,0.20). CCT progressively decreased following CXL (p = .005).
Discussion: Differential tonometry between standard GAT and corneal biomechanical compensating CATS prisms indicates findings consistent with increased corneal rigidity following CXL and may demonstrate a simple and sensitive method for measurement of relative corneal biomechanical changes due to pharmacologic agents and procedures.
Introduction
Corneal Crosslinking (CXL) is a common procedure used clinically to stabilize early progressive keratoconus improving visual acuity and reducing its rate of progression.Citation1 The results have shown corneal flattening and a reduction in corneal surface irregularity with biomechanical changes and stress redistribution of the cornea.Citation2
Corneal biomechanical properties have been measured over the last two decades clinically using an Ocular Response Analyzer (ORA) and more recently with Scheimpflug technology in the Corvis ST. Both are modified pneumotonometers that measure timing or visualization of corneal applanation and use this to calculate energy absorption or characteristic shape changes of the cornea.Citation3,Citation4 Studies using the ORA reported negligible changes in the biomechanical parameter, corneal hysteresis (CH) following CXL.Citation5–7 Likewise, the Corvis ST demonstrated no significant long-term changes in its corneal biomechanical parameters following CXL, except some early changes at one month.Citation8,Citation9 Ex-Vivo inflation tests have clearly demonstrated increased corneal stiffness following CXL.Citation10,Citation11 Several studies have demonstrated decreased Young’s modulus corneal stiffness (E) in eyes with keratoconus.Citation12,Citation13 Furthermore, an increased slope of the IOP sensitivity to CCT with increasing Young’s modulus stiffness (E) has been shown in several studies.Citation14,Citation15
Differential tonometry is the use of two different tonometers to sequentially measure IOP. Several studies have described differential tonometry to measure changes in corneal stress.Citation16–19 Recent clinical studies using a corneal indentation device (CID) to perform continuous differential tonometry have demonstrated in vivo measurement of corneal tangent modulus, by applying variable corneal applanation and determining the slope of the force displacement curve.Citation20–22
A modified curved applanating surface Goldmann prism (CATS) has been developed to replace the standard flat surfaced Goldmann prism on any Goldmann type tonometer. The design differences functionally include a centrally concave and annularly convex applanating surface which has been described in detail previously.Citation14 depicts the CATS prism applanating surface which has clinically demonstrated a decreased sensitivity to the corneal biomechanical factors CCT and CH compared to the GAT prism.Citation14,Citation23–27 The CATS prism applanating surface is a unique parametric solution to a matrix which includes the human probability distributions of the variables: (1) Young’s Modulus(E), (2) Overall Corneal Thickness (CT), and (3) Corneal curvature (CC). The mathematical model solution minimizes the prism surface’s sensitivity to all 3 of the corneal variables.Citation14 Intraocular pressure differences between the CATS and GAT measurements correlated significantly with variations in corneal biomechanical properties such as CH and CCT.Citation14,Citation23–27 Importantly, over a large standard population, no overall IOP bias is demonstrated between the two prisms.Citation14,Citation23–27 Both prisms measure the same pressure for corneas with nominal biomechanical properties, therefore, this provides evidence that the difference in CATS and GAT IOP is a direct measurement (in mm Hg) of the combined corneal biomechanical properties resisting its deformation.Citation23,Citation24 The present study evaluates sequential CATS minus GAT prism differential tonometry to measure changes in corneal biomechanical resistance to applanation following CXL. This study was supported in part by NIH SBIR Grant R43 EY026821-01 and Cornea Associates of Tucson, Tucson, AZ. Mingwu Wang has no relevant financial disclosures and was the principal investigator responsible for independently collecting and maintaining the data. John Berdahl and Sean McCafferty have a relevant financial disclosure as an advisor and officer respectively in Intuor Technologies (Tucson, AZ) owning the technology being examined in this clinical trial.
Figure 1. Photograph of the centrally concave and circumferentially convex applanating surface of the (CATS) prism

Methods
Enrollment included treatment naïve keratoconus patients. Patients were enrolled with demonstrated early progressive keratoconus indicating CXL treatment, except those who were unwilling to participate in the study or unable to commit to all follow-ups during the 6-month postop period. Subjects underwent a series of sequential IOP measurements with the modified (CATS) and standard GAT tonometer prisms. The study was designed as an open-labeled, prospective, controlled, device comparison and was prospectively registered at clinicaltrials.gov (NCT04180111). The clinical trial design and monitoring was approved by Chesapeake IRB (now Advarra). Eligible subject screening, enrollment, and evaluation were in accordance with the study protocol and were recruited from a single site in Tucson, AZ. Study participants included subjects 18 years or older who provided written informed consent and met the protocol criteria.
The clinical study was conducted in accordance with the ethical principles contained in Declaration of Helsinki. The study followed the Code of Federal Regulations (CRF), Institutional Review Boards (21 CFR 56), Obligations of Clinical Investigators (21 CFR 812), and Protection of Human Volunteers (21 CFR 50)
Description of study population
Early progressive keratoconus patients in stage 2 by ABCD classification without prior treatment were enrolled into the study. The present study is comparing GAT and CATS measurements for thin corneas (< 520 um CCT). In a previous study, we found that the difference between GAT and CATS readings had an average of −1.35 mmHg (GAT minus CATS IOP reading) with a standard deviation of 2.38 mmHg across 42 paired readings.Citation23 The 23 eye sample size was determined using preceding study mean differences and standard deviations with a power = 0.85 and an α = 0.05 (2-tailed), β = 0.2.
Exclusionary conditions prevented participation in the study including: Conditions which may confound the study results such as corneal scars, disease, disorders, or infection, or lid conditions, as well as prior ocular surgery. Pregnant or nursing women were also excluded.
Protocol
Enrolled participants received a complete ophthalmic exam from one of the independent investigators, which included a diagnosis progressive keratoconus without prior surgical treatment. Assistant investigators used an Ocular Response Analyzer (ORA) to measure corneal hysteresis (CH) (Reichert, Inc, Depew, NY). Likewise, Central corneal thickness (CCT) was measured using a Zeiss HD-OCT-5000 spectral domain ocular coherence tomographer (Zeiss, Jena, Germany). Corneal crosslinking (S-CXL(3*30) with the epithelium removed was completed with an Avedro model KXL (Waltham, MA) using Photrexa (Avedro, Waltham, MA) riboflavin 5-phosphate in 20% dextran solution (). A standard post-operative regimen of drops was used for 4 weeks with prednisolone acetate 1% four times daily, nepafenac 0.5% once daily, and ofloxacin 0.3% four times daily.
Table 1. Corneal cross-linking procedure parameters
Independent Investigators under the direction of the P.I. measuring IOP remained masked to the CCT and CH results of the assistant investigator. Randomized sequential utilization of CATS and GAT prism devices was completed for IOP measurement. Fluorescein with topical anesthetic (fluorescein sodium and benoxinate hydrochloride ophthalmic solution 0.25%/0.4%, Bausch & Lomb, Tampa, FL) was instilled prior to each measurement. Randomized masked sequential IOP measurement was completed initially with either the CATS or GAT prism placed in a calibrated Haag-Streit model 900 applanation tonometer (Mason, OH) and the prisms were then alternated. Two pressure measurements were made each with a CATS and GAT prism during one determination of the differential tonometry. Each measurement consisted of two averaged measurements at 180 and 90 degrees to correct for astigmatism. All measurements were completed before CXL and at 2 weeks, 2, and 6 months following the CXL procedure.
Endpoints
The primary endpoint consisted of IOP measurement comparisons between the GAT and modified (CATS) prisms before and after CXL. Corneal biomechanical parameters CH and CCT were measured at the same time intervals before and after CXL for corroborating analyses.
Statistical methods
Primary and validating endpoints utilized the Full Analysis Set (FAS) which included all eligible eyes.
Continuous variables formulated all descriptive statistics including mean, standard deviation, median, and range. Paired, 2-tailed t-test (α = 0.05) were used to analyze the primary endpoint. A bivariate linear regression analysis of the paired differential GAT and CATS IOP measurements was correlated to CH and CCT. A general linear mixed effects (GLME) model was used to examine effects of CCT, IOP, CH, age, and gender.
Results
Twenty-three (23) unique eyes were measured from 23 patients, . Five (5) eyes did not complete all measurements. Study completion rates were 2 weeks; n = 23, 2 months; n = 19; 6 months; n = 18. There were 16 (69%) males and 7 (31%) females with an average age of 26±4 years who were enrolled. Patients had an average pre-operative CCT of 482±29 µm and an average CH of 7.5±2.1 mmHg.
Table 2. CXL patient demographics
CATS and GAT differential IOP analysis
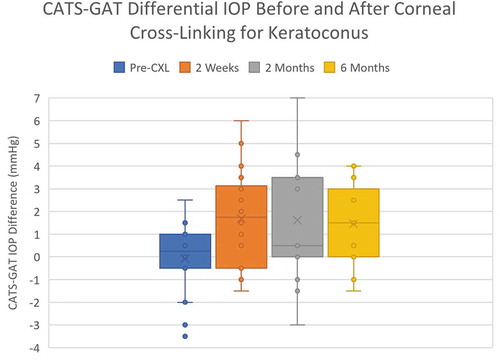
The combined paired differential IOP measurements over all time intervals of CATS minus GAT increased from −0.08 ±1.58 mmHg to an average sustained +1.75±2.15 mmHg following CXL (p = .0009). . Individually, each post-operative time point (2 weeks, 2 months and 6 months) measured an increased differential CATS minus GAT IOP (p = .002, 0.051, 0.062). The average GAT IOP at 2 weeks following CXL increased significantly from 12.5±3.2 mmHg to 17.0±4.0 mmHg (p = .008). The average GAT IOP remained increased albeit lower at 6 months, 12.8±3.1 (p = .11). Likewise, average CATS IOP increased significantly following CXL from 12.8±3.6 mmHg to 18.1 mmHg and reduced but remained elevated at 6 months to 13.59±3.2 mmHg (p = .004,p = .047).
Corneal hysteresis and CCT validation
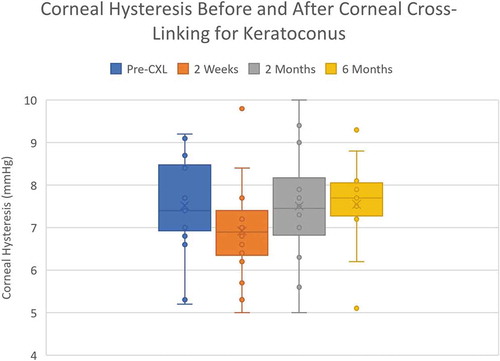
Corneal hysteresis (CH) decreased significantly at 2 weeks following CXL from 7.5±2.1 mmHg to 6.9±1.1 mmHg (p = .033), . However, the CH returned to the sustained pre-op levels at the 2 and 6-month examination times 7.5±1.48 mmHg and 7.5±1.42 mmHg, respectively (p = .20,0.20). Likewise, differential IOPcc-GAT was examined and shown to have a similar response as corneal hysteresis with a transient increase at 2 weeks (p = .081).
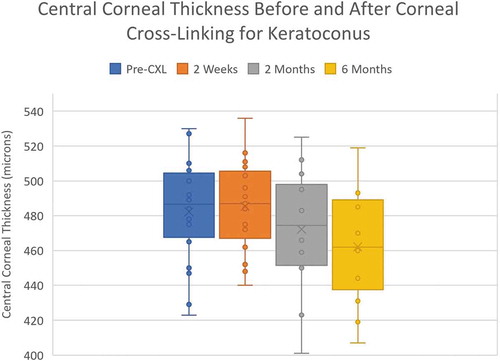
Central corneal thickness (CCT) following CXL significantly and progressively decreased by 6.2% at 6 months from 482±29 µm to 452±31 µm (p = .005), .
General linear mixed effects (GLME) model did not demonstrate significant correlations between CATS and GAT differential IOP measurements except at the two-week period. .
Table 3. Values for coefficient estimates and corresponding p-values are shown in the following tables for each session. P-values < 10% are highlighted orange, yellow for 10–30%, green 30–50%, and >50% blue
Age, time of measurement, CCT, and gender did not correlate to the difference in CATS and GAT IOP measurements (). Age and IOP were within a comparatively narrow range to studies on corneal biomechanics and IOP.Citation14,Citation15
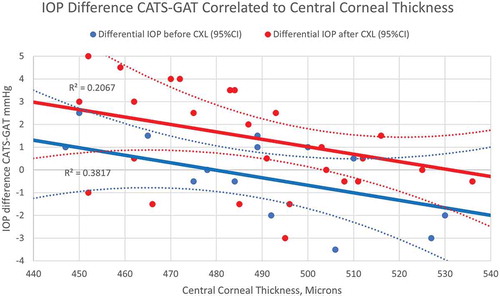
demonstrates an overall elevation of the linear CCT correlation following CXL. There is a correlation to CCT before CXL (R2 = 0.3817,p = .081) and after CXL (R2 = 0.2067, p = .055).
Discussion
Significantly increased and sustained differential IOP was demonstrated following corneal cross linking for early progressive keratoconus. CATS minus GAT differential IOP may measure small corneal biomechanical changes due to procedures affecting corneal biomechanics. The results are consistent with multiple studies exhibiting the same CATS minus GAT differential tonometry correlation to CCT and confirm the CATS-GAT differential tonometry measures a higher CATS compared to GAT IOP with thinner corneas.Citation14,Citation23–27 These results are validated by the concurrent findings of early but unsustained CH changes at 2 weeks and progressive CCT thinning, which are also found in other studies.Citation2,Citation8,Citation9 The transient increase in IOPcc-GAT at 2 weeks is expected as IOPg is calibrated to GAT with a CH correction to obtain IOPcc. These are similar to previous findings of IOPcc and GAT before and after CXL demonstrating a transient differential at 1 month after CXL.Citation28 The moderate progressive decrease in CCT may be a partial reason for the immediate and sustained increase in differential tonometry.
The differential tonometry correlation to CCT, in , demonstrated a statistically significant upward shift following CXL while maintaining the same slope. The upward shift places the CCT correlation differential tonometry similar to what is seen in normal corneas without disease.Citation14,Citation23–27 This suggests that CXL likely re-establishes a more “normal” biomechanical behavior to the keratoconic cornea. Results indicate there is a negligible overall difference between CATS and GAT IOP measurements in the young progressive keratoconic cornea with the exception of the expected CCT correlation. However, following CXL, the slope, orientation and thin CCT bias of CATS-GAT differential tonometry is re-established to that seen in non-diseased corneas.Citation14,Citation23–27
The lack of difference in CATS-GAT IOP in the untreated keratoconic cornea is not consistent with what would be expected in a non-diseased thin cornea.Citation14,Citation23–27 Prior studies indicate a significantly higher CATS IOP than GAT in normal corneas under 500 microns. However, it has been demonstrated by Dynamic Contour Tonometry (DCT) that there is no difference in mean IOP between normal controls and keratoconic corneas despite significant differences in CCT.Citation28 Furthermore, DCT IOP has been shown in normal corneas to be independent of CCT, whereas GAT IOP is correlated to CCT.Citation28 Similarly, CATS IOP has been shown to be nearly independent of CCT whereas GAT IOP is dependent upon CCT.Citation14,Citation23–27
Evidence in this study suggests that the CXL procedure re-establishes the more normal corneal behavior seen previously in CATS-GAT differential tonometry () and this is consistent in most clinical findings with a reduction of corneal irregularity.Citation2 Other studies have shown a significant increase in GAT IOP following CXL suggesting an increase in global corneal stiffness, which is also demonstrated in the present study, albeit reduced at 6 months.Citation29 These results are likely consistent with increased stiffness of the cornea following CXL. This type of corneal surface stress redistribution and increased corneal stiffness has been demonstrated in other treatment modalities which also induce corneal collagen contraction such as CO2 and excimer laser irradiation.Citation30,Citation31
To fully explain these results with increased differential tonometry following CXL, a review of the CATS tonometer prism design is helpful. The CATS Tonometer-applanating surface is a solution to a matrix which includes the human probability distributions of the variables: (1) Young’s Modulus(E), (2) Overall Corneal Thickness (CT), and (3) Corneal curvature (CC). The mathematical model minimizes the prism surface’s sensitivity to all 3 of the corneal variables.Citation14 Optimization of the prism surface to minimize sensitivity to the corneal biomechanical properties produced overall bias between CATS and GAT, which was rectified by increasing the applanation area until both prisms measured the same for a nominal E, CT, and CC. This effectively maintained the reduced sensitivity to E, CT, and CC, but provided equal IOP measurements between CATS and GAT for nominal corneal biomechanical properties, including a CCT of 550 microns.Citation24
Several studies have demonstrated decreased Young’s modulus corneal stiffness (E) in eyes with keratoconus as well as increased corneal stiffness following CXL.Citation12,Citation13 Furthermore, an increased slope of the IOP sensitivity to CCT with increasing Young’s modulus stiffness (E) has been shown in several studies.Citation14,Citation15 is an illustrated adaptation from other studies of GAT and CATS IOP vs. CCT slope findings with increasing corneal stiffness which generally incorporates the average present study’s findings.Citation14,Citation15 It illustrates the increase in IOP following CXL with an increased slope representative of an increase in corneal stiffness. The well-supported increased slope of IOP vs. CCT with increased corneal stiffness leads to an increase in CATS-GAT differential tonometry () in the representative thin keratoconic cornea following CXL. While the cornea is thinner following CXL, it is not the sole contribution to the increase in CATS-GAT differential tonometry. Furthermore, global corneal stiffness is a function of the intrinsic corneal stiffness, Young’s modulus (E), plus the geometric parameter of corneal thickness (CT) which are not separable in measurement by differential tonometry alone.
Figure 6. Illustrated increased IOP vs. CCT slope with increase corneal stiffness depicting the CATS-GAT differential tonometry before and after CXL
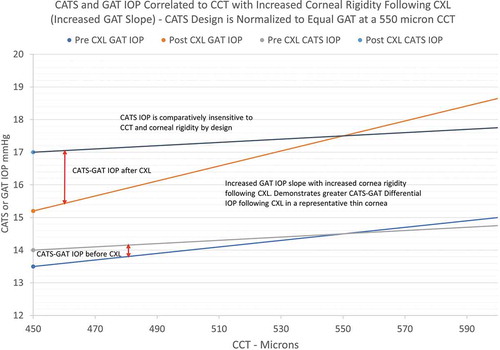
illustrates the representative upward shift in differential tonometry seen in due to the increased global stiffness (E plus CT). also maintains the expected CATS-GAT differential IOP slope to CCT before and after CXL.
Several studies have used differential tonometry to measure changes in corneal rigidity.Citation16–19 The differential tonometry between CATS and GAT IOP may likely demonstrate characteristic changes following pharmacological and surgical interventions which are known to alter corneal biomechanics with the advantage that the CATS and GAT prisms were designed to have zero overall bias using the same measurement device (alternating prisms) and the same measurement technique.Citation19,Citation24 Potential future studies include examining differential CATS-GAT IOP measurement indicating biomechanical changes following LASIK and corneal transplant procedures. Also, the uncharacteristic finding of minimal differential CATS-GAT IOP in a thin cornea may be diagnostic of biomechanical behaviors seen in early keratoconus and ectatic corneal disease processes.
List of abbreviations
| GAT | = | Goldmann applanation tonometer |
| IOP | = | Intraocular Pressure |
| CXL | = | Corneal cross linking |
| CATS | = | Correcting applanation tonometry surface |
| CCT | = | Central Corneal Thickness |
| CH | = | Corneal Hysteresis |
| FDA | = | Food and Drug Administration |
| ANSI | = | American National Standards Institute |
| ISO | = | International Standards Organization |
| CFR | = | Code of Federal Regulations |
| ORA | = | Ocular Response Analyzer |
| IRB | = | Independent Review Board |
| CID | = | Corneal Indentation Device |
Authors’ contributions
All authors meet the ICMJE authorship criteria
M.W. - Design, Data acquisition, drafting, analysis, and interpretation
J.B.- Drafting, analysis, and interpretation
S. M. – Design, drafting, analysis, and interpretation.
Ethics and consent to participate
This clinical study was conducted in accordance with the ethical principles contained within Declaration of Helsinki.
Financial disclosures
Mingwu Wang has a no financial disclosures.
Sean McCafferty is an officer in Intuor Technologies (Tucson, AZ) owning the technology being examined in this clinical trial.
John Berdahl is a consultant to Intuor.
Acknowledgements
Cornea Associates, Tucson, AZ for extensive facilities use. Cynthia Roberts, PhD – methodology and discussion review.
Additional information
Funding
References
- Hersh P, Greenstein S, Fry K. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:149–60. doi:https://doi.org/10.1016/j.jcrs.2010.07.030.
- Roberts C, Dupps W. Biomechanics of corneal ectasia and biomechanical treatments. J Cataract Refract Surg. 2014;40:991–98. doi:https://doi.org/10.1016/j.jcrs.2014.04.013.
- Deol M, Taylor D, Radcliffe N. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26:96–102. doi:https://doi.org/10.1097/ICU.0000000000000130.
- Amano S, Nejima R, Inoue K. Effect of topical prostaglandins on the biomechanics and shape of the cornea. Graefes Arch Clin Exp Ophthalmol. 2019;10:1–7.
- Gkika M, Labiris G, Giarmoukakis A, Koutsogianni A, Kozobolis V. Evaluation of corneal hysteresis and corneal resistance factor after corneal cross-linking for keratoconus. Graefes Arch Clin Exp Ophthalmol. 2012;250:565–73. doi:https://doi.org/10.1007/s00417-011-1897-0.
- Beckman R, Behndig A, Hallberg P, Lindén C. Increased corneal hysteresis after corneal collagen crosslinking: a study based on applanation resonance technology. JAMA Ophthalmol. 2014;132:1426–32. doi:https://doi.org/10.1001/jamaophthalmol.2014.3029.
- Spoerl E, Terai N, Scholz F, Raiskup F, Pillunat L. Detection of biomechanical changes after corneal cross-linking using ocular response analyzer software. J Refractive Surg. 2011;27:452–57. doi:https://doi.org/10.3928/1081597X-20110106-01.
- Bak-Nielsen S, Pedersen IB, Ivarsen A, Hjortdal J. Dynamic Scheimpflug-based assessment of keratoconus and the effects of corneal cross-linking. J Refract Surg. 2014;30:408–14. doi:https://doi.org/10.3928/1081597X-20140513-02.
- Vinciguerra R, Romano V, Arbabi E, Brunner M, Willoughby C, Batterbury M, Kaye S. In vivo early corneal biomechanical changes after corneal cross-linking in patients with progressive keratoconus. J Refract Surg. 2017;33:840–46. doi:https://doi.org/10.3928/1081597X-20170922-02.
- Kling S, Remon L, Pérez-Escudero A, Merayo-Lloves J, Marcos S. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Invest Ophthalmol Vis Sci. 2010;51(8):3961–68. doi:https://doi.org/10.1167/iovs.09-4536.
- Palko J, Tang J, Cruz Perez B, Pan X, Liu J. Spatially heterogeneous corneal mechanical responses before and after riboflavin-ultraviolet-A crosslinking. J Cataract Refract Surg. 2014 Jun;40(6):1021–31. doi:https://doi.org/10.1016/j.jcrs.2013.09.022.
- Edmund C. Corneal elasticity and ocular rigidity in normal and keratoconic eyes. Acta Ophthalmol. 1988;66:134–40. doi:https://doi.org/10.1111/j.1755-3768.1988.tb04000.x.
- Vinciguerra R, Romano V, Arbabi E, Brunner M, Willoughby CE, Batterbury M, Kaye SB. In vivo early corneal biomechanical changes after corneal cross-linking in patients with progressive keratoconus. J Refract Surg. 2017;33:840–46. doi:https://doi.org/10.3928/1081597X-20170922-02.
- McCafferty S, Lim G, Duncan W, Enikov E, Schwiegerling J. Goldmann tonometer prism with an optimized error correcting applanation surface. Transl Vis Sci Technol. 2016;5:1–5. doi:https://doi.org/10.1167/tvst.5.5.4.
- Liu J, Roberts C. Influence of cornea biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cat and Ref Surg. 2005;31:146–55. doi:https://doi.org/10.1016/j.jcrs.2004.09.031.
- Detorakis E, Tsaglioti E, Kymionis G. Non-invasive ocular rigidity measurement: a differential tonometry approach. Acta Medica. 2015;58:92–97. doi:https://doi.org/10.14712/18059694.2015.99.
- Moiseeva I, Stein A, Lyubimov G. Estimating the elastic properties of the eye from differential tonometry by the Schiøtz tonometer: analysis of the measurement procedure on the basis of a two-component mathematical model. BIOPHYSICS. 2016;61:1011–18. doi:https://doi.org/10.1134/S000635091606018X.
- Schneider E, Grehn F. Intraocular pressure measurement-comparison of dynamic contour tonometry and goldmann applanation tonometry. J Glaucoma. 2006;15:2–6. doi:https://doi.org/10.1097/01.ijg.0000196655.85460.d6.
- Radcliffe N, Berdahl J, Ibach M, Schweitzer J, Levine J, McCafferty S. Improved efficacy of topical latanoprost 0.005% demonstrated by corneal biomechanical correcting modified goldmann prism. Clin Ophthalmol. 2020;14:2245–53. doi:https://doi.org/10.2147/OPTH.S264055.
- Lam A, Hon Y, Leung L, Lam D. Repeatability of a novel corneal indentation device for corneal biomechanical measurement. Ophthalmic Physiol Opt. 2015;35:455–61. doi:https://doi.org/10.1111/opo.12219.
- Hon Y, Chen G, Lu S, Lam D, Lam A. In vivo measurement of regional corneal tangent modulus eye. Sci Rep. 2017;7:14974. doi:https://doi.org/10.1038/s41598-017-14750-w.
- Lu SH, Chong IT, Leung SYY, Lam DCC. Characterization of corneal biomechanical properties and determination of natural intraocular pressure using CID-GAT. Transl Vis Sci Technol. 2019;8:10. doi:https://doi.org/10.1167/tvst.8.5.10.
- McCafferty S, Tetrault K, McColgin A, Chue W, Levine J, Muller M. Modified Goldmann prism intraocular pressure measurement accuracy and correlation to corneal biomechanical metrics: multicentre randomised clinical trial. Br J Ophthal. 2019;0:1–5.
- McCafferty S, Tetrault K, McColgin A, Chue W, Levine J, Muller M. Intraocular pressure measurement accuracy and repeatability of a modified Goldmann prism: multi-center randomized clinical trial. Am J Ophthal. 2018;196:145–53. doi:https://doi.org/10.1016/j.ajo.2018.08.051.
- McCafferty S, Lim G, Duncan W, Enikov E, Schwiegerling J. Goldmann tonometer error correcting prism: clinical evaluation. Clin Ophthalmol. 2017;11:835–40. doi:https://doi.org/10.2147/OPTH.S135272.
- McCafferty S, Levine J, Schwiegerling J, Enikov ET. Goldmann and error correcting tonometry prisms compared to intracameral pressure. BMC Ophthalmol. 2018;1:8.
- McCafferty S, Enikov E, Schwiegerling J, Ashley S. Goldmann tonometry tear-film error and partial correction with a shaped applanation surface. Clin Ophthal. 2018;1:5.
- Read S, Collins M. Intraocular pressure in keratoconus. Acta Ophthalmol. 2011;89:358–64. doi:https://doi.org/10.1111/j.1755-3768.2009.01690.x.
- Romppainen T, Bachmann L, Kaufmann C, Kniestedt C, Mrochen M, Thiel M. Effect of riboflavin-UVA–induced collagen cross-linking on intraocular pressure measurement. Invest Ophthalmol Vis Sci. 2007;48:5494–98. doi:https://doi.org/10.1167/iovs.06-1479.
- McCafferty S, Schwiegerling J, Enikov E. Thermal load from a CO2 laser radiant energy source induces changes in corneal surface asphericity, roughness, and transverse contraction. Invest Ophthalmol Vis Sci. 2012;53:4279–88. doi:https://doi.org/10.1167/iovs.12-9579.
- McCafferty S, Schwiegerling J, Enikov E. Corneal surface asphericity, roughness, and transverse contraction after uniform scanning excimer laser ablation. Invest Ophthalmol Vis Sci. 2012;53:1296–305. doi:https://doi.org/10.1167/iovs.11-9267.