ABSTRACT
Objective
To investigate the correlation between serum immunoglobulin E (IgE) levels and severity of Vogt–Koyanagi–Harada (VKH) disease.
Methods
The medical records of patients with VKH disease between 2015 and 2020 were reviewed. Serum immunoglobulins (IgA, IgE, IgG, and IgM), tumor necrosis factor α (TNFα) and C-reactive protein (CRP) were measured. Patients were divided into IgE-positive (IgE ≥ 100 IU/mL) and IgE-negative (IgE < 100 IU/mL) groups. The best-corrected visual acuity (BCVA) and macular morphologic characteristics including foveal thickness (FT), serous retinal detachment (SRD), sensory retinal thickness (SRT), central foveal thickness (CFT), cube volume (V), and cube average thickness (AT) were determined in patients in both groups.
Results
Of 128 patients included in the study, 35 (27.34%) patients were IgE-positive, BCVA (logMAR) was worse in the IgE-positive group. The mean CRP (P= .012) and TNFα (P≤ 0.001) levels were greater in the IgE-positive group than in the IgE-negative group. Regarding macular morphologic characteristics, FT (P= .010), SDR (P= .004), CFT (P= .008), V (P= .013), and AT (P= .006) were significantly greater in the IgE-positive group than in the IgE-negative group.
Conclusions
Elevated serum IgE levels were associated with more severe macular changes in patients with VKH disease. These findings suggest that IgE may be involved in the progression of VKH disease.
Introduction
Vogt–Koyanagi–Harada (VKH) disease is a multisystemic, autoimmune inflammatory disorder with ophthalmic, auditory, dermatologic, and neurologic manifestations.Citation1 Serous retinal detachment (SRD) in the acute phase and sunset glow fundus during the chronic phase are the most common ocular manifestations of VKH disease.Citation1,Citation2 However, the etiology and pathogenesis of VKH disease are complex. Although many aspects of its pathogenesis are unknown, one mechanism appears to involve autoimmune targeting of melanocytes that is mediated by CD4+ T cells.Citation3 These activated T cells may initiate the inflammatory processes in individuals lacking T regulatory cells and altered tolerance to melanocytes by increasing the release of interleukin (IL)-17 and IL-23. However, the trigger that induces the altered tolerance to melanocytes is still unknown.Citation4,Citation5
Immunoglobulin E (IgE) is an immune antibody class that mediates type I hypersensitivity and immunity to parasites. It mainly binds to polyvalent antigens and cross-links to the surface receptors expressed on mast cells and basophils to promote cellular degranulation and inflammatory responses.Citation6 Permin and Wiik demonstrated that IgE autoantibodies are involved in the composition of immune complexes.Citation7 They also reported that IgE may enhance the deposition of immune complexes by mediating the release of vasoactive amines, leading to increased vascular permeability.Citation7 Recent studies have revealed elevated serum IgE levels and detected the presence of IgE autoantibodies in patients with autoimmune diseases, including systemic lupus erythematosus and bullous pemphigoid.Citation6,Citation8 In a study of patients with various forms of autoimmune uveitis, such as acute iris ciliary inflammation, Eales disease, pars planitis, and multifocal choroiditis, it was found that the serum IgE levels were higher in patients with these disorders than in normal control groups.Citation9,Citation10 To our knowledge, however, no studies have reported whether IgE is associated with VKH disease.
Although fundus fluorescein angiography (FFA) plays an important role in the diagnosis of VKH disease, optical coherence tomography (OCT) is becoming increasingly important and is replacing other imaging modalities in the diagnosis of acute and chronic VKH disease by revealing exudative retinal detachment in the acute stage and choroidal thickening or thinning in the chronic stage.Citation3 Using FFA and OCT images, it is possible to determine the severity of exudative retinal detachment and retinal edema, which reflect disease severity.
Therefore, in this study, we reviewed the medical records of patients with primary acute VKH disease who were admitted to our hospital at disease onset. We compared the macular morphologic characteristics determined by OCT between patients with different serum IgE levels to explore the association between IgE levels and severity of VKH disease.
Methods
Patients
We performed a retrospective analysis of patients with newly diagnosed VKH disease who were admitted to Shanghai Xuhui Central Hospital, Shanghai, China, between August 2015 and April 2020. Patients were eligible for this study if they satisfied the diagnostic criteria for acute VKH disease (described in section 2.2). Patients with a history of eye surgery, other systemic immune diseases, developmental abnormalities, and genetic diseases were excluded. Patients who could not cooperate with the examinations or had corneal or lenticular opacity were also excluded. The best-corrected visual acuity (BCVA), anterior segment signs, and time from onset to admission were retrieved from the patients’ medical records. BCVA was converted to logarithms of the minimum angle of resolution (logMAR). This study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Xuhui Central Hospital (2020–179). All of the patients consented to participate in the study.
Diagnostic criteria for acute VKH disease
The diagnostic criteria for acute VKH was as followsCitation2: (A) no history of ocular trauma or intraocular surgery; (B) bilateral ocular involvement (onset within 2 weeks in both eyes); (C) no evidence of infectious uveitis, accompanying systemic rheumatic disease, or other ocular diseases; and (D1) signs of diffuse choroiditis and exudative retinal detachment; (D2) SRD on OCT or B-scan ultrasonography; (D3) choroidal thickening on enhanced depth imaging OCT; (D4) early punctate staining and late subretinal dye pooling on FFA; and (D5) hyperfluorescence of the optic disc on FFA. Only patients who satisfied criteria A–C plus D1, or D2 and D3, or D4 were considered to have acute VKH disease.
Measurement of serum immunoglobulin, C-reactive protein, and tumor necrosis factor α levels
Venous blood samples were obtained from fasting patients. The serum was separated and stored at −20°C until testing. Rate-scattering turbidimetry was used to measure immunoglobulin levels on an automatic analyzer (BN II, Siemens, Germany). C-reactive protein (CRP) and tumor necrosis factor α (TNFα) levels were measured using enzyme-linked immunosorbent assay kits (R&D Systems). The reference range for serum IgE (0–100 IU/mL) was determined according to the reagent specification. Serum IgE levels above the upper limit of the reference range were considered to be elevated, and patients were divided into IgE-positive (IgE ≥ 100 IU/mL) or IgE-negative (IgE < 100 IU/mL) groups.
OCT examination of the macular area
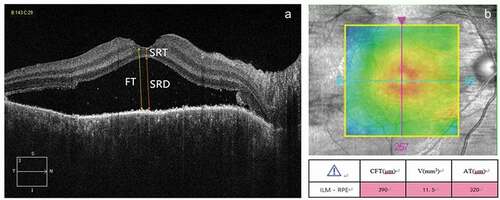
OCT was done at the time of admission to our hospital. The macular area was scanned using an OCT scanner (Cirrus HD-OCT 4000, Zeiss, Germany) with software version 4.0. All patients were scanned in the five-line raster and macular cube modes. In the five-line raster scanning mode, the distance between each line was 0.25 mm and transverse scanning was performed centered on the fovea. The software’s caliper function was used to manually measure foveal thickness (FT), SRD, and sensory retinal thickness (SRT). The boundary for FT was set from the internal limiting membrane to the inner boundary of RPE (). Each measurement was repeated twice, and the average value was recorded. In the macular cube mode (512 × 128), a square region (6.0 × 6.0 mm) centered on the fovea was scanned. The central foveal (central 1 mm subfield) thickness (CFT), cube volume (V), and cube average thickness (AT), which was measured across the entire 6.0 × 6.0 mm scan area, were automatically measured using the software ().
Figure 1. Representative optical coherence tomography images of the fovea. (a) The horizontal B scan was used to manually measure foveal thickness (FT), serous retinal detachment (SRD), and sensory retinal thickness (SRT). (b) The macular cube scan was used to automatically measure the central fovea thickness (CFT), cube volume (V) and cube average thickness (AT) .
Statistical analysis
SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Continuous data are presented as the mean ± standard deviation. Normal distribution was tested using the Kolmogorov–Smirnov test. The independent samples t test was used to compare normally distributed variables and the Mann–Whitney U test was used to compare non-normally distributed variables between the IgE-positive and IgE-negative groups. Pearson’s χ2 test was used to test categorical variables. In all analyses, P< .05 was considered statistically significant.
Results
General clinical characteristics
Our study included 128 patients, of which 59 were males (46.09%). The mean age was 40.91 ± 13.30 years and the mean time between symptom onset and admission was 16.73 ± 16.19 days. The mean BCVA (logMAR) was 0.74 ± 0.54.
The serum IgE level was elevated in 35 patients (27.34%; IgE-positive group), of which 23 were males (65.71%). The other 93 patients were included in the IgE-negative group, of which 36 were males (38.71%). shows the general characteristics of the two groups. There were significant differences in the gender distribution (P = .006) and BCVA (P = .039) between the IgE-positive and IgE-negative groups. The mean age at onset and the time between onset and diagnosis were not significantly different between the two groups (P= .212 and 0.702, respectively). Anterior segment inflammatory signs were found in 26 patients (74.29%) in the IgE-positive group and 74 patients (79.57%) in the IgE-negative group, although this difference was not significant (P= .519).
Table 1. Comparison of general characteristics between the IgE-positive and IgE-negative groups
Serum IgE, CRP, and TNFα levels were elevated in the IgE-positive group
The serum immunoglobulin A (IgA), G (IgG), and M (IgM), and IgE, CRP, and TNFα levels were compared between the IgE-positive and IgE-negative groups, and the results are shown in . There were no significant differences in the levels of IgA (P= .868), IgG (P= .693), and IgM (P= .831) between the two groups. However, the serum CRP (P= .012) and TNFα (P≤ 0.001) levels were significantly greater in the IgE-positive group compared with the IgE-negative group.
Table 2. Comparison of serum immunoglobulin, CRP, and TNFα levels between the IgE-positive and IgE-negative groups
FT, SDR, CFT, V, and AT were greater in the IgE-positive group
Comparisons of FT, SDR, SRT, CFT, V, and AT between the IgE-positive and IgE-negative groups are shown in . FT (P= .010), SDR (P= .004), CFT (P = .008), V (P = .013), and AT (P = .006) were significantly greater in the IgE-positive group compared with the IgE-negative group. SRT was not significantly different between the two groups (P = .089).
Table 3. Comparison of macular area morphologic characteristics between the IgE-positive and IgE-negative groups
BCVA was positively correlated with FT, SDR, CFT, V, and AT
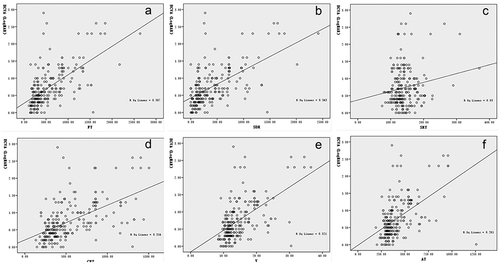
BCVA (logMAR) was positively and significantly correlated with FT, SDR, CFT, V, and AT (r = 0.62, P ≤ 0.001), SDR (r = 0.59, P ≤ 0.001), CFT (r = 0.56, P ≤ 0.001), V (r = 0.57, P ≤ 0.001) and AT (r = 0.51, P ≤ 0.001), but not with SRT (r = 0.17, P= .006). The correlations between BCVA and OCT macular parameters are shown in .
Figure 2. Scatterplots showing correlations between BCVA (logMAR) and macular parameters determined by OCT. (a) Correlation between BCVA (logMAR) and FT. The correlation coefficient was 0.62. (b) Correlation between BCVA (logMAR) and SDR. The correlation coefficient was 0.59. (c) Correlation between BCVA (logMAR) and SRT. The correlation coefficient was 0.17. (d) Correlation between BCVA (logMAR) and CFT. The correlation coefficient was 0.56. (e) Correlation between BCVA (logMAR) and V. The correlation coefficient was 0.57. (f) Correlations between BCVA (logMAR) and AT. The correlation coefficient was 0.51.
Discussion
The proportion of patients with elevated serum IgE levels varies between autoimmune diseases.Citation11,Citation12 Most studies have shown that elevated serum IgE is strongly associated with disease activity.Citation11–13 Onat reported that the mean plasma IgE level was significantly greater in patients with Behçet’s disease compared with controls, and about 44% of the patients had IgE levels exceeding the upper limit of normal.Citation14 Likewise, Cengiz reported that the serum IgE concentrations were greater patients with Behcet’s disease than those in controls, and the serum IgE levels were positively correlated with disease duration.Citation15
In our study of 128 patients with acute VKH disease, 27.34% had elevated serum IgE levels. Despite similar age at onset and time from symptom onset to diagnosis, patients with elevated serum IgE levels had worse visual acuity, suggesting that elevated serum IgE may be associated with more severe acute VKH disease. We also found that males were more likely to have elevated serum IgE.
CRP is a sensitive marker of inflammatory reactive diseases and is elevated in a variety of inflammatory disorders.Citation16 A significant correlation between CRP and disease activity was demonstrated in patients with Crohn’s disease.Citation17 CRP-mediated inflammatory responses include complement pathways, apoptosis, phagocytosis, nitric oxide release, and cytokine production, especially IL-6 and TNFα.Citation18 TNFα is a proinflammatory cytokine. When the body experiences inflammatory stimulation, activated macrophages, T lymphocytes, mast cells, and endothelial cells start producing TNFα.Citation19 TNFα is elevated in a variety of disorders, including idiopathic uveitis, VKH disease, and Behcet’s disease, and its levels are closely correlated with disease activity.Citation20–22 In the present study of patients with acute VKH disease, we found that the serum CRP and TNFα levels were significantly elevated in the IgE-positive group, suggesting that inflammatory activity was more severe in this group than in the IgE-negative group.
Further analysis of the macular morphology revealed that FT, SDR, CFT, V, and AT were all significantly greater in the IgE-positive group than in the IgE-negative group, although there was no significant difference in SRT. These results indicate that retinal leakage, inflammatory activity, and macular edema in patients with acute VKH are more severe in patients with elevated serum IgE. Additionally, we found that BCVA was positively correlated with FT, SDR, CFT, V, and AT. These findings suggest that visual acuity worsens with aggravation of inflammation and macular edema.
IgE may be involved in the pathogenesis of VKH disease by binding of antigen-bound IgE to its high-affinity receptor, FcεRI, which promotes complement inflammatory responses, as well as the recruitment and activation of macrophages, neutrophils, mast cells, and eosinophils. This induces the release of TNFα, VEGF and other inflammatory factors.Citation23 Sustained production of inflammatory factors causes vasculogenesis and damages the blood eye barrier.Citation24 In addition, in patients with uveitis, it was reported that TNFα stimulates choroid endothelial cell production of VEGF, which enhances vascular permeability and leakage.Citation25,Citation26
To our knowledge, this is the first study to explore the association between IgE and VKH disease. However, this study has some limitations. First, we only measured serum total IgE levels in patients with acute VKH disease, without measuring IgE-type autoantibodies. Second, we did not explore whether the IgE levels are elevated in later stages of VKH disease. Third, the study did not include a longitudinal follow-up or examine the correlation between IgE levels and disease activity over time, which require further study. Finally, the OCT 4000 scanner does not provide sufficient resolution to determine the choroidal thickness, so we were unable to measure this parameter or determine its correlation with the IgE level.
Conclusion
Our results suggest that elevated serum IgE levels are associated with more active inflammation and more severe vascular leakage in the primary acute phase of VKH disease. The findings also suggest that IgE may be an involved factor in the progression of VKH disease. The role of IgE and the reason for the elevated IgE in VKH disease need further investigation. Targeting IgE may offer a new therapeutic approach for patients with VKH disease, especially those who fail to respond to corticosteroids or immunosuppressive therapy.
Ethics statement
Ethical approval for this study was obtained from the Shanghai Xuhui Central Hospital Ethics Committee of Shanghai Xuhui Central Hospital.
Acknowledgments
The authors thank Min Zhou (Eye and ENT Hospital of Fudan University) for her clinical advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rao NA, Gupta A, Dustin L. Frequency of distinguishing clinical features in Vogt-Koyanagi-Harada disease. Ophthalmology. 2010;117:591–9,599. e1. doi:https://doi.org/10.1016/j.ophtha.2009.08.030.
- Yang P, Zhong Y, Du L, Chi W, Chen L, Zhang R, Zhang M, Wang H, Lu H, Yang L, et al. Development and evaluation of diagnostic criteria for Vogt-Koyanagi-Harada disease. JAMA Ophthalmol. 2018;136:1025–31. doi:https://doi.org/10.1001/jamaophthalmol.2018.2664.
- O’Keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 2017;62:1–25. doi:https://doi.org/10.1016/j.survophthal.2016.05.002.
- Chen L, Yang P, Zhou H, He H, Ren X, Chi W, Wang L, Kijlstra A. Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci. 2008;49:3475–82. doi:https://doi.org/10.1167/iovs.08-1793.
- Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, Chen L, Zhou H, Huang X, Kijlstra A. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119:1218–24. doi:https://doi.org/10.1016/j.jaci.2007.01.010.
- Sanjuan MA, Sagar D, Kolbeck R. The role of IgE in autoimmunity. J Allergy Clin Immunol. 2016;137:1651–61. doi:https://doi.org/10.1016/j.jaci.2016.04.007.
- Permin H, Wiik A. The prevalence of IgE antinuclear antibodies in rheumatoid arthritis and systemic lupus erythematosus. Acta Pathol Microbiol Scand C. 1978;86c:245–49.
- Messingham KA, Holahan HM, Fairley JA. Unraveling the significance of IgE autoantibodies in organ-specific autoimmunity: lessons learned from bullous pemphigoid. Immunol Res. 2014;59:273–78. doi:https://doi.org/10.1007/s12026-014-8547-7.
- Romero MD, Muiño JC, Bianco GA, Ferrero M, Juarez CP, Luna JD, Rabinovich GA. Circulating anti-galectin-1 antibodies are associated with the severity of ocular disease in autoimmune and infectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1550–56. doi:https://doi.org/10.1167/iovs.05-1234.
- Muiño JC, Juárez CP, Luna JD, Castro CC, Wolff EG, Ferrero M, Romero-Piffiguer MD. The importance of specific IgG and IgE autoantibodies to retinal S antigen, total serum IgE, and sCD23 levels in autoimmune and infectious uveitis. J Clin Immunol. 1999;19:215–22. doi:https://doi.org/10.1023/A:1020516029883.
- Cozzani E, Gasparini G, Di Zenzo G, Parodi A. Immunoglobulin E and bullous pemphigoid. Eur J Dermatol. 2018;28:440–48. doi:https://doi.org/10.1684/ejd.2018.3366.
- Atta AM, Sousa CP, Carvalho EM, Sousa-Atta ML. Immunoglobulin E and systemic lupus erythematosus. Braz J Med Biol Res. 2004;37:1497–501. doi:https://doi.org/10.1590/S0100-879X2004001000008.
- Lamb PM, Patton T, Deng JS. The predominance of IgG4 in prodromal bullous pemphigoid. Int J Dermatol. 2008;47:150–53. doi:https://doi.org/10.1111/j.1365-4632.2008.03361.x.
- Onat AM, Buyukhatipoglu H, Yilmaz M, Geyik R, Celik A, Ozturk MA. Immunoglobulin E: a new diagnostic clue for Behcet’s disease? IgE and Behcet’s disease. Clin Rheumatol. 2007;26:81–83. doi:https://doi.org/10.1007/s10067-006-0335-x.
- Cengiz K. Serum IgE concentrations in complete Behcet’s disease. J Clin Pathol. 1990;43:262. doi:https://doi.org/10.1136/jcp.43.3.262-a.
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi:https://doi.org/10.1172/JCI200318921.
- Jones J, Loftus EV Jr, Panaccione R, Chen LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG, Harmsen SW, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–24. doi:https://doi.org/10.1016/j.cgh.2008.06.010.
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:https://doi.org/10.3389/fimmu.2018.00754.
- Cordero-Coma M, Sobrin L. Anti-tumor necrosis factor-α therapy in uveitis. Surv Ophthalmol. 2015;60:575–89. doi:https://doi.org/10.1016/j.survophthal.2015.06.004.
- El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SS, Opdenakker G, Geboes K, Damme JV. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139:177–84. doi:https://doi.org/10.1016/j.clim.2011.01.014.
- Chen W, Zhao B, Jiang R, Zhang R, Wang Y, Wu H, Gordon L, Chen L. Cytokine expression profile in aqueous humor and sera of patients with acute anterior uveitis. Curr Mol Med. 2015;15:543–49. doi:https://doi.org/10.2174/1566524015666150731100012.
- Ozdamar Y, Berker N, Bahar G, Soykan E, Bicer T, Ozkan SS, Karakaya J. Inflammatory mediators and posterior segment involvement in ocular Behcet disease. Eur J Ophthalmol. 2009;19:998–1003. doi:https://doi.org/10.1177/112067210901900616.
- Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi:https://doi.org/10.1038/nrrheum.2015.169.
- Khera TK, Dick AD, Nicholson LB. Mechanisms of TNFα regulation in uveitis: focus on RNA-binding proteins. Prog Retin Eye Res. 2010;29:610–21. doi:https://doi.org/10.1016/j.preteyeres.2010.08.003.
- Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, Klagsbrun M, Ferrara N, Bussolino F. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998;273:22128–35. doi:https://doi.org/10.1074/jbc.273.34.22128.
- Hangai M, He S, Hoffmann S, Lim JI, Ryan SJ, Hinton DR. Sequential induction of angiogenic growth factors by TNF-alpha in choroidal endothelial cells. J Neuroimmunol. 2006;171:45–56. doi:https://doi.org/10.1016/j.jneuroim.2005.09.018.
