ABSTRACT
Purpose
The lamina cribrosa (LC) is a layer of fenestrated connective tissue tethered to the posterior sclera across the scleral canal in the optic nerve head (ONH). It is located at the interface of intracranial and intraocular compartments and is exposed to intraocular pressure (IOP) anteriorly and intracranial pressure (ICP) or Cerebrospinal fluid (CSF) pressure (CSFP) posteriorly. We hypothesize that the pressure difference across LC will determine LC position and meridional diameter of scleral canal (also called Bruch’s membrane opening diameter; BMOD).
Methods
We enrolled 19 human subjects undergoing a medically necessary lumbar puncture (LP) to lower CSFP and 6 anesthetized pigs, whose ICP was increased in 5 mm Hg increments using a lumbar catheter. We imaged ONH using optical coherence tomography and measured IOP and CSFP/ICP at baseline and after each intervention. Radial tomographic ONH scans were analyzed by two independent graders using ImageJ, an open-source software. The following ONH morphological parameters were obtained: BMOD, anterior LC depth and retinal thickness. We modeled effects of acute CSFP/ICP changes on ONH morphological parameters using ANOVA (human study) and generalized linear model (pig study).
Results
For 19 human subjects, CSFP ranged from 5 to 42 mm Hg before LP and 2 to 19.4 mm Hg after LP. For the six pigs, baseline ICP ranged from 1.5 to 9 mm Hg and maximum stable ICP ranged from 18 to 40 mm Hg. Our models showed that acute CSFP/ICP changes had no significant effect on ONH morphological parameters in both humans and pigs.
Conclusion
We conclude that ONH does not show measurable morphological changes in response to acute changes of CSFP/ICP. Proposed mechanisms include compensatory and opposing changes in IOP and CSFP/ICP and nonlinear or nonmonotonic effects of IOP and CSFP/ICP across LC.
Introduction
Intracranial pressure (ICP) measurement is important in the management of catastrophic neurological conditions (traumatic brain injury and intracranial hemorrhage), as well as chronic diseases, such as idiopathic intracranial hypertension and normal pressure hydrocephalus.Citation1 Current methods for ICP measurement, such as external ventricular drainage, parenchymal monitoring or lumbar puncture (LP), are invasive, require trained personnel and clinical settings, such as hospitals or clinics, and have increased risks for serious complications, such as chronic pain, infections, and neurological deficits.Citation2 Thus, there is a critical need to develop noninvasive clinical methods for ICP estimation. The optic nerve head (ONH) may be an ideal site to explore biomarkers for such clinical methods.
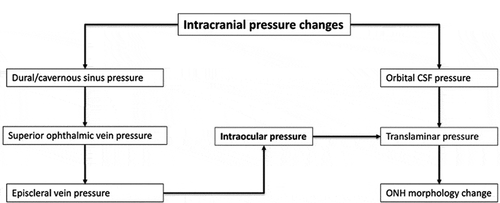
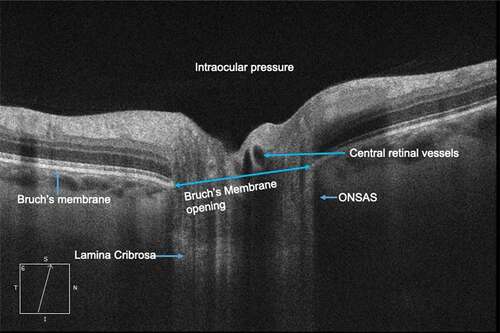
The anatomic and physiologic relationships between intracranial and ocular compartments makes the ONH an ideal site for noninvasive biomarkers for acute ICP changes.Citation3 The ONH is comprised of a fenestrated band of connective tissue called lamina cribrosa (LC) tethered to the sclera at its posterior opening called scleral canal or Bruch’s membrane opening (BMO). Neurovascular structures of the globe-axons, central retinal artery, and vein transit through this opening (). The optic nerve is enclosed by a CSF-filled meningeal tissue called the optic nerve sheath (ONS), which maintains hydrostatic continuity with the intracranial subarachnoid space.Citation4 Studies in a dog model have shown that pressure within the optic nerve subarachnoid space (ONSAS) is equivalent to the lateral ventricular CSF pressure (CSFP).Citation5 The ONS inserts into the posterior sclera and transmits CSFP to ONH along the ONSAS. Exposure of the LC to intraocular pressure (IOP) anteriorly and CSFP posteriorly produces translaminar pressure difference (TLPD) across the LC. The TLPD is defined as the difference between IOP and CSFP.Citation5 The IOP is postulated to produce a pressure load perpendicular to the inner surface of the eye wall, generating an in-wall circumferential stress known as the hoop stress, which pulls the LC taut across the scleral canal.Citation6 The CSFP produces an anteriorly directed pressure on the posterior globe and LC. Mathematical models show that a drop in IOP results in reduced hoop stress, which produces anterior LC displacement and scleral canal narrowing, while increase in CSFP results in anterior LC displacement and increased diameter of scleral canal through its effect on the posterior globe.Citation7
Figure 1. Anatomy of the optic nerve head as seen on optical coherence tomography imaging. ONSAS- Optic nerve subarachnoid space
While chronic ICP elevation produces papilledema or ONH swelling from axoplasmic stasis and intraneuronal edema,Citation8 the effects of acute ICP changes on ONH are less clear. In a monkey model, mild optic disc edema was noted on stereoscopic color fundus photography 48 hours after acute ICP increase.Citation8 However, in a dog model, immediate anterior displacement of the optic disc surface following acute ICP increase was observed using confocal scanning laser tomography.Citation9 In a recent in-vitro study of three porcine eyes, increased CSFP at a steady IOP resulted in distension of the ONS and deformations within the LC and, more prominently, in the retrolaminar neural tissue.Citation10
Optical coherence tomography (OCT) has renewed interest in quantifying ONH morphology following ICP changes. OCT imaging in patients with papilledema from idiopathic intracranial hypertension (IIH) demonstrates asymmetric anterior deformation of peripapillary retina, which resolves within days of lowering ICP.Citation11,Citation12 However, acute ICP changes in 16 eyes of eight nonglaucomatous subjects undergoing LP did not result in any observable changes of ONH morphology on OCT imaging at 5, 60, and 360 minutes after LP.Citation13 In another small study of five subjects (five eyes) with IIH, all eyes showed small but statistically insignificant changes of retinal nerve fiber layer thickness, scleral canal opening, and a significant change of the Bruch’s membrane angle on OCT imaging.Citation14
We hypothesize that the acute CSFP changes will alter TLPD, resulting in measurable changes in ONH morphology as defined by the position of LC and diameter of scleral canal or BMO diameter (BMOD) (). Increased CSFP will reduce TLPD resulting in an anterior shift of LC and reduction of BMOD through reduced circumferential scleral canal hoop stress. CSFP reduction will produce a posterior shift of LC through increased TLPD and increased BMOD through increased scleral canal hoop stress. We tested this hypothesis in a cohort of patients undergoing LP (CSFP reduction) and in an animal model of elevated ICP.
Methodology
We conducted two different interventional studies to assess the effects of acute ICP changes on ONH.
Lumbar puncture study
The University of Nebraska Medical Center’s Institution Review Board reviewed and approved the human study protocol and administration. To model the effects of acute CSFP decrease on ONH, we enrolled 19 consecutive human subjects undergoing a medically necessary LP. These subjects were being evaluated for multiple sclerosis (8), idiopathic intracranial hypertension (5), dementia (4), neuropathy (1), and headache (1). All LP procedures were performed by a single expert neurologist in the left lateral decubitus position using standard clinical methods. The head, neck, and extremities were relaxed for CSF opening and closing pressure measurements, which were obtained using a column manometer. CSFP was defined as the height of the CSF column in the manometer as it oscillates about a mean. Study parameters described below were obtained for both eyes except for two patients who were one-eyed (subject 3 – right eye only; subject 5 – left eye only). The following parameters were assessed before and 30 minutes after LP in 36 eyes of 19 patients.
Intraocular pressure
IOP was measured using iCare rebound tonometer (iCare USA, Raleigh NC) in the sitting position by a single experienced ophthalmologist. IOP was defined as the average of two measurements, which were within 2 mm Hg of each other. If there was a difference of greater than 2 mm Hg between measurements, a third reading was obtained. The median value was used as IOP value.
Ophthalmoscopic examination was performed by two masked experienced ophthalmologists to characterize disc margins, presence of papilledema, and spontaneous venous pulsation.
Optic disc imaging
Using spectral domain OCT (SD-OCT; Cirrus HD-OCT (Carl Zeiss Meditec Inc.)), 12 radial scans with enhanced depth imaging (EDI) centered on the ONH were obtained in the seated position. Each B-scan was acquired at 938 × 625 pixels and an aspect ratio of 0.5 (). Optic disc cube (6 mm by 6 mm; 200 × 200 pixels) scan was used to obtain optic disc morphological parameters and the peri-papillary Retinal nerve fiber layer (RNFL) using the proprietary software provided by Carl-Zeiss Meditec Inc. The pre-LP images were used as reference for the post-LP images for all subjects. Pharmacological pupil dilation was not used for the human subjects. describes the baseline characteristics of the enrolled subjects.
Table 1. Baseline data for the cohort of patients undergoing lumbar puncture
Pig model study
To model the effects of acute ICP increase on ONH, we used a pig model developed by our group.Citation15,Citation16 This model was chosen due to prior surgeon experience with lumbar and intracranial CSF access in the species (Sus domesticus) and comparable intracranial, intraorbital, and ocular anatomy, including multilayer LC with humans.Citation17
Seven female domestic pigs weighing between 65 and 75 kg were used in the experiments. We excluded one pig due to difficulty in placing the lumbar drain and frontal intraparenchymal monitor.Citation15 All studies were conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and approved by the Institution’s Animal Care and Use Committee. After anesthetization and positioning of the animals, ICP was increased using a lumbar catheter inserted into the lumbar cistern. The lumbar catheter was connected to a normal saline bag using IV tubing and adjustable flow valve. Continuous ICP was recorded using an intraparenchymal ICP monitor probe (Integra Camino fiberoptic ICP monitor) placed in the frontal lobe. Correct placement was confirmed by the pulsatile ICP waveform on the monitor. ICP was serially increased in approximately 5–10 mm Hg increments until a maximum ICP of 40 mm Hg or until it was impossible to maintain a steady ICP reading (within 2 mm Hg) for at least 5 minutes. After ICP was stabilized (less than ±2 mm Hg fluctuation around the intended ICP), we performed these study measurements:
IOP (right eye) using pneumatonometry (Model 30, Reichert USA).
Episcleral vein pressure (EVP; right eye) using standard techniques of venomanometry (Eyetech USA).
Optic disc imaging (left eye) using an SD-OCT (Spectralis, Heidelberg, Germany; 12 radial EDI scans centered on the optic disc).
Posterior segment examination (left eye; direct and indirect ophthalmoscopy by a neuro-ophthalmologist) and posterior pole photographs and videos using the Volk InView Camera (Volk, Germany).
The left pupil was pharmacologically dilated to aid examination and imaging of the optic nerve and retinal blood vessels. The right pupil was not pharmacologically dilated as the eye was used to image and measure the EVP, which might be affected by the topical medications.
Image analysis
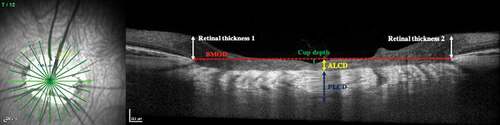
All 12 radial scans from both eyes of human subjects and the left eye of the pig model were downloaded as TIFF images, assigned a code, and randomized prior to analysis using ImageJ 1.50i (National Institutes of Health, USA). Two trained, masked graders independently performed segmentation of ONH structures and identified BMO and anterior LC surface. They also measured the BMOD and anterior LC depth (ALCD). Posterior LC depth (PLCD), cup depth, and retinal thickness (defined as thickness to the internal limiting membrane at either edge of BMO) were measured in the pig images ().
Figure 3. Optic nerve morphological parameters measured on OCT images (ALCD: Anterior lamina cribrosa depth; PLCD: posterior lamina cribrosa depth; BMOD: Bruch’s membrane opening diameter)
If discrepancy between the two graders was greater than 10% of parameter average, a third expert grader independently analyzed the images. If discrepancy between any two graders was within 10% of parameter average, an average between the two graders was used as parameter value; otherwise, the image parameter was classified as ungradable. Intragrader reliability was tested by having each grader grade a standard image five times at different times. Graders analyzed 1224 OCT images (864 human eye and 360 pig eye images). Images of poor quality (signal quality <7) and those with discrepancy >10% between graders were excluded from analysis ().
Table 2. Quality control analysis for the optic nerve head imaging using SD-OCT
Statistical methods
ONH parameters (ALCD, BMOD, and retinal thickness) were the primary outcome measures, while cup volume/depth and spontaneous venous pulsations were secondary outcome measures. Sample size for the LP study was calculated using data from a proof of concept (unpublished) study by our group. In 20 normal volunteers performing Valsalva, we found that subjects had a change in ALCD of approximately 20 µm with a standard deviation of 14 µm. A sample size of 28 eyes would achieve 80% power to detect a difference of 15 µm difference in the ALCD with an estimated standard deviation of 20 µm at a significance level (alpha) of 0.05 using two-sided one-sample paired t-test. Since the pig study was exploratory, a sample size was not calculated.
Intergrader and intragrader reliability was assessed by measuring accuracy, precision, and intraclass correlation (ICC). Summary statistics are provided for the parameters using mean, standard deviation, and range for continuous variables and percentages for nominal variables. The influence of ICP on ONH parameters was determined as follows: for the LP study, repeated measures ANOVA was used to evaluate differences in ONH parameters over time (pre- vs. post-procedures) in both the right and left eyes. Covariates to explain linear sources of variation include gender, age, body mass index, and changes in ICP and IOP. For the pig model, change in optic nerve parameters was modeled using a fixed effect model for longitudinal data. Slopes were evaluated to determine if changes in ICP were associated with changes in outcomes. The effect of blood pressure and IOP at each ICP level was controlled in the model. We used SAS software version 9.4 (SAS Institute Inc., Cary, NC) for the analysis.
Results
Lumbar puncture study
There were no significant baseline interocular differences for IOP, RNFL, cup volume, disc area, or rim area (). Four subjects (eight eyes) had papilledema at baseline. There were no other ocular abnormalities, eye symptoms, or structural changes in other patients. The CSF opening pressure showed a significant correlation with age (Pearson correlation coefficient −0.64 (p = .001)) and BMI (Pearson correlation coefficient 0.72 (p = .003)). Following CSFP reduction after LP, there were no significant changes for any of the ONH clinical parameters (). There was slight reduction of IOP in both eyes, with greater reduction being noted for patients with a diagnosis of IIH. Using repeated measures ANOVA, we did not find significant effects of CSFP reduction on differences in ONH parameters over time (pre- vs. post-LP) in both the right and left eyes after controlling for gender, age, body mass index, and changes in CSFP and IOP.
Table 3. The effect on ophthalmic parameters of acute ICP lowering following lumbar puncture. Values depicted are mean (standard deviation)
Pig model study
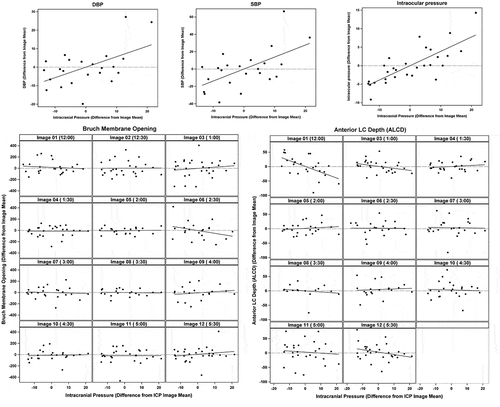
At baseline, average ICP was 5.4 ± 2.7 mm Hg (range 1.5–9 mm Hg) and IOP was 12.6 ± 2.1 mm Hg (mean ± standard deviation). ICP was successfully increased in six pigs in 5–10 mm Hg increments using normal saline infusion through the lumbar drain. Maximum stable ICP achieved ranged from 18 to 40 mm Hg. Fixed effects model demonstrated significant changes in systolic blood pressure (β = 1.383, p = .008), diastolic blood pressure (β = 0.566, p = .019), and IOP (β = 0.388, p < .001) with acute ICP changes. There were no significant effects of ICP change on optic nerve morphology ().
Figure 4. Top panel: Slopes generated using linear effects model demonstrate a linear relationship between changes in ICP and changes in diastolic blood pressure (DBP), systolic blood pressure (SBP), and intraocular pressure. Lower panel: The effects of acute ICP changes on optic nerve head morphology are not significant. The variables on the horizontal and vertical are shown as mean centered values for each pig at each image meridian
Discussion
Acute changes in CSFP/ICP did not result in measurable changes of ONH morphology in humans and in pig model. We propose that the following factors might mitigate the effects of acute CSFP/ICP changes on ONH.
The biomechanical effects of acute ICP changes on ONH may be mitigated by compensatory changes in IOP.Citation7 The IOP response to acute ICP changes is likely mediated by pressure changes in the orbital veins including episcleral veins, which drain into the cavernous sinus. We recently reported a significant linear association between acute changes in ICP and IOP such that each unit increase of ICP was associated with a 37% increase in IOP and 31% increase in episcleral venous pressure.Citation15 A linear relationship between acute changes in ICP and IOP was also demonstrated in a dog model, where ICP was reduced using intraventricular CSF drainage.Citation18 Repeated simultaneous measurements of ICP and IOP in longitudinally designed studies of human subjects also demonstrate coupling of ICP and IOP such that acute changes of ICP result in compensatory changes in IOP.Citation19–22 A recent study of 36 human subjects showed a mean IOP reduction of 0.8 mm Hg following acute ICP reduction after LP. The magnitude of IOP reduction was slightly greater in subjects who had ICP >20 mm Hg.Citation23 In our LP study, we found similar reduction of IOP by 0.6 mm Hg following LP. Although the magnitude of the IOP reduction was not statistically significant, it was perhaps sufficient to mitigate some of the effects of acute ICP reduction on the LC.
LC may not be the site of maximum impact from acute changes in CSFP/ICP, which could explain the lack of observable morphological changes in our studies. Finite element mathematical models of ONH show that while IOP produces a direct posterior force on the LC, ICP appears to have maximal impacts on strain distributions within the retrolaminar nerve tissue rather than LC itself.Citation24,Citation25 Acute ICP increase may result in compression of retrolaminar neural tissue, distension of dural sheath, and deformation of posterior globe surface by exerting an anterior force on the peripapillary sclera.Citation26 In an in-vivo study of a glaucomatous eye of a brain-dead subject, alteration of IOP and CSFP resulted in deformation (strain) of LC and peripapillary sclera with regional variation. The deformation from elevation of CSFP was similar in magnitude but opposite in direction to those secondary to elevation in IOP.Citation27
The magnitude of ONH morphological changes from acute changes in ICP and/or IOP may vary with specific tissue anatomy and mechanical properties.Citation26 Using in-vivo OCT imaging of ONH in eyes of a rhesus monkey, while acutely changing IOP and ICP, Tran et. al. found that the effects of pressure changes on LC were localized, nonlinear, and nonmonotonic.Citation28 Through in-vivo studies of five nonhuman primate (NHP) monkeys, Wang et al. showed that the effects of acute ICP and IOP on the 3-D microstructure of LC were small (5–10%), heterogenous across the LC and variable across different animals.Citation29 This is consistent with our observations of variable meridional changes in BMOD and LCD with acute ICP changes within the same eye of an individual and between eyes of different individuals.
It is also unclear if acute changes of ICP is transmitted with high fidelity along the ONS to ONH as is suggested by the principle of hydrostatic continuity of all contiguous CSF compartments. Anatomic variations of the ONS may affect the transmission of acute ICP changes to the ONH and thus produce variable effects on its morphology. Although a significant linear relationship between ONS pressure and lateral ventricle CSFP was demonstrated in a dog model, there are no published human studies demonstrating such a relationship.Citation30 In 16 patients who were undergoing removal of a blind eye, researchers measured the optic nerve subarachnoid pressure to be 4–14 mm Hg, which falls within the known physiologic ICP range. In five patients who were placed in Trendelenburg position, the optic nerve pressure was found to increase by 1–2 mm Hg, which suggests that ICP increase due to gravity was transmitted to the optic nerve.Citation31 Both studies are limited by a lack of CSFP measurement. In an unpublished study, investigators simultaneously catheterized the ONS and lumbar cistern subarachnoid spaces in six patients (seven eyes), who were undergoing orbital procedure for different disease conditions. By varying CSFP through the lumbar drain, the investigators found that the initial pressure was equal in both compartments in four of seven eyes while ONS pressure was higher in three of seven eyes. The response of ONS pressure to ICP changes, however, was highly variable with significant differences in the rates of rise and fall of ONS pressure being noted within the same eye as well as between eyes, which suggests that communication between the intracranial and ONS subarachnoid space might be inconsistent (personal communication Gregory Kosmorski D.O.). There is significant positional fluctuation of IOP and CSFP, which may alter the effect of these parameters on LC. Simultaneous in-vivo measurements of IOP and CSFP in NHP animal models demonstrated reduced TLPD during sleep, which was driven primarily by increased CSFP during sleep.Citation32 Moreover, the diurnal fluctuations of IOP, CSFP, and TLPD varied across individual animals.Citation33 These anatomic and physiologic variabilities may limit consistent and measurable morphologic changes within the ONH, especially when pooled across subjects.
We acknowledge the limitations of our study. We had a small sample size in both parts of our study, and we might have missed a small effect size. However, the variability of morphological parameters within the same eye, between eyes, and across different experimental time points suggests a true lack of effect rather than a small true effect. We did not measure all known morphological parameters, such as the peripapillary Bruch’s Membrane shape, which was recently proposed as a useful biomarker for ICP changes.Citation34 We had to exclude approximately 12–25% of our OCT images, which were deemed ungradable due to image quality () despite our best attempts at quality control. Finally, in the LP study, we measured CSFP in lateral decubitus position and IOP in sitting position. In sitting or upright position, gravity causes a caudal shift of CSF toward the lumbar cistern, resulting in differential CSFP along the neuraxis.Citation35 In this scenario, CSF opening pressure obtained in the lateral decubitus position may not accurately reflect pressure within the ONS in a sitting position. This, however, was not the case in the animal model where both pressures were obtained simultaneously in the prone position, which suggests that the effect of gravity on ONH morphology is minimal.
In conclusion, we were unable to detect any significant alteration of the measured ONH parameters secondary to acute ICP increase in the pig model or acute CSFP decrease in patients undergoing LP. This null result is important to further our understanding of the homeostatic mechanisms, which preserve the integrity of ONH morphology during states of acute ICP fluctuation. ONH morphology may not be a viable biomarker for acute ICP changes.
Study support
Lisa Reid and Toni Goeser (Department of Ophthalmology UNMC), Marsha Morien, Nathan Bills and Crystal Krause (Center for Advanced Surgical Techniques, UNMC), Neil Jouvenat (Department of Neurological Sciences).
Commercial relationships
Deepta Ghate MBBS, MD: Licensed technology with EON Reality Inc.
Sachin Kedar MBBS, MD: Licensed technology with EON Reality Inc.
Shane Havens MD: None
Shan Fan MD: None
William Thorell MD: None
Carl Nelson PhD: None
Linxia Gu PhD: None
Junfei Tong PhD: None
Vikas Gulati MD: None
Robin High: None
John Bader: None
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nakagawa K, Smith WS. Evaluation and management of increased intracranial pressure. Continuum (Minneap Minn). 2011 Oct 01;17( 5Neurologic Consultation in the Hospital):1077–93.
- Bruce BB. Noninvasive assessment of cerebrospinal fluid pressure. J Neuroophthalmol. 2014 Sept 01;34(3):288–94. doi:https://doi.org/10.1097/WNO.0000000000000153.
- Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci. 2003 Dec 01;44(12):5189–95. doi:https://doi.org/10.1167/iovs.03-0174.
- Hayreh SS. The sheath of the optic nerve. Ophthalmologica. 1984;189(1–2):54–63. doi:https://doi.org/10.1159/000309386.
- Morgan WH, Yu DY, Alder VA, Cringle SJ, Cooper RL, House PH, Constable IJ. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci. 1998 Jul 01;39(8):1419–28.
- Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008 Jun 01;85(6):425–35. doi:https://doi.org/10.1097/OPX.0b013e31817841cb.
- Tong J, Ghate D, Kedar S, Gu L. Relative contributions of intracranial pressure and intraocular pressure on lamina cribrosa behavior. J Ophthalmol. 2019 Mar;17(2019):3064949.
- Hayreh SS. Optic disc edema in raised intracranial pressure. V. pathogenesis. Arch Ophthalmol. 1977 Sept 01;95(9):1553–65. doi:https://doi.org/10.1001/archopht.1977.04450090075006.
- Morgan WH, Chauhan BC, Yu DY, Cringle SJ, Alder VA, House PH. Optic disc movement with variations in intraocular and cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2002 Oct 01;43(10):3236–42.
- Feola AJ, Coudrillier B, Mulvihill J, Geraldes DM, Vo NT, Albon J, Abel RL, Samuels BC, Ethier CR. Deformation of the lamina cribrosa and optic nerve due to changes in cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2017 Apr 01;58(4):2070–78. doi:https://doi.org/10.1167/iovs.16-21393.
- Kupersmith MJ, Sibony P, Mandel G, Durbin M, Kardon RH. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011 Aug 22;52(9):6558–64. doi:https://doi.org/10.1167/iovs.10-6782.
- Sibony P, Kupersmith MJ, Honkanen R, Rohlf FJ, Torab-Parhiz A. Effects of lowering cerebrospinal fluid pressure on the shape of the peripapillary retina in intracranial hypertension. Invest Ophthalmol Vis Sci. 2014 Nov 18;55(12):8223–31. doi:https://doi.org/10.1167/iovs.14-15298.
- Poli M, Denis P, Sellem E, Aho-Glele LS, Bron AM. Is the optic nerve head structure impacted by a diagnostic lumbar puncture in humans? J Glaucoma. 2017 Nov 01;26(11):1036–40.
- Anand A, Pass A, Urfy MZ, Tang R, Cajavilca C, Calvillo E, Suarez JI, Venkatasubba Rao CP, Bershad EM. Optical coherence tomography of the optic nerve head detects acute changes in intracranial pressure. J Clin Neurosci. 2016 Jul;01(29):73–76. doi:https://doi.org/10.1016/j.jocn.2015.12.016.
- Ghate D, Kedar S, Havens S, Fan S, Thorell W, Nelson C, Gu L, Tong J, Gulati V. The effects of acute intracranial pressure changes on the episcleral venous pressure, retinal vein diameter and intraocular pressure in a pig model. Curr Eye Res. 2020 Aug;17:1–8.
- Twedt M, Pfeifer C, Thorell W, Bashford G. Measuring hemodynamic changes in the ophthalmic artery during applied force for noninvasive intracranial pressure monitoring: test results in a porcine model. Milmed. 2017;182:72–77.
- Ghoshal NG, Zguigal H. Dural sinuses in the pig and their extracranial venous connections. Am J Vet Res. 1986 May 01;47(5):1165–69.
- Hou R, Zhang Z, Yang D, Wang H, Chen W, Li Z, Sang J, Liu S, Cao Y, Xie X, et al. Intracranial pressure (ICP) and optic nerve subarachnoid space pressure (ONSP) correlation in the optic nerve chamber: the beijing intracranial and intraocular pressure (iCOP) study. Brain Res. 2016 Mar 15;1635:201–08. doi:https://doi.org/10.1016/j.brainres.2016.01.011.
- Sheeran P, Bland JM, Hall GM. Intraocular pressure changes and alterations in intracranial pressure. Lancet. 2000 Mar 11;355(9207):899. doi:https://doi.org/10.1016/S0140-6736(99)02768-3.
- Lashutka MK, Chandra A, Murray HN, Phillips GS, Hiestand BC. The relationship of intraocular pressure to intracranial pressure. Ann Emerg Med. 2004 May 01;43(5):585–91. doi:https://doi.org/10.1016/j.annemergmed.2003.12.006.
- Spentzas T, Henricksen J, Patters AB, Chaum E. Correlation of intraocular pressure with intracranial pressure in children with severe head injuries. Pediatr Crit Care Med. 2010 Sept 01;11(5):593–98. doi:https://doi.org/10.1097/PCC.0b013e3181ce755c.
- Sajjadi SA, Harirchian MH, Sheikhbahaei N, Mohebbi MR, Malekmadani MH, Saberi H. The relation between intracranial and intraocular pressures: study of 50 patients. Ann Neurol. 2006 May 01;59(5):867–70. doi:https://doi.org/10.1002/ana.20856.
- Gonzalez-Camarena PI, San-Juan D, Gonzalez-Olhovich I, Rodriguez-Arevalo D, Lozano-Elizondo D, Trenado C, Anschel DJ. Dynamic changes of the intraocular pressure and the pressure of cerebrospinal fluid in nonglaucomatous neurological patients. Acta Ophthalmol. 2017 Mar 01;95(2):e138–43. doi:https://doi.org/10.1111/aos.13236.
- Feola AJ, Myers JG, Raykin J, Mulugeta L, Nelson ES, Samuels BC, Ethier CR. Finite element modeling of factors influencing optic nerve head deformation due to intracranial pressure. Invest Ophthalmol Vis Sci. 2016 Apr 01;57(4):1901–11. doi:https://doi.org/10.1167/iovs.15-17573.
- Hua Y, Tong J, Ghate D, Kedar S, Gu L. Intracranial pressure influences the behavior of the optic nerve head. J Biomech Eng. 2017 Mar 01;139(3). doi:https://doi.org/10.1115/1.4035406.
- Hua Y, Voorhees AP, Sigal IA. Cerebrospinal fluid pressure: revisiting factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2018 Jan 01;59(1):154–65. doi:https://doi.org/10.1167/iovs.17-22488.
- Fazio MA, Clark ME, Bruno L, Girkin CA. In vivo optic nerve head mechanical response to intraocular and cerebrospinal fluid pressure: imaging protocol and quantification method. Sci Rep. 2018 Aug 23;8(1):12639–018. doi:https://doi.org/10.1038/s41598-018-31052-x.
- Tran H, Grimm J, Wang B, Smith MA, Gogola A, Nelson S, Tyler-Kabara E, Schuman J, Wollstein G, Sigal IA. Mapping in-vivo optic nerve head strains caused by intraocular and intracranial pressures. Proc SPIE Int Soc Opt Eng. 2017 Feb 01;10067. doi:https://doi.org/10.1117/12.2257360.
- Wang B, Tran H, Smith MA, Kostanyan T, Schmitt SE, Bilonick RA, Jan NJ, Kagemann L, Tyler-Kabara EC, Ishikawa H, et al. In-vivo effects of intraocular and intracranial pressures on the lamina cribrosa microstructure. PLoS One. 2017 Nov 21;12(11):e0188302. doi:https://doi.org/10.1371/journal.pone.0188302.
- Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ. The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci. 1995 May 01;36(6):1163–72.
- Liu D, Michon J. Measurement of the subarachnoid pressure of the optic nerve in human subjects. Am J Ophthalmol. 1995 Jan 01;119(1):81–85. doi:https://doi.org/10.1016/S0002-9394(14)73817-6.
- Jasien JV, Samuels BC, Johnston JM, Downs JC. Diurnal cycle of translaminar pressure in nonhuman primates quantified with continuous wireless telemetry. Invest Ophthalmol Vis Sci. 2020 Feb 07;61(2):37. doi:https://doi.org/10.1167/iovs.61.2.37.
- Jasien JV, Fazio MA, Samuels BC, Johnston JM, Downs JC. Quantification of translaminar pressure gradient (TLPG) with continuous wireless telemetry in nonhuman primates (NHPs). Transl Vis Sci Technol. 2020 Nov 12;9(12):18. doi:https://doi.org/10.1167/tvst.9.12.18.
- Malhotra K, Patel MD, Shirazi Z, Moss HE. Association between peripapillary bruch’s membrane shape and intracranial pressure: effect of image acquisition pattern and image analysis method, a preliminary study. Front Neurol. 2018 Dec 21;9:1137. doi:https://doi.org/10.3389/fneur.2018.01137.
- Magnaes B. Body position and cerebrospinal fluid pressure. part 2: clinical studies on orthostatic pressure and the hydrostatic indifferent point. J Neurosurg. 1976 Jun 01;44(6):698–705. doi:https://doi.org/10.3171/jns.1976.44.6.0698.