ABSTRACT
Purpose
Impaired tear production – a common sign of keratoconjunctivitis sicca (KCS) – is associated with qualitative or quantitative tear deficiency. OTX-101 0.09% is a novel, nanomicellar formulation of cyclosporine A approved in the US for increasing tear production in patients with KCS. We present a pooled analysis of the phase 2b/3 and phase 3 studies evaluating the effect of OTX-101 on tear production in a subgroup of patients with keratoconjunctivitis sicca with severely impaired tear production (Schirmer’s score <5 mm in either eye at baseline).
Methods
In these randomized, double-masked studies, patients instilled 1 drop OTX-101 or vehicle per eye twice daily for 84 days. Pooled efficacy endpoints included percent (%) of patients with ≥10 mm change from baseline and mean change from baseline in Schirmer’s score at day 84. Pooled safety endpoints included adverse event monitoring.
Results
Subgroup analyses included 133 and 113 patients receiving OTX-101 and vehicle, respectively. Mean baseline (BL) Schirmer’s score ± standard deviation was 2.7 ± 1.2 for OTX-101 and 2.5 ± 1.1 mm for vehicle (P = .3203). On day 84, number (%) of patients with ≥10 mm Schirmer’s score change from baseline was 30 (22.6%) and 12 (10.6%, P = .0168); mean change from baseline ± standard deviation was 5.5 ± 8.0 and 3.6 (6.0, P = .0405) mm for OTX-101 and vehicle, respectively. Adverse events were mostly mild and did not require treatment.
Conclusion
OTX-101 administered twice daily for 84 days significantly improved tear production vs vehicle in patients with severely impaired tear production, as evidenced by significantly larger proportion of patients with ≥10 mm increases from baseline and higher mean change from baseline in Schirmer’s scores
Introduction
Dry eye disease (DED), also known as keratoconjunctivitis sicca (KCS), is a multifactorial disease of the ocular surface, characterized by insufficiency of tear production and loss of homeostasis of the tear film.Citation1,Citation2 Impaired tear production and increased tear film instability cause a qualitative or quantitative deficiency of tears, symptoms of discomfort, and visual disturbance.Citation2,Citation3 KCS is often accompanied by increased activities of inflammatory cells and the release of inflammatory mediators, such as cytokines. Hyperosmolarity and subsequent inflammation lead to ocular surface damage.Citation2 Because tear dynamics play a crucial role in maintaining the health of the optical surface and providing a clear vision, KCS can negatively impact patients’ daily functioning and quality of life. Unanesthetized Schirmer’s test estimates stimulated reflex tear flow, and is used to diagnose loss of tear volume, which may be a key pathogenic mechanism in KCS.Citation4
Cyclosporine A (CsA) is an immunomodulatory agent with anti-inflammatory properties that block T-cell activation and subsequent release of proinflammatory cytokines.Citation2,Citation5 The topical actions of CsA in the conjunctival cells promote tear production and ocular homeostasis. However, owing to its lipophilic nature, CsA ophthalmic emulsion 0.05% (Restasis; Allergan, Irvine, CA) limits drug solubility and provides a low bioavailability in ocular tissue.Citation6 OTX-101 (CsA 0.09%, CEQUA™; Sun Pharmaceutical Industries, Inc., Cranbury, NJ) is a novel, nanomicellar aqueous solution of cyclosporine. The nanomicellar technology offers improved physicochemical stability and delivers enhanced bioavailability in ocular tissue to address the underlying inflammation, potentially resulting in better efficacy and safety.Citation6,Citation7
Both phase 2b/3 and phase 3 clinical trials demonstrated that OTX-101 0.09% was superior to vehicle in improving tear production in patients with KCS and was well tolerated.Citation8,Citation9 This pooled analysis evaluated the effect of OTX-101 0.09% vs vehicle on tear production in patients with severe tear deficiency (defined as a baseline Schirmer’s score <5 mm) and compared the results from this subgroup with those from an intent-to-treat (ITT) population.
Methods
Study design
This pooled analysis of a phase 2b/3 and phase 3 study evaluated the effect of OTX-101 0.09% vs vehicle. Both studies were registered (ClinicalTrials.gov: phase 2b/3, NCT02254265; phase 3, NCT02688556), and their design methods were published previously in detail.Citation8,Citation9 All enrolled patients provided written informed consent, and all study documents were approved by the Institutional Review Board. Both studies were conducted in accordance with the International Council for Harmonisation Good Clinical Practices guidelines.
Both studies included a 14–20 day run-in period in which all patients received 1 drop of vehicle per eye twice daily (BID). The vehicle formulation was identical to that of OTX-101 0.09% except for the omission of the cyclosporine, and unit-dose/single-use vehicle vials were indistinguishable in appearance from those containing active drug. The vehicle included the same nanomicelle formulation as OTX-101 0.09%. During the treatment period, patients were randomized from 1:1 to either OTX-101 or vehicle 1 drop per eye BID for 84 days. In the phase 2b/3 study, patients received OTX-101 0.09% or 0.05%; this analysis includes only the 0.09% dose. The phase 3 study only included the approved OTX-101 0.09% dose. The study visits occurred at baseline and days 14 (phase 2b/3 only), 28, 42 (phase 2b/3 only), 56, and 84 (or early discontinuation).
Study population
Key inclusion and exclusion were previously described.Citation8,Citation9 The pooled analysis included data from adult patients with a self-reported history of KCS for a period of at least 6 months, supported by a clinical diagnosis of bilateral KCS at the time of screening. During both the screening and baseline visits, patients were required to have a lissamine green conjunctival staining sum score of 3–9 out of a total possible score of 12 (excluding the superior zones) in the same eye and a Snellen visual acuity better than 20/200 in each eye. Patients were also required to discontinue any current therapy for dry eye disease (DED), including artificial tears or other ocular lubricants, for the duration of the study.
Patients were excluded if they used cyclosporine ophthalmic emulsion 0.05% within 3 months before screening or reported previous treatment failure with cyclosporine ophthalmic emulsion 0.05%, had a diagnosis of Sjögren’s syndrome >5 years before screening, underwent a corneal refractive surgery within 6 months of screening visit, or had current eye disease other than DED that required the use of ophthalmic medications. Overall, there were no significant differences in key inclusion or exclusion criteria between the studies.
Assessments
The primary efficacy endpoint in this analysis was the proportion of eyes with a clinically meaningful improvement – defined as an increase in Schirmer’s score of >10 mm at day 84 or early discontinuation relative to baseline. An unanesthetized Schirmer’s test was performed in both studies at baseline, and on day 84 or at early discontinuation testing strips of filter paper were placed in both eyes at the same time and removed after 5 minutes; the amount of wetting was recorded in millimeters (as a measure of tear flow). If the test strip demonstrated <5 mm of wetness after 5 minutes in either eye, severe tear deficiency was designated. The worst eye was defined as the eye with a lower Schirmer’s score or the right eye if equal scores were observed from both eyes. The secondary efficacy endpoint was the change in Schirmer’s score from BL to day 84 in the designated worst eye.
Safety assessments in both studies included a collection of self-reported treatment-emergent adverse events (TEAEs) and monitoring at each visit.
Statistical analysis
Data from randomized patients in the ITT populations who received either OTX-101 0.09% or vehicle were pooled at baseline and at day 84 or early discontinuation. Results were analyzed on the worst eyes of the ITT population and the subgroup of patients with severely aqueous deficient KCS (defined as Schirmer’s score <5 mm in either eye at baseline). Pooling the studies allowed for a larger subgroup population with Schirmer’s score <5 mm at baseline. Observed Schirmer’s scores and arithmetic changes from baseline were described using summary statistics (n, mean, median, minimum, and maximum). Mean changes were expressed as mean (standard deviation [SD]). Numerical comparisons between OTX-101 0.09% and vehicle control groups were made using two statistical tests: Cochran-Mantel-Haenszel test for the proportion of eyes with an increase in Schirmer’s score ≥10 mm at day 84, and Wilcoxon rank sum test for the difference between treatments in observed Schirmer’s scores and Schirmer’s score change from baseline to day 84. A P-value of <0.05 was considered statistically significant.
Results
Baseline patient demographics and characteristics
Pooled baseline demographic characteristics have been previously described in detail.Citation8,Citation9 Baseline demographic characteristics for the subgroup population with a baseline Schirmer’s score <5 mm, in comparison with those of the ITT population, are summarized in . Briefly, of 1048 patients included in this pooled analysis, 523 received OTX-101 0.09% and 525 received vehicle. In the subgroup population, 133 and 113 patients received OTX-101 0.09% and vehicle, respectively. In the ITT population, the mean (SD) age was 58.6 (14.2) years for the OTX-101 group and 59.5 (14.4) years for the vehicle group. The majority of patients were women (averaged 82.8%) and white (averaged 82.2%). The subgroup population followed the same demographic patterns. There was no significant difference in baseline Schirmer’s scores between OTX-101 treatment and vehicle control groups: 12.0 (8.6) vs 12.1 (8.4) mm in the ITT population, P = .4687; and correspondingly 2.7 (1.2) vs 2.5 (1.1) mm in the subgroup patients, P = .3203. Overall, demographic characteristics and baseline conditions were well balanced across treatment groups ().
Table 1. Pooled patient demographics and baseline characteristics
Schirmer’s score
Patients treated with OTX-101 0.09% experienced greater improvement in Schirmer’s score vs those treated with vehicle. As shown in , a significantly higher proportion of eyes treated with OTX-101 0.09% achieved an increase in Schirmer’s scores >10 mm at day 84 or early discontinuation than eyes treated with vehicle. In the ITT population, 19.1% of eyes in OTX-101 group reached this efficacy outcome vs 11.0% of eyes in vehicle group (P = .0003). Likewise, in the subgroup population with baseline Schirmer’s score <5 mm, the corresponding values were 22.6% and 10.6% for OTX-101 vs vehicle, respectively (P = .0168).
Figure 1. Percentage of worse eyes with an increase in Schirmer’s score ≥10 mm at day 84 or early discontinuation relative to baseline
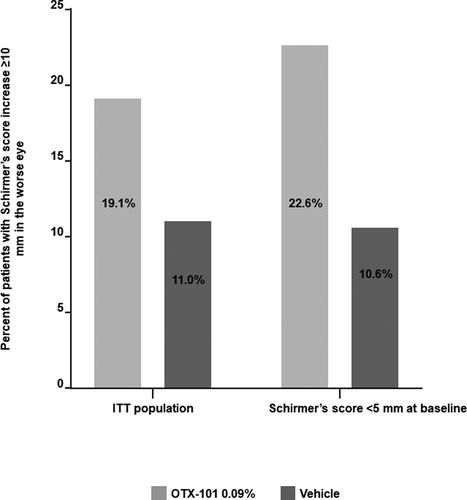
Treatment with OTX-101 0.09% for 84 days led to a significantly higher increase in Schirmer’s scores from baseline in the worst eye vs vehicle controls (). In the ITT population, the mean change from baseline (SD) was 3.6 (8.2) and 1.7 (7.3) mm for the OTX-101 and vehicle groups, respectively (P < .0001). In the subgroup with BL Schirmer’s score <5 mm, the corresponding values were 5.5 (8.0) and 3.6 (6.0) mm, OTX-101 vs vehicle, respectively (P = .0405). Overall, OTX-101 0.09% was significantly more effective than vehicle in improving Schirmer’s score from baseline to day 84 among both ITT and subgroup populations.
Table 2. Change from baseline in Schirmer’s scores in the worse eye at day 84
Safety
Most AEs were mild to moderate in severity and resolved without treatment. The most common AE was instillation site pain, reported in 21.8% and 4.0% of patients receiving OTX-101 and vehicle, respectively. This pain was mostly mild, transient, and resolved within 5 minutes following instillation. The most common TEAEs are categorized as ocular and non-ocular in nature, and a brief summary of these AEs is presented in , also as previously documented.Citation10,Citation11 Notably, there were no clinically or systematically significant reactions in all treatment groups during the 12-week course of the studies.
Table 3. Summary of common (≥1%) ocular and non-ocular treatment-emergent adverse events in the pooled safety population
Discussion
The major findings of this pooled analysis indicate that OTX-101 0.09% significantly improved tear production in patients with severe KCS, especially in the subgroup of patients with a baseline Schirmer’s score <5 mm. In addition, OTX-101 was well tolerated; most TEAEs were mild to moderate in severity and resolved without treatment. Our results show that the effect of OTX-101 on tear production, particularly in patients with severely aqueous deficient DED, adds to the clinical evidence and understanding accumulated from the OTX-101 studies to date.
Data from the present analysis are consistent with those of other studies supporting efficacy and safety of OTX-101 0.09% in patients with KCS.Citation8–12 In each of these clinical trials multiple outcome measures have been applied. Both studies demonstrate OTX-101 0.09% is superior to vehicle when evaluated by several signs of KCS, including increased tear production. In a pooled analysis of the aforementioned two trials examining an ITT population and a subgroup of patients with a baseline Schirmer’s score <10 mm, Sheppard et al. found OTX-101 significantly improves tear production compared with vehicle in patients with KCS even in the subgroup of patients with severe eye dryness.Citation11 Other pooled analyses show OTX-101 0.09% improves conjunctival staining and corneal fluorescein staining scores, suggesting improved cornea/ocular surface integrity and associated visual function.Citation10,Citation12 The safety and tolerability profile of OTX-101 0.09% is also confirmed. Building on these prior studies, this pooled analysis demonstrates OTX-101 0.09% may improve tear production in patients with a much greater severity of KCS.
Currently, there is no gold standard diagnostic test for DED.Citation13 The Schirmer test is commonly used in ophthalmic examination and/or clinical trials to measure tear production for the diagnosis of conditions, such as KCS, that generally refer to dry eye. It is an easy and economic clinical test to perform, and an abnormal finding indicates a tear deficiency. A score of greater than 10 mm in 5 minutes is accepted as normal. As defined in our pooled analysis, the unanesthetized Schirmer’s score <5 mm after 5 minutes is highly suggestive of severely aqueous deficient dry eye. Overall, the Schirmer test score is one of the proven criteria for outcome measures in other ophthalmological studies, despite the controversies concerning its variations and repeatability.Citation4,Citation14
The limitations of the present analysis include a relatively short treatment duration (12 weeks) with the lack of a long-term follow-up to examine confirmed or continuous improvement if the study length were extended; the lack of an active comparator (such as the currently marketed topical cyclosporine emulsion 0.05%); and a limited range of tools and/or tests to evaluate other clinical outcomes. Because OTX-101 contains cyclosporine, an immunomodulating drug with anti-inflammatory properties, future studies with efficacy endpoints (such as inflammatory biomarkers or correlated tests) may provide insights into its influence on the inflammatory status of the eyes.
In conclusion, OTX-101 may significantly improve tear production in patients with KCS, including those with severe aqueous deficient dry eye (with a baseline Schirmer’s score <5 mm). The combined efficacy and safety profile of OTX-101 0.09% appears to offer clinical benefits for patients with severe KCS.
Acknowledgments
This study was sponsored and funded by Ocular Technologies, SARL (now a wholly owned subsidiary of Sun Pharmaceutical Industries), which participated in the design, conduct, monitoring, data collection, data management, and data analysis of the study. Writing and editorial support for manuscript preparation were provided by Jennifer Meyering RN, MS, CMPP, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc. All authors met the International Council of Medical Journal Editors criteria and received neither honoraria nor payment for authorship.
Disclosure statement
MT reports consultant fees from Bausch & Lomb; Eyevance; Iridex; Mallinkrodt; Shire; Sun Pharmaceutical Industries, Inc.; and Zeiss; speaker fees from Bausch & Lomb; Iridex; Lumenis; Mallinkrodt; Shire; Sun Pharmaceutical Industries, Inc.; and Zeiss; research fees from Bausch & Lomb; DigiSight; Dompé Farmaceitici; Iridex; Kala Pharmaceuticals; Lumenis; Magellan; Mallinkrodt; MIXTO Lasering; Ocular Tech; Oculos; Opternative; ReVitaLid; Shire; Sun Pharmaceutical Industries, Inc.; and Zeiss; and miscellaneous fees from MIXTO Lasering.
PG is a consultant to Allergan; Alcon; Aurea; Bausch & Lomb; Johnson & Johnson Vision; Kala Pharmaceuticals; Novartis; Ocular Science; RegGenRxTree; Shire; Sight Sciences; TearLab; TearScience; and Zeiss. BM is an employee of Sun Pharmaceutical Industries, Inc. PK reports consultant fees from Akorn, Alcon Labs, Aldeyra, Allergan/Abbvie, Azura Pharmaceuticals, Bausch + Lomb, BioTissue, BlephEx, Bruder Healthcare, Bruno Pharmaceuticals, Cambium Pharmaceuticals, Dompe, Eyedetec, Eyegate, Eyevance, Healthe, Imprimis, Johnson & Johnson Vision, Kala pharmaceuticals, KEPLR Vision, Konan Medical, Mallinckrodt, Neurolens, Novartis, Oasis Medical, Ocuphire, Ocular Sciences, Oculus, OcuMedic, Osmotica, Oyster Point, RegenerEyes, Science Based Health, Sentiss Pharmaceuticals, Sight Sciences, Silk Technologies, Sun Pharmaceutical Industries, Inc., Surface Pharmaceuticals, Tarsus Medical, TearClear, Visant Medical, Vital Tears
Additional information
Funding
References
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83.
- Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (CEQUA®) for dry eye disease. Pharm Res. 2019;36:36.
- Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510.
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–74.
- Yavuz B, Bozdag Pehlivan S, Unlu N. An overview on dry eye treatment: approaches for cyclosporin a delivery. Sci World J. 2012:194848.
- Jerkins GW, Pattar GR, Kannarr SR. A review of topical cyclosporine a formulations-a disease-modifying agent for keratoconjunctivitis sicca. Clin Ophthalmol. 2020;14:481–89.
- Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):422–37.
- Tauber J, Schechter BA, Bacharach J, Toyos MM, Smyth-Medina R, Weiss SL, Luchs JI. A phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–29.
- Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126:1230–37.
- Smyth-Medina R, Johnston J, Devries DK, Jasper A, Kannarr SR, Schechter BA, Shen Lee B, Varghese G, Ogundele A, Darby CH, et al. Effect of OTX-101, a novel nanomicellar formulation of cyclosporine A, on conjunctival staining in patients with keratoconjunctivitis sicca: a pooled analysis of phase 2b/3 and 3 clinical trials. J Ocul Pharmacol Ther. 2019;35(7):388–94.
- Sheppard J, Kannarr S, Luchs J, Malhotra R, Justice A, Ogundele A, Darby C, Bacharach J. Efficacy and safety of OTX-101, a novel nanomicellar formulation of cyclosporine a, for the treatment of keratoconjunctivitis sicca: pooled analysis of a phase 2b/3 and phase 3 study. Eye Contact Lens. 2020;46(Suppl 1):S14–S19.
- Malhotra R, Devries DK, Luchs J, Kabat A, Schechter BA, Shen Lee B, Shettle L, Smyth-Medina R, Ogundele A, Darby C, et al. Effect of OTX-101, a novel nanomicellar formulation of cyclosporine a, on corneal staining in patients with keratoconjunctivitis sicca: a pooled analysis of phase 2b/3 and phase 3 studies. Cornea. 2019;38(10):1259–65.
- Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, de Paiva CS, Gomes JAP, Hammitt KM, Jones L, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–12.
- Wood SI, Mian SD. Diagnostic tools for dry eye disease. Eur Ophthalmic Rev. 2016;10(2):101-7.
