ABSTRACT
Purpose
The formation of fibrovascular membranes (FVMs) is a serious sight-threatening complication of proliferative diabetic retinopathy (PDR) that may result in retinal detachment and eventual blindness. During the formation of these membranes, neurite/process outgrowth occurs in retinal neurons and glial cells, which may both serve as a scaffold and have guiding or regulatory roles. To further understand this process, we investigated whether previously identified candidate proteins, from vitreous of PDR patients with FVMs, could induce neurite outgrowth in an experimental setting.
Materials and methods
Retinal explants of C57BL6/N mouse pups on postnatal day 3 (P3) were cultured in poly-L-lysine- and laminin-coated dishes. Outgrowth stimulation experiments were performed with the addition of potential inducers of neurite outgrowth. Automated analysis of neurite outgrowth was performed by measuring β–tubulin-immunopositive neurites using Image J. Expression of PDGF receptors was quantified by RT-PCR in FVMs of PDR patients.
Results
Platelet-derived growth factor (PDGF) induced neurite outgrowth in a concentration-dependent manner, whilst neuregulin 1 (NRG1) and connective tissue growth factor (CTGF) did not. When comparing three different PDGF dimers, treatment with PDGF-AB resulted in the highest neurite induction, followed by PDGF-AA and -BB. In addition, incubation of retinal explants with vitreous from PDR patients resulted in a significant induction of neurite outgrowth as compared to non-diabetic control vitreous from patients with macular holes, which could be prevented by addition of CP673451, a potent PDGF receptor (PDGFR) inhibitor. Abundant expression of PDGF receptors was detected in FVMs.
Conclusion
Our findings suggest that PDGF may be involved in the retinal neurite outgrowth, which is associated with the formation of FVMs in PDR.
Introduction
Diabetic retinopathy (DR) is a chronic and progressive eye disease and a leading cause of blindness amongst the working-age population. Its proliferative stage, with retinal neovascularization, can result in severe intraocular hemorrhage as well as retinal traction and detachment, with vascular endothelial growth factor (VEGF) as the main driver.Citation1 In addition to VEGF, many other pro-angiogenic growth factors and pro-inflammatory cytokines have been reported to play a role in PDR, including PlGF, interleukins, angiopoietin-2, MMP-9 and CTGF.Citation2–5
Besides vascular pathogenesis, neuroglial cell migration/proliferation and fibrosis at the vitreoretinal interface are linked to proliferative DR (PDR). Importantly, fibrovascular membrane (FVM) formation is a serious sight-threatening complication of PDR as it may result in tractional retinal detachment.Citation6 Extensive neurite sprouting occurs during the formation of fibrovascular membranes (FVMs) and may originate from any type of neuron including photoreceptors, bipolar, amacrine and ganglion cells, as well as from retinal glial cells.Citation7–9 The role of these neurites in FVM formation is unknown, but it has been speculated that vascular growth is stimulated by neurotrophic factors derived from neurons and Müller cells and that the neurite outgrowths may also serve as a scaffold for the newly formed vessels.Citation7,Citation8,Citation10 During progression of the disease, in which myofibroblasts play a central role in a wound-healing-like response, fibrosis leads to FVM formation. In addition, Müller glial–mesenchymal transition has been postulated as an alternative fibrinogenic mechanism associated with membrane formation in PDR.Citation11 More precise knowledge of the molecular mechanisms underlying FVM development and progression may enable novel pharmacological interventions to be identified.
To date, the neurotrophic factors which initiate the neurite outgrowth in FVMs are unknown. In a previous study, we used a custom antibody array that included antibodies against various neurotrophic factors to analyze the protein content of vitreous samples of PDR patients with FVMs, as compared to PDR patients without FVMs.Citation12 Based on these expression data and on published associations with neurite outgrowth or formation of fibrovascular membranes, we selected two proteins that were highly elevated (>2.5-fold) in vitreous of PDR patients and which may have the potential to induce retinal neurite extension: neuregulin-1 (NRG1β1) and platelet-derived growth factor (PDGF). An additional finding in our previous study was that the PDGF axis has a central role in a protein cluster of co-regulated proteins.Citation12 NRG1β1 is a neurotrophic factor that is highly expressed in the central nervous system and that was found to stimulate neurite outgrowth in dorsal root ganglia explantsCitation13 and in the developing rat retina.Citation14 PDGF is a dimeric glycoprotein that can consist of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB). In addition, two other dimeric proteins exist that consist of two C or two D subunits (PDGF-CC and PDGF-DD), which need proteolytic cleavage before receptor binding.Citation15 PDGF stimulates proliferation and differentiation of cells of mesenchymal origin, including fibroblasts, smooth muscle cells and Müller cells.Citation15 In vitro, PDGF has been shown to stimulate neuronal and Müller cell proliferation and neurite outgrowth in rat brain cultures.Citation16–19 In addition, we selected connective tissue growth factor (CTGF), which is highly elevated in vitreous of PDR patients and functionally linked to the formation of fibrous membranes, fibrosis and the angio-fibrotic switch.Citation3,Citation20 Whereas others were able to demonstrate elevated levels of BDNF, GDNF, NT-3 and NT-4,Citation21 we were unable to detect these neurotrophins in our previous studyCitation12 and therefore did not include them in the present study.
In the present study, we investigated the role of these selected growth factors on neurite outgrowth using ex vivo cultures of retinal explants isolated from neonatal mice. After 3 days of exposure to accumulating concentrations of growth factor, the total area of immunolabeled neurites was quantified per 100 µm segment by automatic morphometric analysis.Citation22 This organotypic model has been well characterized and applied to explore the potential of compounds of interest in supporting neurite outgrowth.Citation23–26 In this model, we subsequently tested the effect of vitreous from PDR patients on neurite outgrowth as compared to vitreous from non-diabetic patients undergoing vitrectomy for macular holes.
Materials and methods
Animals
Experiments were performed using C57BL6/N mice of both sexes on postnatal day 3 (P3). Breeding pairs and litters were housed under standard laboratory conditions, raised in 12/12 hours light/dark cycle, with food and water ad libitum. All animal experiments were approved by the Institutional Ethical Committee of KU Leuven and followed the European Communities Council Directive of 22 September 2010 (2010/63/EU).
Human samples/tissue
Pooled vitreous samples were used from both control patients that had undergone vitrectomy for macular hole (n = 5) and separately from PDR patients showing high vitreal PDGF levels (n = 7). Pooling was necessary to obtain a sufficient sample with equal amounts of PDGF. The concentrations of PDGF-AA and PDGF-BB protein in the vitreous samples were quantified by using an array-based multiplex immunoassay (Human Quantibody array) performed by the manufacturer (RayBiotech, Norcross, GA, USA) with 200 µl of each vitreous sample. PDGF-AB was not determined in these samples.
The vitreoretinal membranes were harvested during pars plana vitrectomy from a separate set of patients that were operated on for removal of primary epiretinal membranes from non-diabetic patients (n = 5) or for removal of fibrovascular membranes from PDR patients (n = 4). Retinal tissue was isolated from donor eyes (n = 6), provided anonymously by the Cornea Bank Beverwijk, The Netherlands (http://www.eurotissuebank.nl/comeabank/), as described previously.Citation12 The study followed the tenets of the Declaration of Helsinki, with approval from the National Health Service Research Ethics Committee (South East Coast – Surrey research ethics committee reference 12/LO/0130). Signed informed consent was obtained from the subjects after explaining the nature of the study. Patient data was treated confidentially, and samples were anonymized.
Compounds
The following neurogenic and angiogenic factors, dissolved in culture medium, were used: neuregulin 1 β1 human recombinant (NRG1β1, CYT-733) used at 10, 50 and 100 ng/ml; connective tissue growth factor human recombinant (CTGF, CYT-541) used at 10, 50 and 100 ng/ml; and platelet-derived growth factor (PDGF). From the latter compound, three isoforms were investigated: PDGF-AA human recombinant (CYT-590), PDGF-AB human recombinant (CYT-342) and PDGF-BB human recombinant (CYT-501), respectively, used at concentrations of 1, 10, 50 or 100 ng/ml. All compounds were derived from ProspecBio (Rehovot, Israel). CP673451 (Tocris, Bio-techne, Abingdon, UK) was used as a potent and selective PDGFRα/β inhibitor at a concentration of 100 nM.Citation27 A combination of recombinant brain-derived neurotrophic factor (BDNF, cat# 450–02, 5 ng/ml) and recombinant ciliary neurotrophic factor (CNTF, cat# 450–50, 1 ng/ml), both purchased from Preprotech (London, UK), was used as a positive control.
Retinal explant culture
Retinal explant cultures were performed as described previously.Citation23 Briefly, retinas were dissected from postnatal day 3 (P3) mouse pups and 750 μm diameter explants were punched from the retinas. Explants were cultured in poly-L-Lysine- (0.25 mg/ml) and laminin (2 μg/ml)-coated 4-well plates (NunclonTM dElta Surface, ThermoFisher Scientific, Waltham, MA, USA) for 3 days, oriented with the retinal ganglion cell (RGC) layer facing the coated surface to allow RGC axons to grow out from the retinal tissue. Explants were cultured in Neurobasal-A medium supplemented with 1 mM L-glutamine, 0.25 μg/ml Fungizone, 100 U/ml penicillin, 100 μg/ml streptomycin, 2% B27 supplement (all from Invitrogen, Carlsbad, CA, USA), and 0.4% methylcellulose (Sigma-Aldrich, St. Louis, MO, USA). In addition, the culture medium was supplemented with either 250 µl neurogenic/angiogenic compounds or vitreous samples from control or PDR patients (1/5 diluted in PBS). During the 3-day incubation period, half of the medium was replaced every 24 hours by fresh medium, containing the same concentration of compound or vitreous sample.
Immunological staining
After 3 days in culture, explants were rinsed with cold phosphate-buffered saline (PBS) and fixed in 4% phosphate-buffered paraformaldehyde (PFA) for 1 hour. The explants were rinsed and incubated in blocking solution for 45 minutes. Neurite outgrowth was visualized using a mouse anti-β-tubulin primary antibody (Sigma-Aldrich) overnight and an Alexa-488-labeled secondary antibody (Invitrogen) for 2 hours, as previously described.Citation23 Explants were counterstained with 4ʹ,6-diamidino-2-phenylindole (DAPI, 1 µg/ml) in PBS for 30 minutes and mounted with mowiol (Sigma-Aldrich). All explant pictures were taken on a confocal microscope (Olympus, FV1000) and analyzed using an in-house ImageJ script, all as described.Citation23,Citation28
Image analysis
Automated analysis of neurite outgrowth was performed by measuring the Immunodetected Neurite Area (INA), identified as the area containing outgrowing β-tubulin-immuno-positive neurites attached to the explant body. The INA was normalized for explant size by dividing by the perimeter of the DAPI-stained explant body and normalized further by equating the control explants per experiment to 100%. Next, the acquired INA was divided into four segments, using concentric circles, each 100 µm further from the explant body. This provides three ring segments and an outer segment with the remaining immune-labeled neurites, allowing the effect of the compounds on axonal growth to be investigated by making a distinction between neurite outgrowth initiation (close to explant body; 0–100 µm and 100–200 µm) and neurite elongation (further from the explant body; 200–300 µm and >300 µm).Citation23
Quantitative RT-PCR
RNA was isolated using TRIzol reagent (ThermoFisher Scientific). RNA integrity was verified using an Experion Automated Electrophoresis System (Bio-Rad, Hercules CA, USA). All samples had clear ribosomal RNA bands with minimal sign of degradation. Complementary DNA (cDNA) was generated from 1 μg RNA per sample. cDNA and RNA were stored at −80°C.
Primer sequences were as follows: PDGFRA forward 5ʹ-ACATCGGAGGAGAAGTTTCCCAGA-3ʹ and reverse 5ʹ-TTAGGCTCAGCCCTGTGAGAAGA-3ʹ; PDGFRB forward 5ʹ-AGCCAGCTCCACCCTGAATG-3ʹ; and reverse 5ʹ-CGAATCCGGCAACTGTTCCAG-3ʹ. Real-time quantitative PCR was performed on 20x diluted cDNA samples using a CFX96 system (Bio-Rad, Hercules, CA). Ct values were converted to arbitrary absolute amounts (2−Ct × 1e12) and normalized to tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) mRNA levels for each sample, forward 5ʹ-ACTTTTGGTACATTGTGGCTTCAA-3ʹ, and reverse 5ʹ-CCGCCAGGACAAACCAGTAT-3ʹ.
Statistical analysis
Retinal explant data are presented as mean ± SEM. The normal distribution was verified using a Kolmogorov–Smirnov test, and parallel equal variance between groups was tested. Outliers were identified and excluded, based on a Grubbs’ test (extreme studentized deviate (ESD) method). One-way ANOVA followed by Dunnett’s multiple comparisons test or unpaired t-tests was performed. Gene expression data are presented as mean ± SD, and statistical differences between groups were analyzed with the Mann–Whitney U test. All statistical tests were performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California, USA). Differences were considered statistically significant with a probability level of α <0.05.
Results
Platelet-derived growth factor (PDGF) as an important initiator of neurite outgrowth
As demonstrated in , treatment with the positive controls, BDNF and CNTF, effectively induced neurite outgrowth in retinal explants, while treatment with increasing concentrations of NRG1 did not stimulate neurite outgrowth as compared to the control condition. As with NRG1, incubation with CTGF also did not augment neurite outgrowth.
Figure 1. Neurite outgrowth in retinal explants incubated with selected growth factors. Retinal explants were incubated with increasing concentrations of recombinant human neuregulin 1 β1 (NRG1; 10, 50 and 100 ng/ml), recombinant human connective tissue growth factor (CTGF; 10, 50 and 100 ng/ml) and recombinant human platelet-derived growth factor (PDGF; 1, 10 and 50 ng/ml). A combination of recombinant brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) was used as positive control (B/C), and untreated explants served as a negative control (CON). (a) Total neurite outgrowth area was determined by automated morphometric analysis of β-tubulin immunostained mouse retinal explants. Results are presented as mean ± SEM of 8–13 explants of 2 biological repeats. One-way ANOVA with Dunnett’s multiple comparison test was used to calculate statistical differences (** P < .01, *** P < .001). (b) Representative images of explants of each condition are shown with NRG1, CTGF and PDGF-BB at a concentration of 50 ng/ml. Scale bar is 500 µm
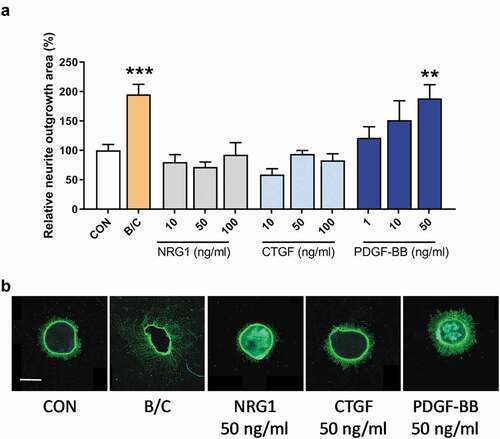
However, when mouse retinal explants were exposed to PDGF-BB, a concentration-dependent increase in neurite outgrowth was observed using a concentration of 1–50 ng/ml with the highest concentration of PDGF-BB showing a significant difference as compared to control retinas. For this initial experiment, we chose PDGF-BB since it has previously been shown to induce Müller cell proliferation,Citation19 neuronal differentiationCitation16 and neurite outgrowth from primary rat brain cultures.Citation18
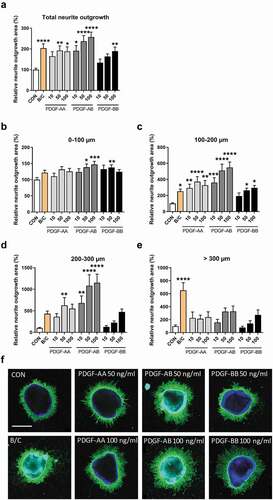
To further investigate the effects of PDGF on neurite outgrowth, we tested the effect of not only PDGF-BB but also PDGF-AA and -AB ligands on neurite outgrowth and at higher concentrations ranging from 10 to 100 ng/ml, as the dose-dependent increase observed in our initial experiments did not reach a plateau (). PDGF-AA treatment showed a 1.9-fold increase in total neurite outgrowth at a concentration of 50 and 100 ng/ml, as compared to control (P = .0054 and 0.0370, respectively). PDGF-AB demonstrated a concentration-dependent increase in total neurite outgrowth of 1.9-fold at 10 ng/ml (P = .0108), 2.4-fold at 50 ng/ml (P < .0001) and 2.6-fold at 100 ng/ml (P < .0001), while PDGF-BB addition resulted in a 1.9-fold increase in total neurite outgrowth at 100 ng/ml (P = .0078) (). When we distinguished neurite outgrowth initiation and elongation using the concentric rings described in the methods, we confirmed that treatment of explants with BDNF/CNTF robustly stimulated neurite elongation, which was clearly visible in the outer segment (>300 µm).Citation25 Of all ligands, PDGF-AB (at 100 ng/ml) showed the largest effect on neurite outgrowth initiation and elongation with a 1.5-fold, 5.5-fold and 11.5-fold difference as compared to controls in regions of 0–100 µm (P = .0006; ), 100–200 µm (P < .0001; ) and 200–300 µm (P < .0001; ), respectively. The effects of PDGF-AA were somewhat less robust and most pronounced at a concentration of 50 ng/ml with 3.7-fold in the regions of 100–200 µm (P < .0001; ) and 6.3-fold in the regions of 200–300 µm (P = .0059; ). PDGF-BB at 50 mg/ml showed an increase in neurite outgrowth initiation with a difference of 1.4-fold as compared to controls in regions of 0–100 µm (P = .0033; ) and at 100 ng/ml an increase of 2.9-fold in regions of 100–200 µm (P = .0146; ). None of the ligands showed a significant effect in the region >300 µm (representative images of the two highest concentrations that show the largest differences as compared to control are given in ). Overall, PDGF-AB showed the most pronounced effects on initiation of neurite outgrowth, but even more on neurite elongation, which was especially evident by the 11.5-fold increase in the region 200–300 µm.
Figure 2. Neurite outgrowth in retinal explants incubated with three different PDGF dimers. Retinal explants were incubated with increasing concentrations (10, 50 and 100 ng/ml) of PDGF-AA, -AB or -BB. A combination of recombinant brain-derived neurotrophic factor (BDNF) and recombinant ciliary neurotrophic factor (CNTF) was used as positive control (B/C). Relative neurite outgrowth area was determined as compared to control conditions (CON) in total (a) and 100 µm segments of 0–100 µm (b), 100–200 µm (c), 200–300 µm (d) and >300 µm segments (e). Results are presented as mean ± SEM of 14–36 explants of three biological repeats. One-way ANOVA with Dunnett’s multiple comparison test was used to calculate statistical differences (* P < .05, ** P < .01, *** P < .001, **** P < .0001). (f) Representative images of explants of each condition are shown with each PDGF-dimer at a concentration of 50 and 100 ng/ml. Scale bar is 500 µm
Pharmacological inhibition of PDGF signaling is sufficient to prevent neurite outgrowth induced by vitreous of PDR patients
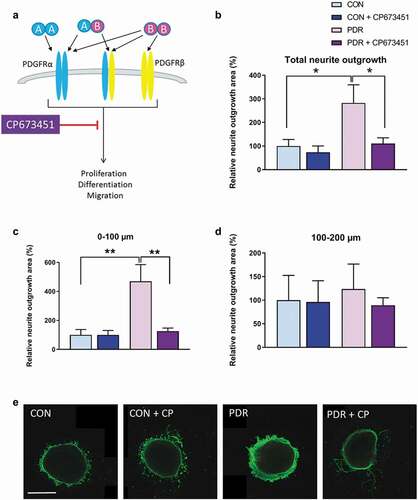
Next, we wanted to investigate whether PDGF in vitreous samples of PDR patients was able to induce neurite outgrowth and whether this could be blocked with a specific PDGF inhibitor. For this purpose, we used pooled vitreous samples from seven PDR patients with relatively high PDGF-AA protein levels and pooled vitreous samples from five control patients. Together, the pooled vitreous samples from PDR patients contained 5249 pg/ml PDGF-AA and 1.8 pg/ml PDGF-BB. The pooled vitreous from control patients contained 41.7 pg/ml PDGF-AA and no detectable PDGF-BB.
Retinal explants incubated with vitreous from PDR patients showed, on average, a 2.8-fold greater induction of neurite outgrowth as compared to control vitreous (). To investigate whether specific inhibition of PDGF receptor signaling in vitreous from PDR patients was able to prevent this induced neurite outgrowth in the retinal explants, we used the pharmacological inhibitor CP673451, which is directed against both alpha and beta PDGF receptor types, thereby inhibiting downstream signaling of PDGF ligand binding (). When we simultaneously added the potent PDGFR inhibitor, the stimulation of neurite outgrowth by the PDR vitreous was completely abrogated, except for some sporadic single neurites (). This was especially observed in the region up to 100 µm away from the explant (), but not in regions at a longer distance () and in regions >200 µm (data not shown). Neurite outgrowth measured after culturing explants in control vitreous with PDGFR inhibitor was comparable to that without inhibitor (–e). Together, these results suggest that vitreous samples of PDR patients with high protein levels of PDGF-AA induce neurite outgrowth and that pharmacologic inhibition of PDGF signaling is able to prevent this.
Figure 3. Neurite outgrowth in retinal explants incubated with vitreous of PDR patients or non-diabetic controls with macular holes in the presence or absence of the PDGFR inhibitor CP673451. (a) CP673451 blocks downstream signaling of both PDGFRα and -β leading to processes such as proliferation, differentiation and migration. Relative neurite outgrowth area was determined as compared to control conditions (CON) in total (b), 100 µm segments of 0–100 µm (c) and 100–200 µm (d). Results are presented as mean ± SEM of 6–10 explants of two biological repeats. Unpaired t-tests were used to calculate statistical differences (* P < .05, ** P < .01). (e) Representative images of explants cultured with pooled control vitreous or pooled vitreous of PDR patients in the presence or absence of CP673451 (CP). Scale bar is 500 µm
Expression of PDGF receptors in vitreoretinal membranes and retina
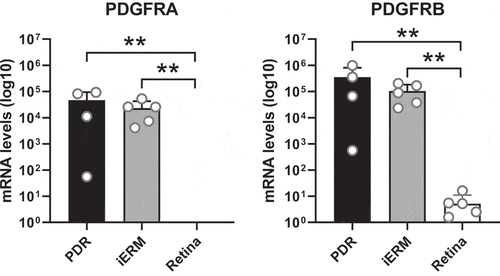
To further investigate the cellular mechanisms by which PDGF signaling may contribute to neurite/process outgrowth and FVM development, gene expression levels of PDGFA and PDGFB ligands and their receptors PDGFRα and -β were evaluated in FVMs from patients with PDR via quantitative RT-PCR. Whereas very low or undetectable mRNA levels of PDGFA and -B were found in FVMs of PDR patients,Citation12 PDGFRα and -β were found to be abundantly expressed (). For comparison, we investigated mRNA levels in idiopathic epiretinal membranes (iERMs) from the eyes of non-diabetic patients with macular pucker and in whole retinae from non-diabetic donors. Expression of both receptors in iERMs was comparable to that of FVMs in PDR, whereas absent or low levels of PDGFRα and -β were found in non-diabetic retina.
Figure 4. Expression of PDGFRA and PDGFRB in vitreoretinal membranes and human retina. Levels of PDGFRA and PDGFRB mRNA was determined by real-time quantitative PCR and presented as absolute amounts in Log10 scale. Mean ± SD are given of fibrovascular membranes of patients with PDR (PDR, n = 4), idiopathic epiretinal membranes of non-diabetic patients with a macular pucker (iERM, n = 5) and non-diabetic retina (Retina, n = 6). All data points of PDGFRA and one data point of PDGFRB in retina was below 1 and therefore not visible in the graph. The Mann–Whitney U test was used to compare statistical differences between groups (**P < .01)
Discussion
Of the three tested candidate growth factors, PDGF was found to be an important stimulator of neurite outgrowth in murine retinal explants, whereas no effect was observed for NRG1 or CTGF. In addition, we showed that neurite outgrowth is induced by vitreous of PDR patients with high protein levels of PDGF-AA, which could be prevented by a specific inhibitor of PDGF receptor-mediated signaling. Furthermore, our study revealed that PDGFRα and -β mRNA expression is highly abundant in FVMs of PDR patients, whereas expression in non-diabetic human retina is absent or low. Together, these results indicate that PDGF may be a prominent initiator of neurite outgrowth leading to fibrovascular membranes in PDR. PDGF signaling may play a role in the formation of not only FVMs but also iERMs in non-diabetic patients with macular pucker since highly abundant expression of both receptors was found in both membrane types.
PDGF receptors are cell surface receptor tyrosine kinases that have 10 phosphorylation sites, which upon activation can interact with a substantial number of downstream acting proteins, including signaling kinases Src, Fer, phosphoinositide 3-kinase (PI3K), Src homology 2 (SH2) domain–containing phosphatase 2 (SHP2) and phospholipase C (PLC), thereby leading to activation of several signaling pathways, including the PI3K pathway, mitogen-activated protein (MAP) kinase pathways and the PLC pathway.Citation29 Exactly which pathways are involved in the formation of FVMs remains elusive and needs future investigation.
The selection of PDGF as a candidate for induction of neurite outgrowth was based on expression levels in vitreous of PDR patientsCitation12 and their published activity on neurite proliferation. In our previous exploratory study with vitreous samples from PDR patients with FVMs, the PDGF axis was shown to play a central role in a network of co-regulated proteins.Citation12 Our hypothesis-driven approach identified PDGF as a major inducer of neurite outgrowth, but this does not exclude that other growth factors and cytokines may also play an important role in neurite outgrowth in PDR. For example, two recent studies showed the involvement of the chemokine axis CXCL16/CXCR6 and the metalloproteinases ADAM10 and ADAM17Citation30 and the involvement of CD146Citation31 in vitreous of PDR patients, FVMs and in Müller cell activation.
The observed effects of PDGF on neurite outgrowth confirm the results from previous studies. Cultured primary rat neuronal cells treated with PDGF-BB for 2 days resulted in extended cell survival and increased outgrowth of neurites.Citation18 In addition, prolonged incubation of more than 10 days with PDGF-BB resulted in an extended survival of cultured GABAergic interneurons.Citation32 PDGF-BB was also found to stimulate proliferation of Müller cells and PDGF receptor phosphorylation, which was blocked by a PDGFR-selective tyrosine kinase inhibitor.Citation19 Müller cells may play a role in the formation of FVMs in PDR. Proliferating Müller cells are considered to be a scaffold for neurites to grow on,Citation7,Citation33 and recently, Müller glial–mesenchymal transition was postulated as an alternative fibrinogenic mechanism associated with membrane formation in PDR.Citation11 Another recent study showed that proliferation and migration of cultured Müller cells were stimulated by vitreous of PDR patients (Rezzola et al. 2021).Citation34 Together, this provides further evidence for a role of Müller cells in the formation of FVMs in PDR.
Since treatment of PDR as well as neovascular AMD with anti-VEGF therapy may be ineffective or incomplete,Citation35 additional therapies have been sought including anti-PDGF therapy. In the cancer biology field, it was found that a combination therapy targeting PDGF and VEGF pathways was more effective as an anti-angiogenic treatment than anti-VEGF therapy alone, which could largely be subscribed to the role of pericytes in this process.Citation36–40 Also in ocular models of corneal and choroidal neovascularization, increased vascular regression was observed using a combination of an anti-VEGF aptamer and an anti-PDGFR-β antibody as compared to an anti-VEGF aptamer alone.Citation41 In the laser-induced CNV model in mice, it was found that PDGFR-expressing mesenchymal (pericyte-like) cells infiltrated into the wound to form a scaffold for endothelial cells to develop new vessels, which could be prevented by inhibiting PDGF signaling.Citation42
Dual targeting against PDGF (Fovista) and VEGF (ranibizumab) in a Phase 2B trial in neovascular AMD showed promising results as compared to ranibizumab alone, including reduced macular fibrosis and a higher gain in visual acuity.Citation43 This has prompted many other companies to invest in the development of novel applications using this dual-target approach. However, a follow-up phase 3 study by Fovista failed to show the beneficial effects of the combination therapy over the monotherapy, and likewise, other combination therapies targeting PDGF and VEGF signaling showed disappointing results.Citation44
The lack of treatment efficacy in this study may have been due to the wide range of pleiotropic effects of PDGF and its role in survival of multiple cell types, including endothelial cells, pericytes, ganglion cells, neurons and Müller cells.Citation45,Citation46 Müller glia, which closely interact with pericytes, express high levels of PDGF and PDGFRα and -β,Citation45,Citation46 suggesting an important role for a PDGF-mediated cross talk between Müller cells and pericytes during angiogenesis and vascular homeostasis. This has been confirmed with Müller cell-specific PDGFRα knockout in the laser-induced choroidal neovascularization (CNV) model in mice although PDGFRα deletion also showed adverse effects on Müller cell homeostasis.Citation47 Knockout mice showed reduced vascular leakage and smaller CNV lesion areas as compared to wild-type animals. However, under conditions of hypo-osmotic stress, in which Müller cells normally do not swell, PDGFRα-deficient Müller cells immediately increase in volume. More research is clearly needed on the effect of PDGF inhibition on Muller cell viability and homeostasis.
In conclusion, our study supports the role of PDGF signaling in neurite outgrowth during the formation of FVMs in PDR. A possible involvement of Müller cells in this process needs to be further established. The development of novel therapies based on these findings is not immediately evident, given the protective properties of PDGF on neurons, Müller cells and many other retinal cells. However, our study provides important new insights into the molecular mechanisms of FVM formation in PDR.
Data availability statement
Raw data were generated at the Department of Biology, KU Leuven, and the Department of Ophthalmology, Amsterdam UMC. Derived data supporting the findings of this study are available from the corresponding author [I.K.] on request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016 Mar;51:156–86.
- Deuchler S, Schubert R, Singh P, Chedid A, Brui N, Kenikstul N, Kohnen T, Ackermann H, Koch F. Vitreous expression of cytokines and growth factors in patients with diabetic retinopathy – an investigation of their expression based on clinical diabetic retinopathy grade. PLoS One. 2021;16(5):e0248439. doi:https://doi.org/10.1371/journal.pone.0248439.
- Ma T, Dong LJ, Du XL, Niu R, Hu BJ. Research progress on the role of connective tissue growth factor in fibrosis of diabetic retinopathy. Int J Ophthalmol. 2018;11:1550–54.
- Opdenakker G, Abu El-Asrar A. Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell Mol Life Sci. 2019 Aug;76(16):3157–66. doi:https://doi.org/10.1007/s00018-019-03177-3.
- Van Bergen T, Etienne I, Cunningham F, Moons L, Schlingemann RO, Feyen JHM, Stitt AW. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog Retin Eye Res. 2019 Mar;69:116–36.
- Berrocal MH, Acaba LA. Surgical management of fibrovascular membranes. Advances in technique are improving patient outcomes. Retinal Physician. 2018;15:20–23.
- Lesnik Oberstein SY, Lewis GP, Chapin EA, Fisher SK. Ganglion cell neurites in human idiopathic epiretinal membranes. Br J Ophthalmol. 2008 Jul;92(7):981–85. doi:https://doi.org/10.1136/bjo.2007.132332.
- Lesnik Oberstein SY, Lewis GP, Dutra T, Fisher SK. Evidence that neurites in human epiretinal membranes express melanopsin, calretinin, rod opsin and neurofilament protein. Br J Ophthalmol. 2010;95(2):266–72. doi:https://doi.org/10.1136/bjo.2010.180679.
- Kim LA, Wong LL, Amarnani DS, Bigger-Allen AA, Hu Y, Marko CK, Eliott D, Shah VA, McGuone D, Stemmer-Rachamimov AO, et al. Characterization of cells from patient-derived fibrovascular membranes in proliferative diabetic retinopathy. Mol Vis. 2015;21:673–87.
- Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honoré JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008 Oct;14(10):1067–76. doi:https://doi.org/10.1038/nm.1873.
- Wu D, Kanda A, Liu Y, Noda K, Murata M, Ishida S. Involvement of Müller glial autoinduction of TGF-β in diabetic fibrovascular proliferation via glial-mesenchymal transition. Invest Ophthalmol Vis Sci. 2020 Dec 1;61(14):29. doi:https://doi.org/10.1167/iovs.61.14.29.
- Klaassen I, De Vries EW, Vogels IMC, van Kampen AHC, Bosscha MI, Steel DHW, Van Noorden CJF, Lesnik-Oberstein SY, Schlingemann RO. Identification of proteins associated with clinical and pathological features of proliferative diabetic retinopathy in vitreous and fibrovascular membranes. PLoS One. 2017;12(11):e0187304. doi:https://doi.org/10.1371/journal.pone.0187304.
- Liu Z, Gao W, Wang Y, Zhang W, Liu H, Li Z. Neuregulin-1β regulates outgrowth of neurites and migration of neurofilament 200 neurons from dorsal root ganglial explants in vitro. Peptides. 2011 Jun;32(6):1244–48. doi:https://doi.org/10.1016/j.peptides.2011.04.005.
- Bermingham-McDonogh O, McCabe KL, Reh TA. Effects of GGF/neuregulins on neuronal survival and neurite outgrowth correlate with erbB2/neu expression in developing rat retina. Development. 1996 May;122(5):1427–38. doi:https://doi.org/10.1242/dev.122.5.1427.
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999 Oct;79(4):1283–316. doi:https://doi.org/10.1152/physrev.1999.79.4.1283.
- Park N, Yoo JC, Ryu J, Hong SG, Hwang EM, Park JY. Copine1 enhances neuronal differentiation of the hippocampal progenitor HiB5 cells. Mol Cells. 2012 Dec;34(6):549–54. doi:https://doi.org/10.1007/s10059-012-0235-7.
- Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014 Mar;9(2):168–81. doi:https://doi.org/10.1007/s11481-013-9479-z.
- Smits A, Kato M, Westermark B, Nistér M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8159–63. doi:https://doi.org/10.1073/pnas.88.18.8159.
- Moon SW, Chung EJ, Jung SA, Lee JH. PDGF stimulation of Müller cell proliferation: contributions of c-JNK and the PI3K/Akt pathway. Biochem Biophys Res Commun. 2009 Oct 9;388(1):167–71. doi:https://doi.org/10.1016/j.bbrc.2009.07.144.
- Klaassen I, Van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO. The role of CTGF in diabetic retinopathy. Exp Eye Res. 2015 Apr;133:37–48. doi:https://doi.org/10.1016/j.exer.2014.10.016.
- Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5594–603. doi:https://doi.org/10.1167/iovs.17-21973.
- Gaublomme D, Buyens T, Moons L. Automated analysis of neurite outgrowth in mouse retinal explants. J Biomol Screen. 2013 Jun;18(5):534–43. doi:https://doi.org/10.1177/1087057112471989.
- Buyens T, Gaublomme D, Van Hove I, De Groef L, Moons L. Quantitative assessment of neurite outgrowth in mouse retinal explants. Methods Mol Biol. 2014;1162:57–71.
- Bollaerts I, Van Houcke J, Andries L, De Groef L, Moons L. Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediators Inflamm. 2017;2017:9478542. doi:https://doi.org/10.1155/2017/9478542.
- Van Hove I, Lefevere E, Moons L. ROCK inhibition as a novel potential strategy for axonal regeneration in optic neuropathies. Neural Regen Res. 2015 Dec;10(12):1949–50. doi:https://doi.org/10.4103/1673-5374.172311.
- Van de Velde S, De Groef L, Stalmans I, Moons L, Van Hove I. Towards axonal regeneration and neuroprotection in glaucoma: rho kinase inhibitors as promising therapeutics. Prog Neurobiol. 2015 Aug;131:105–19. doi:https://doi.org/10.1016/j.pneurobio.2015.06.002.
- Roberts WG, Whalen PM, Soderstrom E, Moraski G, Lyssikatos JP, Wang HF, Cooper B, Baker DA, Savage D, Dalvie D, et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP-673,451. Cancer Res. 2005 Feb 1;65(3):957–66.
- Bollaerts I, Veys L, Geeraerts E, Andries L, De Groef L, Buyens T, Salinas-Navarro M, Moons L, Van Hove I. Complementary research models and methods to study axonal regeneration in the vertebrate retinofugal system. Brain Struct Funct. 2018 Mar;223(2):545–67.
- Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014 Jun;25(3):273–83. doi:https://doi.org/10.1016/j.cytogfr.2014.03.003.
- Abu El-Asrar AM, Nawaz MI, Ahmad A, De Zutter A, Siddiquei MM, Blanter M, Allegaert E, Gikandi PW, De Hertogh G, Van Damme J, et al. Evaluation of proteoforms of the transmembrane chemokines CXCL16 and CX3CL1, their receptors, and their processing metalloproteinases ADAM10 and ADAM17 in proliferative diabetic retinopathy. Front Immunol. 2020;11:601639. doi:https://doi.org/10.3389/fimmu.2020.601639.
- Abu El-Asrar AM, Nawaz MI, Ahmad A, Siddiquei MM, Allegaert E, Gikandi PW, De Hertogh G, Opdenakker G. CD146/soluble CD146 pathway is a novel biomarker of angiogenesis and inflammation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021 Jul 1;62(9):32. doi:https://doi.org/10.1167/iovs.62.9.32.
- Smits A, Ballagi AE, Funa K. PDGF-B-B exerts trophic activity on cultured GABA interneurons from the newborn rat cerebellum. Eur J Neurosci. 1993 Aug 1;5(8):986–94. doi:https://doi.org/10.1111/j.1460-9568.1993.tb00950.x.
- Lewis GP, Betts KE, Sethi CS, Charteris DG, Lesnik-Oberstein SY, Avery RL, Fisher SK. Identification of ganglion cell neurites in human subretinal and epiretinal membranes. Br J Ophthalmol. 2006;91(9):1234–38. doi:https://doi.org/10.1136/bjo.2006.104612.
- Rezzola S, Guerra J, Krishna Chandran AM, Loda A, Cancarini A, Sacristani P, Semeraro F, Presta M. VEGF-independent activation of Müller cells by the vitreous from proliferative diabetic retinopathy patients. Int J Mol Sci. 2021 Feb 22;22(4). doi:https://doi.org/10.3390/ijms22042179.
- Low A, Kansagara D, Freeman M, Fu R, Bhavsar K, Faridi A, Kondo K, Paynter R. Comparative Clinical and Economic Effectiveness of Anti-vascular Endothelial Growth Factor Agents [Internet]. Washington (DC): Department of Veterans Affairs (US); 2017 Jan. PMID: 29369569.
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003 Oct;112(8):1142–51. doi:https://doi.org/10.1172/JCI200318549.
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003 May;111(9):1287–95. doi:https://doi.org/10.1172/JCI200317929.
- Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004 Feb;18(2):338–40. doi:https://doi.org/10.1096/fj.03-0271fje.
- Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006 Oct;116(10):2610–21. doi:https://doi.org/10.1172/JCI24612.
- Mabry R, Gilbertson DG, Frank A, Vu T, Ardourel D, Ostrander C, Stevens B, Julien S, Franke S, Meengs B, et al. A dual-targeting PDGFRbeta/VEGF – a molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo. MAbs. 2010 Jan-Feb;2(1):20–34. doi:https://doi.org/10.4161/mabs.2.1.10498.
- Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006 Jun;168(6):2036–53. doi:https://doi.org/10.2353/ajpath.2006.050588.
- Strittmatter K, Pomeroy H, Marneros AG. Targeting platelet-derived growth factor receptor β(+) scaffold formation inhibits choroidal neovascularization. Am J Pathol. 2016 Jul;186(7):1890–99. doi:https://doi.org/10.1016/j.ajpath.2016.02.018.
- Jaffe GJ, Ciulla TA, Ciardella AP, Devin F, Dugel PU, Eandi CM, Masonson H, Monés J, Pearlman JA, Quaranta-El Maftouhi M, et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: a Phase IIb, multicenter, randomized controlled trial. Ophthalmology 2017 Feb;124(2):224–34. doi:https://doi.org/10.1016/j.ophtha.2016.10.010.
- Dunn EN, Hariprasad SM, Sheth VS. An overview of the fovista and rinucumab trials and the fate of anti-PDGF medications. Ophthalmic Surg Lasers Imaging Retina. 2017 Feb 1;48(2):100–04. doi:https://doi.org/10.3928/23258160-20170130-02.
- Cox OT, Simpson DA, Stitt AW, Gardiner TA. Sources of PDGF expression in murine retina and the effect of short-term diabetes. Mol Vis. 2003 Dec;10(9):665–72.
- Biswas SK, Zhao Y, Nagalingam A, Gardner TW, PDG SL. F- and insulin/IGF-1-specific distinct modes of class IA PI 3-kinase activation in normal rat retinas and RGC-5 retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3687–98. doi:https://doi.org/10.1167/iovs.07-1455.
- Díaz-Lezama N, Wolf A, Koch S, Pfaller AM, Biber J, Guillonneau X, Langmann T, Grosche A. PDGF Receptor Alpha Signaling Is Key for Müller Cell Homeostasis Functions. Int J Mol Sci. 2021 Jan 25;22(3):1174. doi:https://doi.org/10.3390/ijms22031174
