ABSTRACT
Purpose/Aim of the study
To evaluate the improvement of ocular signs and symptoms in patients suffering from Demodex blepharitis using a combined treatment approach: use of eyelid wipes impregnated with 2.5% terpinen-4-ol (T4O) and 0.2% hyaluronic acid (HA) in the initial treatment period and investigation of maintenance of the treatment effect with the use of eyelid cleansing wipes.
Materials and Methods
Fifty patients with Demodex blepharitis were treated in the initial treatment period with sterile eyelid T4O impregnated wipes for 28 days. In the following four-week maintenance period, 82% patients received sterile eyelid maintenance wipes, while 16% continued treatment with T4O impregnated wipes. Global ocular discomfort, adapted TOSS, SANDE score, and individual blepharitis symptoms were assessed by patients at day 28 and day 56. Ocular signs were evaluated by the investigator at the study visits. Investigator’s assessment of the overall treatment performance, patient’s assessment of treatment satisfaction, and tolerability were evaluated with questionnaires.
Results
All global ocular discomfort symptoms and disease specific symptoms assessed by patients as well as all parameters evaluated by the investigators significantly improved in the initial treatment period with the application of eyelid wipes impregnated with 2.5% terpinen-4-ol until day 28. The therapeutic effect was maintained or even improved during the maintenance period under administration of mainly eyelid maintenance wipes until day 56. Both products were well tolerated. No adverse events and no clinically relevant changes in visual acuity were observed during both periods.
Conclusions
Once daily treatment with T4O impregnated eyelid wipes in the initial treatment period significantly improved the ocular symptoms and signs and reduced the mite count in patients with Demodex blepharitis within four-weeks administration. Subsequent maintenance treatment with maintenance wipes for another 4 weeks preserved or further intensified the treatment success. The products were well tolerated and were convenient to use.
Introduction
Demodex mites, D. folliculorum and D. brevis, have been implicated to have a significant impact on the pathophysiology of anterior and posterior blepharitis, blepharoconjunctivitis, blepharokeratitis, and beyond. There are studies showing a correlation between an increased number of Demodex and subjective symptoms of blepharitis.Citation1,Citation2 It was shownCitation1 that the number of Demodex mites is associated and related to the presence of cylindrical dandruffCitation3, ocular discomfort and tear film instability and may also be correlated with poor ocular hygiene. Affected patients usually present ocular symptoms such as itching, redness, burning, foreign body sensation, eyelid crusting, and blurred vision.
One of the primary goals in the treatment of Demodex blepharitis is to reduce parasitic overpopulation in the lids and lashes to decrease inflammation and to provide a healthier environment for the ocular surface. Traditional therapies for blepharitis include warm compresses and antibiotic/steroid combinations; however, these therapies do not eradicate Demodex, and they even often cause the condition to persist.
There are several effective treatment options for patients with Demodex blepharitis, and the selection is based on the severity of the condition. The most effective and recent treatment agent for Demodex is tea tree oil (TTO).Citation4–6 It has been reported that lid scrubs with different concentrations of TTO are effective in reducing Demodex mite counts and ocular surface inflammation associated with blepharitis, conjunctivitis, and keratitis.Citation2
With regard to biological activity, it has been shown that the antimicrobial activity of TTO is attributed mainly to terpinen-4-ol (T4O), a major component of the essential oil.Citation4,Citation5 The potency of T4O was greater than that of TTO at an equivalent concentration, and it possesses anti-inflammatory, antimicrobial, antiviral, and antifungal properties.Citation7 In a study with 24 patients displaying Demodex infestation, 2.5% T4O was the most effective treatment to kill D. folliculorum. These observations led to the development of T4O impregnated wipes additionally containing hyaluronic acid (HA).Citation8–10 A recent study with these sterile wipes revealed improvement of ocular symptoms and signs of Demodex blepharitis after application once or twice daily over 4 weeks.Citation11
However, while treatment methods including TTO are effective at reducing Demodex, studies have shown that no single management option fully eradicates Demodex after 4 weeks of therapy, highlighting the chronic nature of the condition and the requirement for long-term therapy.Citation12
The aim of this study is to evaluate the therapeutic benefit and safety of a long-term treatment regimen consisting of 2 subsequent treatment phases with different products: a so-called ‘initial treatment period’ with use of sterile 2.5% T4O-containing eyelid wipes for 4 weeks followed by a ‘maintenance period’ with administration of sterile preservative-free eyelid maintenance wipes containing HA and plant extracts from Iris florentina and Centella asiatica for another 4 weeks.
Materials and methods
Study design
This prospective, open label, multicenter, post market study with two medical devices was performed at two sites in Germany between Sep 2019 and Mar 2020. The study was conducted in accordance with Good Clinical Practise, Declaration of Helsinki, and local health regulations. Ethics committee approval was obtained for all centers prior to enrollment of the first subject. All participants provided written informed consent.
Participants
Subjects were regarded as eligible for enrollment in the study if they met the following inclusion criteria and none of the exclusion criteria as evaluated by the investigator, i.e., an ophthalmologist at screening and before the start of the treatment. Investigators decided for each patient if the planned study specific treatment regimen was suitable for the patient. Patients, who presented a very severe blepharitis and were deemed more suitable for alternative treatment regimes, e.g., antibiotics such as topical azithromycin or systemic tetracycline derivates, were not included in the study.
Females of child-bearing potential had to agree to a pregnancy test at screening with a negative test result.
Treatments and assessments
Treatments
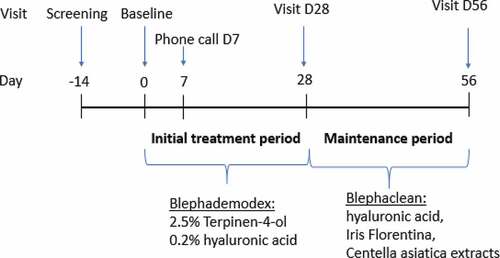
The study comprised two subsequent treatment periods with different products ().
During the first treatment period (initial treatment period), patients used one 2.5% T4O and 0.2% hyaluronic acid containing eyelid wipe (Blephademodex® also referred to as 2.5% T4O-wipes in the text) per eye every evening to clean the eyelids of both eyes for 28 days. At the end of the initial treatment period (day 28 ± 3), the patients evaluated the treatment effect using the Global Discomfort Scale (GDS) and the ophthalmologist assessed the signs and symptoms of blepharitis and Demodex infestation as described above for the inclusion. The decision on the treatment for the second study period (maintenance period) was based on the subject’s assessment of the GDS at day 28 (improvement of GDS by at least one point on the severity scale on day 28 compared to Baseline) and on investigator’s evaluation at day 28 if the condition was resolved and managed accordingly. If both conditions were fulfilled, the subject changed to sterile eyelid cleansing wipes containing hyaluronic acid and Iris florentina and Centella asiatica extracts (Blephaclean®, referred to as maintenance wipes in the text) every evening to clean the eyelids of both eyes according to manufacturer’s instructions for the second study period (maintenance period) of 28 days. If one of the two conditions was not fulfilled (either no GDS improvement by at least one point or the investigator judged the condition as not appropriately managed), the subject should continue with 2.5% T4O-wipes for another 28 days. At day 56 ± 3 (final visit), patients returned to the study site and the ocular signs and symptoms were evaluated. If patients used lubricating eye drops for the treatment of dry eyes at the day of enrollment, they were allowed to continue using these. For the days of study visits, no creams, lotions, or detergents were permitted to be used on the area to be assessed.
Assessments
The following assessments were done by the patients at day 0, day 28, and day 56:
The GDS of the eyes related to Demodex blepharitis experienced at the present day was verbally evaluated using the 0–10 numeric rating scale (NRS) ranging from 0 (no ocular discomfort) to 10 (worst ocular discomfort imaginable).
The adapted Total Ocular Symptom Score (TOSS) for eight different symptoms (itchy eyelids, eyelid redness, red eyes generally, swollen/puffy eyes, tearing eyes, crusting around the lashes, burning or stinging sensations, and noticed eyelash loss) was evalua. Patients rated with an overall score (0 = none of the time, 1 = some of the time, 2 = half of the time, 3 = most of the time, and 4 = all of the time) for each symptom if they experienced it during the last week. The adapted TOSS (ranging from 0–100) was calculated as follows: (1) add the scores from all eight symptoms, (2) divide this sum by the maximum score (i.e., if eight questions are answered divide by 32, etc.), and (3) multiply by 100.
The Symptom Assessment iN Dry Eye (SANDE) score to evaluate symptoms of ocular discomfort and dryness was assessed by a 100 mm horizontal visual analog scale (VAS). The frequency of symptoms ranged from “rarely” to “all of the time” and the severity of the symptoms ranged from “very mild” to “very severe”
The severity of five blepharitis symptoms (eyelid itching, redness, swelling, crusting in the morning, and missing eyelashes) was rated on the day of visit using 10-grade scales (from 1 = none to 10 = severe).
At each study visit, the investigator assessed the following:
The lid margin redness and swelling as well as the ocular surface redness for both eyes using the Efron grading scale ranging from 0 to 4 (normal to severe).
Demodex count by means of the lash manipulation technique recording the extent and severity/intensity for 10 eyelashes.Citation13 The presence of mites per eyelash was evidenced by answering yes or no. The intensity per eyelash was assessed by a three-point severity from 1 to 3 (1 = one tail, 2 = two tails, or 3 = more than two tails). The extent sum score was derived as the number of eyelashes with mites present (between 0 and 10). The severity sum score was derived as the sum of the severity scores (ranging between 0 and 30) per eyelash.
Cylindrical dandruff (CD) with the grade of lash line coverage in percent (from 0 to 100%) and severity (mild = CD present on fewer than five lashes, moderate = CD present on 5–9 lashes, and severe = CD present on 10 or more lashes) for upper and lower lid of each eye individually.
Surface damage to the exposed eye, assessed by ocular surface staining and graded by using the 0–5 point Oxford scale.
The overall performance of the products in terms of sufficient effectiveness of the treatment was assessed by the investigator using a four-point scale (strongly disagree, disagree, agree, and strongly agree) at the end of each treatment period.
Patient satisfaction and compliance
Patient’s impression of treatment satisfaction with the test products was assessed at day 28 and 56 with a five-point scale ranging from completely satisfied to very dissatisfied. Ease of use was rated by the patient at day 28 and day 56 for the test product used in the respective treatment period on a five-point scale ranging from very difficult to very easy.
For assessment of treatment compliance, patients were interviewed for adherence with treatment instructions and they were instructed to return the unused study products at each post-baseline visit. Returned products were accounted for and compared with the reported number of applications. Patients were regarded as compliant if they didn’t miss more than six applications until the last visit and did not report two or more missing consecutive applications.
Safety assessment
The assessment of safety included documentation of adverse events (AE) throughout the study and evaluation of visual acuity of both eyes using the Snellen chart at day 0, day 28, and day 56. The patient assessed the overall tolerability at day 28 and day 56 on a four-point scale (from 0 = intolerable to 3 = fully tolerable). The investigator evaluated the overall tolerability of the treatment regimen at the end of the treatment on day 56 with the same four-point scale.
Statistical analysis
Statistical analysis was performed using the Statistical Analysis System (SAS®) Analytics Pro, version 9.4. Continuous parameters were described by means, standard deviation, median, minimum, maximum, and 95% confidence limits for raw data and differences in the baseline. Categorical data are presented by means, standard deviation, median, minimum, and maximum. Differences in the baseline are assessed with a paired t-Test or with a Wilcoxon signed-rank test where p-values less than 0.05 were considered statistically significant. Missing data were only computed for the GDS with last information available carried forward (LOCF). The main objective was to compare the ocular symptoms from day 0 to day 28 of treatment with eyelid T4O wipes followed by showing no change of the ocular symptoms from day 28 to day 56 of treatment with maintenance wipes based on the GDS. Another focus of interest was the change of all efficacy parameters in the treatment period and from day 28 to day 56.
Results
Participants
Fifty patients fulfilling all eligibility criteria were enrolled in the trial and treated with the T4O-wipes in the initial treatment period. The average age of the trial participants was 60.9 ± 18.7 years (range 23–84 years). The majority of the enrolled patients in this trial were female (66%), while 34% were male participants. One patient prematurely discontinued the trial during the first treatment period (day 0 to day 28) due to a planned surgery (which was not reported by the patient at screening). Forty nine (98%) patients completed the trial. In the second treatment period (from day 28 to day 56), most patients (n = 41, 82%) were treated with maintenance wipes, whereas eight patients (16%) continued treatment with T4O-wipes.
Compliance was assessed by the number of wipes returned at the post baseline visits and the interview with the patients for adherence with treatment instructions. All patients were regarded as compliant as none of the patients had more than six missing applications until the last visit or two or more missing consecutive applications.
Global ocular discomfort and individual symptoms assessed by patients
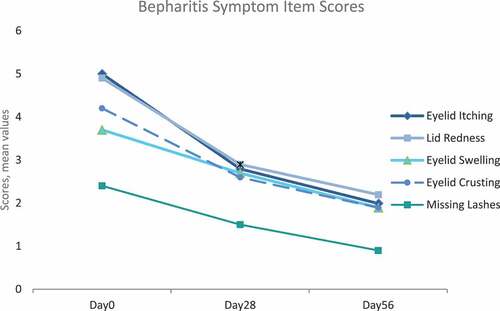
There was a statistically significant improvement in GDS and individual symptoms assessed in the TOSS and SANDE from day 0 to the end of the initial treatment period (day 28) (). For the GDS, a statistically significant mean change of −1.9 ± 1.9, p < .0001, was observed. For the adapted TOSS (mean change −18,7 ± 16.2, p < .0001) and SANDE (mean change −1,9 ± 2.2, p < .0001), the differences to day 0 were also statistically significant (). Compared to day 0, all five blepharitis symptom item scores showed a reduction in the severity score with a statistically significant change until day 28, indicating an improvement of all symptoms ().
Table 1. Differences between day 0 to day 28 as well as differences between day 28 to day 56 for GDS, TOSS, and SANDE scores
Figure 2. Mean (± SD) scores for global discomfort score (GDS), SANDE, and TOSS at day 0 (baseline), day 28, and day 56
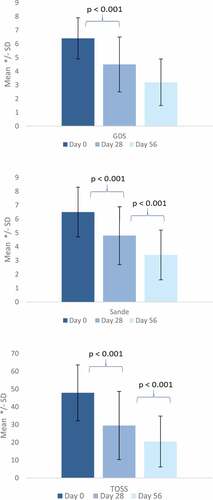
During the second treatment period from day 28 to day 56, the mean scores of GDS, adapted TOSS, SANDE, and blepharitis symptoms further decreased, indicating continuing improvement of the symptoms ( and ).
Ocular signs and symptoms assessed by the investigator
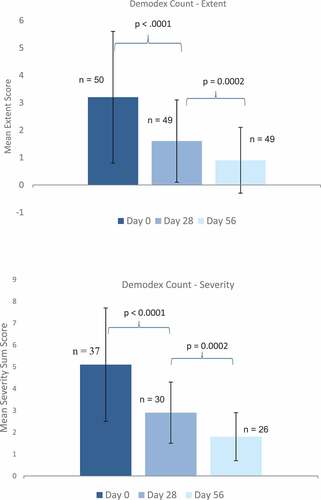
The Demodex count decreased statistically significantly during the initial treatment period until day 28 in terms of the number of affected eyelashes (extent score; mean change of −1.5 ± 1.7, p < .0001) as well as the severity score of the affected eyelashes (mean change of −2.4 ± 2.7, p < .0001), and further improvement was observed in the maintenance period until day 56 ().
Figure 4. Mean (±SD) scores for Demodex count at day 0 (baseline), day 28, and day 56
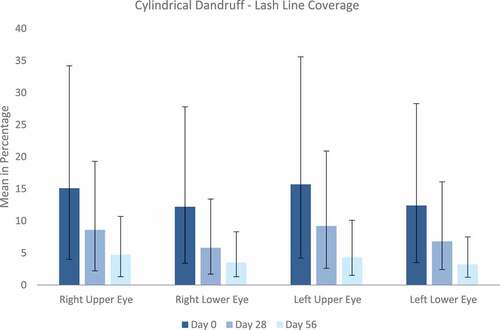
As displayed in , the cylindrical dandruff improved for both eyes showing a statistically significant reduction in upper and lower lash line coverage from day 0 to day 28 and further decrease until day 56. The severity score also improved during both treatment periods.
Figure 5. Cylindrical dandruff. The mean (±SD) grade of lash line coverage in percent was assessed for upper and lower lids of each eye individually
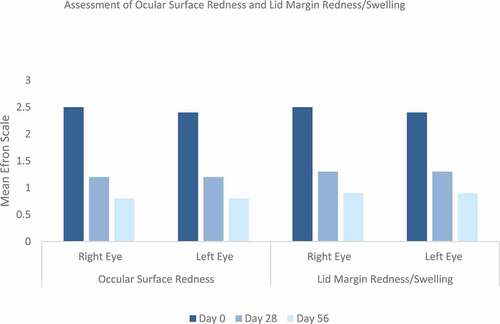
Ocular surface redness and lid margin redness/swelling improved during the initial treatment period statistically significantly (). The mean change for ocular surface redness was observed with −1.2 for the right eye and −1.3 for the left eye (p value <.0001, each) and that for lid margin redness/swelling with −1.1 for the right and left eyes (p-value <.0001, each). During the second treatment period, a further reduction or maintenance was observed.
Subgroup analysis
A subgroup efficacy analysis was performed to evaluate the influence of the severity of Demodex infestation measured by the Demodex count on several efficacy parameters. Three subgroups were defined regarding the Demodex count at day 0 as mild, moderate, or severe and evaluated for the efficacy parameters. Thirteen patients were excluded from subgroup analysis due to either missing extent or severity of the Demodex count at the baseline.
Improvement of all symptoms was observed in all subgroups during the initial treatment phase from day 0 to day 28 with further improvement during the maintenance period. Ocular surface redness and lid margin redness /swelling improved statistically significantly for all subgroups during the initial treatment period (Supplementary table 1).
Treatment effectiveness and satisfaction
In the majority of the patients, the investigator agreed (38%) or strongly agreed (52%) that treatment in the initial treatment period was sufficiently effective. Evaluation of investigator’s assessments in the period from day 28 to day 56 indicated that the investigator agreed (20%) or strongly agreed (74%) that the maintenance treatment was sufficiently effective.
Patient’s impression on treatment satisfaction revealed that the majority of the patients were satisfied with the treatment in both treatment periods [rated as completely satisfied (42%) or very satisfied (24%) for the initial treatment period and as completely satisfied (58%) or very satisfied (22%) for treatment in the maintenance period].
Safety
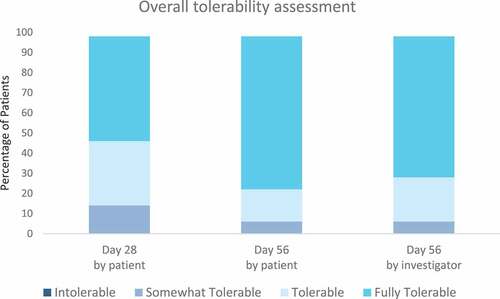
Both the products were well tolerated. Eighty six percent of the patients rated the treatment with T4O-wipes during the initial treatment period as tolerable or fully tolerable and 92% of them assessed the subsequent maintenance treatment as tolerable or fully tolerable. Investigators assessed the treatment regimen of T4O-wipes and maintenance wipes as tolerable or fully tolerable for 92% of the patients ().
Figure 7. Overall tolerability assessed by the patient at day 28 and day 56 and by the investigator at day 56
No adverse events were reported during the whole study period. No clinically significant change in visual acuity was observed.
Discussion
The present study aimed to investigate the clinical improvement in signs and symptoms of Demodex blepharitis with a new therapeutic scheme comprising a treatment regimen of a four-week initial treatment phase with T4O impregnated wipes for reduction of Demodex mites followed by a maintenance phase with maintenance wipes for four weeks to continue a sufficient lid margin hygiene and to prevent reoccurrence of the mites’ overgrowth and symptoms. The duration of the four-week initial treatment period with T4O impregnated wipes was selected to cover at least two life cycles of the mites as one life cycle is approximately 14–18 days from an egg to the adult stage (Citation14, Citation15).
Data from our study showed a statistically significant improvement of global ocular discomfort and all individual blepharitis symptoms as evaluated by the patients within the four-week initial treatment period with the T4O wipes. In addition, the investigator-assessed symptoms CD, ocular surface redness, and lid margin redness/swelling also improved significantly under this treatment. This is in line with previous results from a study investigating eyelid scrubbing with T4O impregnated wipes.Citation11 Furthermore, it supports the use of T4O containing products, which is currently part of the best practise for treatment of Demodex blepharitis.
The Demodex count revealed a significant reduction in extent and severity, which correlates with the decrease in CD. Correlation between the presence of CD and infestation with Demodex has been shown in several studies earlier.Citation3 Our results also indicate that the notable decrease in lash coverage of CD and in mites count is associated with the improvement of ocular symptoms, as reported from previous studies.Citation1
After the first four-week initial treatment phase with T4O impregnated eyelid wipes, 41 (82%) patients changed to treatment with maintenance wipes for another 28 days. During this maintenance period with mainly warm compresses and eyelid massage and cleaning the global discomfort, the score improved further. Additionally, all individual blepharitis symptoms, as evaluated by the patient and those assessed by the ophthalmologists, maintained as they have been or even further improved. Even though several studies were published showing that complete eradication of the mites is often not achieved after four weeks of therapy and long-term therapy is required,Citation4 no studies were published investigating the effect of subsequent eyelid cleansing measures. The results from this study indicate the importance of implementing a combination of two subsequent treatment phases, starting treatment with T4O for the initial treatment phase followed by a subsequent phase with at least routine eyelid hygiene, involving warm compresses, lid margin massage, and cleansing to prevent relapsing and to maintain the treatment effect.
No direct correlation between the severity of the Demodex count and statistically significant improvement of symptoms was observed in the subgroup analysis. Nevertheless, the results of this analysis indicate that reduction in Demodex count and improvement of symptoms can be achieved by treatment with T4O wipes in mildly, moderately, and also severely affected subjects. In addition, maintenance or even improvement of the treatment effect during subsequent maintenance treatment was shown for all subgroups.
As Demodex blepharitis is a chronic disease, patients’ therapy compliance remains a key part for successful treatment. It is of the utmost importance to inform patients about the severity and intricacies of their condition, as well as to explain the necessity to use the recommended therapy as prescribed and improve compliance.Citation16 However, potential discomfort with any product for eyelid treatment can have an impact on the compliance. Previous reports about the use of TTO generally proved efficacy in reducing ocular signs and symptomsCitation2,Citation17,Citation18 but also reported compliance issues, which are possibly related to adverse effects caused by the ingredients. Studies reported that poor compliance was observed when TTO had to be applied on a daily basis.Citation2,Citation17 In contrast, non-compliance was reported for 0% of patients with one-time daily application of T4O impregnated wipes,Citation11 potentially caused by less adverse effects compared to other Demodex treatments. This is consistent with the results from our study in which all patients were considered compliant, i.e., they did not miss more than six applications or two subsequent applications, and no adverse effects were reported. Eighty six percent of patients rated the four-weeks treatment with the T4O-wipes as tolerable or fully tolerable, and 92% of them assessed the subsequent maintenance treatment as tolerable or fully tolerable. Also in patients who administered the T4O containing eyelid T4O-wipes for up to 56 days, no adverse effects were reported. Therefore, it can be assumed that good compliance and good tolerability are correlated and resulted in a long-lasting therapeutic benefit.
A limitation of the study design is that no clear distinction between patients treated with a combination of both eyelid wipes in the two subsequent treatment phases and the few patients who administered only T4O eyelid wipes in both treatment phases is possible. It would have been of interest to follow up the patients treated with T4O eyelid wipes alone, but this was not included in this study design. Furthermore, a longer follow-up of patients would be interesting to assess the long-term effect of a single period of T4O treatment followed by the maintenance treatment period. However, the study results are promising and further research is needed beyond the maintenance period to determine whether the therapy effect will persist.
Conclusion
In summary, an initial treatment phase with one-time daily application of T4O-impregnated eyelid wipes for 28 days significantly improved ocular symptoms and signs and reduced the mite count in patients with Demodex blepharitis. Subsequent treatment with maintenance wipes for another four weeks preserved or further intensified the treatment success. The products were well tolerated and were convenient to use. This approach to manage Demodex blepharitis by the combination of an initial treatment phase with T4O-containing eyelid wipes and a subsequent maintenance therapy phase with maintenance eyelid wipes seems to have a positive effect in the treatment of Demodex blepharitis and is promising for patients mildly, moderately, and also severely affected by Demodex infestation.
Considering that the administration of this combined therapy is well-tolerated and effective for all severities of Demodex infestation, it can be implemented also in the daily routine at general practitioners. As a consequence, treatment could start as soon as blepharitis symptoms such as eyelid crusting, CD, and lid margin redness or swelling are observed. With this promising treatment regimen, long-term consequences such as redness, inflammation, telangiectasia, Meibomian gland disease (MGD), and ocular allergy can most likely be reduced and safely and effectively treated.
Declaration of conflict of interest
C.J. is a consultant for Alcon, Bausch & Lomb, Novaliq, Novartis, OmniVision, Santen, Théa, Ursapharm, and Visufarma. S.D. is a consultant for Alcon, Bausch & Lomb, Horus, Santen, and Théa. F.C. is a consultant for Alcon, Allergan, Horus, Novartis, Santen, and Théa. T.K. is a consultant for Alcon, Bausch & Lomb, Ebiga, Johnson & Johnson, Novaliq, OmniVision, Santen, Théa, and Ursapharm. The authors report no other financial or non-financial competing interests.
Supplemental Material
Download PDF (313.2 KB)Acknowledgments
The authors wish to thank the patients for their participation in this study and proDERM (Hamburg, Germany) for contribution in conducting the study, data collection, management, and analysis. They especially thank Dr. Dorothea Wilhelm (director scientific research, proDERM GmbH) for her contribution to the concept, review, and discussion of the manuscript.
The study was sponsored and funded by Laboratoires Théa (Clermont-Ferrand, France), whose staff participated in study design, interpretation of data, and revising the manuscript.
All of the authors approved the manuscript to be published and agree to be accountable for all aspects of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (V.P.) upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Lee SH, Chun YS, Kim ES, Kim JC. The relationship between Demodex and ocular discomfort. Invest Ophtalmol Vis Sci. 2010;51:2906–11. doi:10.1167/iovs.09-4850.
- Koo H, Kim TH, Kim KW, Wee SW, Chun YS, Kim JC. Ocular surface discomfort and Demodex: effectof tea tree oil eyelid scrub in Demodex blepharitis. J Korean Med Sci. 2012;27(12):1574–79. doi:10.3346/jkms.2012.27.12.1574.
- Gao -Y-Y, Di Pascuale MA, Li W, Liu DT-S, Baradaran-Rafii A, Elizondo AUA. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005 September;46(9):3089–94. doi:10.1167/iovs.05-0275.
- Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom. 2018;10:57–63. doi:10.2147/OPTO.S142708.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006 January;19(1):50–62. doi:10.1128/CMR.19.1.50-62.2006.
- Navel V, Mulliez A, Benoist d’Azy C, Baker JS, Malecaze J, Chiambaretta F, Dutheil F. Efficacy of treatments for Demodex blepharitis: a systematic review and meta-analysis. Ocul Surf. 2019 Oct;17(4):655–69. doi:10.1016/j.jtos.2019.06.004.
- Tighe S, Gao -Y-Y, Tseng SCG. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl Vis Sci Technol. 2013 November;2(7):2. doi:10.1167/tvst.2.7.2.
- Rah MJ. A review of hyaluronan and its ophthalmic applications. Optometry. 2011;82(1):38–43. doi:10.1016/j.optm.2010.08.003.
- Chen LH, Xue JF, Zheng ZY, Shuhaidi M, Thu HE, Hussain Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: a review of recent developments and critical appraisal of preclinical and clinical investigations. Int J Biol Macromol. 2018;116:572–84. doi:10.1016/j.ijbiomac.2018.05.068.
- Bukhari SNA, Roswandi NL, Waqas M, Habib H, Hussain F, Khan S, Sohail M, Ramli NA, Thu HE, Hussain Z, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120(Pt B):1682–95. doi:10.1016/j.ijbiomac.2018.09.188.
- Messaoud R, El Fekih L, Mahmoud A, Amor HB, Bannour R, Doan S, Khairallah M. Improvement in ocular symptoms and signs in patients with Demodex anterior blepharitis using a novel terpinen-4-ol (2.5%) and hyaluronic acid (0.2%) cleansing wipe. Clinl Ophthalmol. 2019;13:1043–54. doi:10.2147/OPTH.S198585.
- Ngo W, Jones L, Bitton E. Short-term comfort responses associated with the use of eyelid cleasning products to manage Demodex folliculorum. Eye Contact Lens. 2018;44(6):87–92. doi:10.1097/ICL.0000000000000415.
- Muntz A, Purslow C, Wolffsohn JS, Craig JP. Improved Demodex diagnosis in the clinical setting using a novel in situ technique. Cont Lens Anterior Eye. 2020 Aug;43(4):345–49. doi:10.1016/j.clae.2019.11.009.
- Lacey N, Kavanagh K, Tseng SC. Under the lash: Demodex mites in human diseases. Biochem (Lond). 2009 Aug 1;31(4):2–6.
- Rather PA, Hassan I. Human demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014 Jan;59(1):60–66. doi:10.4103/0019-5154.123498.
- Bitton E, Ngo W, Dupont P. Eyelid hygiene products: a scoping review. Contact Lens Anterior Eye. 2019;42:591–97. doi:10.1016/j.clae.2019.09.008.
- Nicholls SG, Oakley CL, Tan A, Vote BJ. Demodex treatment in external ocular disease: the outcomes of a tasmanian case series. Int Ophtalmol. 2016;36(5):691–96. doi:10.1007/s10792-016-0188-5.
- Gao YX, Xu DL, Huang LJ, Wang R, Tseng SC. Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea. 2012;13(1):14–17. doi:10.1097/ICO.0b013e31820ce56c.