Abstract
Purpose
Abnormal lipid metabolism has been proved to be implicated in the complex pathogenesis of diabetic retinopathy (DR). 12-lipoxygenase (12-LOX) is a member of lipoxygenase family responsible for the oxygenation of cellular polyunsaturated fatty acids to produce lipid mediators which modulate cell inflammation. This review explores the role of 12-lipoxygenase and its products in the pathogenesis of DR.
Methods
A comprehensive medical literature search was conducted on PubMed till September 2021.
Results
Emerging evidence has demonstrated that 12-LOX and its main product 12- hydroxyeicosatetraenoic acid (12-HETE) activate retinal cells, especially retinal vascular endothelial cells, through the activation of NADPH oxidase and the subsequent generation of reactive oxygen species (ROS), mediating multiple pathological changes during DR. Genetic deletion or pharmacological inhibition models of 12-LOX in mice show protection from DR.
Conclusion
12-LOX and its product 12-HETE take important part in DR pathogenesis and show their potential as future therapeutic targets for DR. Further studies are needed on the specific mechanism including 12-LOX pathway related molecules, 12-HETE receptors and downstream signaling pathways.
Introduction
Diabetic retinopathy (DR), a severe microvascular complication in both type 1 and type 2 diabetes, is the main cause of vision loss in working-age people.Citation1 Despite the existence of diverse strategies (e.g., intravitreal drugs, laser photocoagulation, and vitreous surgery), the treatment of DR remains a challenge due to its complex pathogenesis. A better understanding of the role of inflammation and the related molecular pathways may help determine early goals for the prevention and intervention of DR.
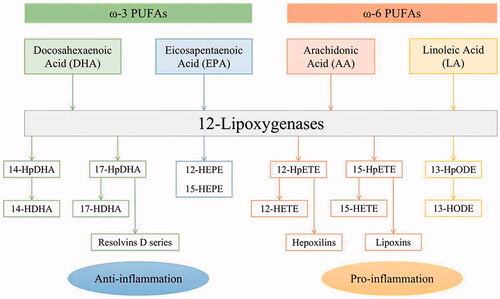
In recent years, a growing number of studies have emphasized on the close relationship between abnormal lipid metabolism and DR development.Citation2,Citation3 Polyunsaturated fatty acids (PUFAs) are a class of essential fatty acids that cannot be synthesized endogenously in required amounts and rely on dietary intake.Citation4 Among them, ω-3 and ω-6 PUFAs have attracted much attention, and the imbalance of ω-3/ω-6 PUFAs is associated with many chronic conditions, such as cardiovascular disease, diabetes, cancer, obesity, autoimmune diseases, rheumatoid arthritis, asthma and depression.Citation5 In the retina, the primary ω-3 and ω-6 PUFA is docosahexaenoic acid (DHA) and arachidonic acid (AA), respectively.Citation6 The membrane-bound PUFAs are both released from the second carbon group of glycerol by activated phospholipase A2 (PLA2), and then further converted to functionally active metabolites through 3 major enzymatic pathways – the cyclooxygenases (COXs), lipoxygenases (LOXs) and cytochrome P450 oxidases (CYPs).Citation7–9 LOX is a family of non-heme iron-containing enzymes widely distributed in the plant and animal kingdoms, which can stereospecifically insert molecular oxygen into certain PUFAs.Citation10 According to the position of the carbon atom that binds molecular oxygen, LOX is classified into 5-, 8-, 12-, and 15-LOX.Citation11 Among these, 12-LOXs will be the focus of this review, whose products generated from PUFAs take part in anti- and pro-inflammatory activities. Some of the metabolizing pathways are presented in .
Experimental studies have reported a protective role of ω-3 PUFAs against DR or pathological retinal angiogenesis.Citation12–14 In the PREDIMED (Prevención con Dieta Mediterránea) trial, a 6-year follow-up analysis in middle-aged and older individuals with type 2 diabetes adhering to the Mediterranean diet showed that intake of ≥500 mg/d of dietary ω-3 PUFAs is connected with a reduced incidence of sight-threatening DR.Citation15 The protective effect of ω-3 PUFAs was abrogated in 5-LOX−/− mouse models of oxygen-induced ischemic retinopathy (OIR) but not in 12/15-LOX−/− ones, suggesting that the 5-LOX metabolite 4-hydroxydocosahexaenoic acid (4-HDHA) mediates the major antiangiogenic effect.Citation16 A recent study found that DHA and its 12-LOX oxylipins (11-HDHA and 14-HDHA in platelets) could inhibit platelet activity and thrombus formation.Citation17 However, 12-LOX-derived ω-3 oxylipins have not been examined in the retina and additional studies are needed to clarify their particular role in neovascular eye diseases.
Contrary to the protective effect of ω-3 PUFAs on DR, ω-6 PUFAs were found increased in diabetic patients, especially AA, whose pro-inflammatory products are important lipid mediators to regulate DR development.Citation18,Citation19 12-LOX converts AA into 12-hydroperoxyeicosatetraenoic acid (12-HpETE), which is then reduced by glutathione peroxidase to 12-hydroxyeicosatetraenoic acid (12-HETE). Many studies have shown that 12-LOX and its product, 12-HETE, promote inflammation and oxidative stress, playing a key role in the pathogenesis of diabetes and its complications.Citation20,Citation21
Here, based on the findings of previous studies, we investigated the biological functions of 12-LOX and 12-HETE, and summarized their role in DR pathogenesis.
An overview of 12-LOX family and its main product 12-HETE
Different types of 12-LOXs
Four subtypes of 12-LOX have been discovered in mice: platelet-type 12-LOX (P-12-LOX), leukocyte-type 12-LOX (L-12-LOX), epidermal-type 12-LOX (E-12-LOX), and 12(R)-LOX.Citation22 Except for 12(R)-LOX, the other three are 12(S)-LOXs and produce the corresponding products in the S-stereo configuration. The isoforms are named after the cells where they were originally identified and subsequently detected in various cell types, such as endothelial cells, smooth muscle cells, glial cells, etc.Citation23–26 In fact, the phylogenetic correlations among these isomers are quite low.Citation27 Murine L-12-LOX is more closely related to human reticulocyte type 15-LOX-1 than to murine P-12-LOX.Citation22 Human 15-LOX-1 (encoded by ALOX15) and murine L-12-LOX (encoded by Alox15) share 73% sequence homology and are collectively called 12/15-LOX.Citation28 Both of them can produce 12-HETE and 15-HETE; however, the product preference varies as per species, with 9:1 15-HpETE/12-HpETE in humans and 1:4 in mice, considering AA as the main substrate.Citation28 Therefore, even if these two proteins are derived from a homologous gene family, they may not produce the same oxylipins. This must be considered during the development and selection of potential LOX enzyme inhibitors.Citation20 In comparison, human 12(S)-LOX (encoded by ALOX12) and murine P-12-LOX (encoded by Alox12) share 85% sequence homology and both produce only 12-HETE.Citation20 The general expression patterns of different types of 12-LOXs are listed in .
Table 1. General expression patterns and products of 12-LOXs
Biological actions of 12-HETE
The impact of 12-LOX is mainly attributed to the production of 12-HETE, which takes part in a wide range of biological processes, such as thrombogenesis, atherosclerosis, and neurodegeneration.Citation29–32 It also plays an important role in cancer biology as it promotes cell invasion, adhesion, growth, and metastasis.Citation30,Citation33,Citation34 Moreover, 12-HETE is a pro-inflammatory lipid metabolite that has been implicated in diabetes and vascular complications.Citation35–37 High glucose treatment increases the 12-HETE production in vascular endothelial and smooth muscle cells, which in turn upregulates the expression of vascular endothelial growth factor (VEGF), a key factor associated with pathogenic neovascularization.Citation38,Citation39
Pathogenic roles of 12-LOX and 12/15-LOX in DR
Experimental studies have proved the critical role of 12-LOXs and its metabolite 12-HETE in the pathogenesis of DR. For convenience, in the following contents of this review, the simplified term “12-LOX” will refer to the enzyme encoded by human ALOX12 and murine Alox12 genes, while “12/15-LOX” will refer to the one encoded by human ALOX15 and murine Alox15 genes.
The effect of 12/15-LOX in DR
Extensive research has focused on the role of 12/15-LOX in the DR pathogenesis, whose effects are mediated by the occurrence and development of the disease.
Immunolocalization has shown that 12/15-LOX is expressed in retinal cells, including retinal microvascular endothelial and retinal pigment epithelial cells.Citation40 The earliest study has explored the role of 12/15-LOX in retinal angiogenesis, which is one of the most destructive pathological events occurring during advanced DR.Citation40 In a mouse model of OIR, the retinal levels of 12/15-LOX, 12-HETE, and 15-HETE were found to be increased significantly. In addition, the 12-HETE and 15-HETE levels were significantly elevated in the vitreous of diabetic patients with proliferative DR (PDR) compared with those without PDR. The blockade of 12/15-LOX with baicalein, a non-specific LOX inhibitor, or deletion of the 12/15-LOX gene could attenuate the expression of VEGF and ameliorate the retinal neovascularization. In addition, 12-HETE induced and inhibited the expression of VEGF and pigment epithelium-derived factor (PEDF) in rat Müller cells and murine primary astrocytes, respectively.Citation40 These results indicate that 12-HETE and 15-HETE produced by 12/15-LOX could promote retinal angiogenesis by disrupting the balance between VEGF and PEDF.
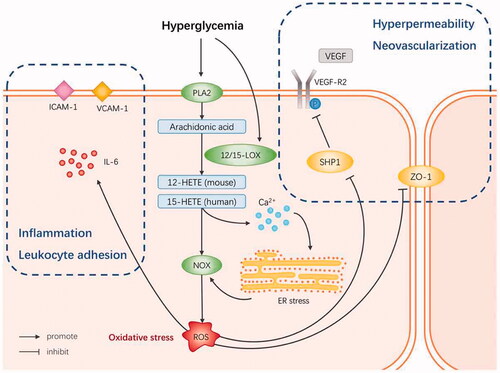
It is well known that inflammatory damage to the retina is involved in the early development of DR. Therefore, subsequent research was concentrated on studying the effect of 12/15-LOX on the barrier function of retinal vascular endothelial cells (RECs). Othman et al. found that the 12/15-LOX metabolites promote high permeability of the retinal vascular barrier, which is connected with the enhanced NADPH oxidase (NOX2) expression and reactive oxygen species (ROS) generation and decline in the level of zonula occludens-1 (ZO-1), while NADPH oxidase (NOX) inhibitors could prevent the 12/15-LOX pro-permeability effect.Citation41 Treatment of Ins2Akita mice with baicalein significantly diminished the levels of 12-HETE, inflammatory molecules (ICAM-1, VCAM-1, and IL-6), ROS production, NOX2, and pVEGFR2 expression in the retina, while restoring the protein tyrosine phosphatase pSHP1 expression.Citation41 This suggests that 12/15-LOX leads to vascular hyperpermeability during DR through a NOX-dependent mechanism, involving the inhibition of protein tyrosine phosphatase and activation of the VEGFR2 signaling pathway. In another study, LC/MS lipidomics analysis of human RECs incubated with high glucose (30 mM) found that 12/15-LOX products were significantly upregulated. 12/15-LOX activates RECs with the help of NOX system, resulting in leukocyte adhesion, high permeability, and ultimately increased neovascularization.Citation42 To further understand the pathogenesis of the LOX-NOX pathway in DR, Elmasry et al. investigated the role of endoplasmic reticulum (ER) stress in mediating the effect of 12/15-LOX metabolites on REC dysfunction.Citation43 They found that knocking out the 12/15-LOX gene could significantly mitigate the ER stress in the retina caused by diabetes, whereas 15-HETE could induce ER stress in human RECs. Inhibition of NOX or deletion of NOX2 did not attenuate 12/15-LOX-induced ER stress, while inhibition of ER stress downregulated the NOX activity and the expression of NOX2 and P47phox, its catalytic and regulatory subunits, respectively. It also lowered 15-HETE-induced leukocyte adhesion and VEGFR2 phosphorylation. In addition, 15-HETE enhances the intracellular calcium levels in human RECs.Citation43 These findings support the hypothesis that ER stress is upstream of the NOX pathway, promoting 12/15-LOX-induced retinal inflammation in DR by activating NOX and VEGFR2. The destruction of Ca2+ homeostasis may be an indispensable step in DR to initiate this signaling pathway.
Thus, hyperglycemia activates PLA2 to release AA from the retinal cell membrane, which is then converted into 12-HETE or 15-HETE. Then, ROS is generated through NOX, thereby forming a state of oxidative stress. Oxidative stress activates RECs through various inflammatory signaling pathways, leading to pathological changes in DR at different stages ().
In the aforementioned studies, the authors emphasized that considering the species differences in 12/15-LOX, they used 12-HETE in all murine experiments and 15-HETE in all human cell experiments.Citation44 This action ensured the consistency of 12/15-LOX, the research object, in both species. However, a key issue may have been overlooked, that is, it is the product of the enzyme that exerts the biological effect, not the enzyme itself. There is no guarantee that the role of 12-HETE in mice is the same as that of 15-HETE in humans. As reported, 12-HETE is an effective vasodilator, whereas 15-HETE can cause vasoconstriction.Citation19,Citation45 In addition, the studies used baicalein to block the function of 12/15-LOX, which is a nonspecific LOX inhibitor and may have off-target effects.Citation19 On one hand, such an inhibitor with antioxidant properties could affect cell redox homeostasis, making it difficult to determine whether LOX inhibition or redox homeostasis is the main reason for the observed biological results.Citation46 On the other hand, not all the effects that cause DR microvascular disease are attributed to 12/15-LOX, because other studies have demonstrated the role of other LOXs, such as 5-LOX, and their products in DR, which can also be blocked by baicalein.Citation20,Citation47
The effect of 12-LOX in DR
It has been confirmed that 12-LOX participates in diabetes and its vascular complications such as diabetic nephropathy and diabetic heart disease.Citation21,Citation48,Citation49 A previous study reported the existence of 12-LOX in the retina, and its expression was significantly increased in the OIR mouse model compared with the control.Citation40 Moreover, an obvious increase in 12-LOX protein levels was observed in the retinas of diabetic subjects compared with non-diabetic subjects.Citation40 However, no follow-up experimental investigations were conducted on the relationship between 12-LOX and DR. Recently, a multiplatform metabolomics study revealed a significantly augmented level of serum 12-HETE in DR subjects compared to those with diabetes without DR, and predicted it to be a promising diagnostic biomarker of DR.Citation50 Unlike in mice, the main source of 12-HETE in humans is 12-LOX, not 12/15-LOX. Therefore, the significantly elevated level of 12-HETE in DR patients suggests a strong association between 12-LOX and DR. On some occasions, there is even a statement that the murine 12/15-LOX is functionally closest to the human 12-LOX, which produces 12-HETE almost exclusively.Citation37
Pharmacological inhibition of 12-LOX and 12/15-LOX
Nonspecific LOX inhibitors are easy to discover, many of which are utilized widely and effectively in animal models of various diseases including DR, like baicalein and cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate (CDC).Citation51,Citation52 Nevertheless, using nonspecific inhibitors could easily confuse the interpretation of experimental results due to the too many targets (as discussed in Section 3.1). Thus, great efforts have been made to identify selective inhibitors of certain LOXs.
The specific human 12/15-LOX inhibitor ML351 which was discovered by high-throughput screening (HTS) showed its effectiveness in inhibiting murine 12/15-LOX.Citation53 This attribute is important because the distinct product preference between the two enzymes implies their different active sites, while ML351 inhibits both. That means the effects of ML351on human versus mouse are not exactly the same, which should be paid attention to when utilizing ML351 in preclinical studies.
ML355, the specific inhibitor of 12-LOX, efficiently improves the functions of cytokine-treated human islets and type 2 diabetic islets in vitro 55. ML355 is a benzenesulfonamide also identified by HTS with high potency (IC50 = 0.3 μM) and selectivity.Citation54,Citation55 It has been employed in research in multiple fields, including diabetes, thrombosis, LPS-induced inflammation, and ischemia-reperfusion-induced hepatic injury, displaying good pharmacokinetic characteristics in mice without observable toxic effects.Citation56–58 It has advantages over other LOX inhibitors in terms of explaining 12-LOX-related molecular mechanisms and drug development.
Conclusion and prospect
In this review, we have briefly introduced the 12-LOX family and its primary metabolite 12-HETE, elaborated the functions of 12/15-LOX in the onset and progression of DR, and indicated the necessity for further research on 12-LOX in DR.
Existing studies have shown (as described in Section 3.1) that 12-HETE activates the NOX system to generate ROS, causing oxidative stress and triggering inflammation in the retina. Nevertheless, the specific mechanism by which 12-HETE activates NOX remains unclear. An unresolved issue regarding 12-HETE is whether its cellular effects are mediated by its binding to a receptor or acting as a second messenger. G protein-coupled receptor 31 (GPR31) is a specific receptor with high affinity for 12-HETE and renamed as 12-HETER.Citation33 12-HETE–GPR31 mediates a variety of signaling pathways, such as MAPK, MEK, and NF-κB.Citation34 It is involved in thrombosis, hepatic ischemia-reperfusion injury, and regulation of cancer proliferation, migration, and invasion, implicating its relevance in inflammation and angiogenesis.Citation58–62 As the development of 12-LOX inhibitors has been challenged by the structural and functional differences of enzymes among species, it can be worth concentrating on 12-HETE and its receptors.
To summarize, an in-depth exploration of the role of lipid metabolism such as 12-LOX and 12-HETE in DR would help elucidate the pathogenesis of this disease, thereby, providing a potential new strategy for treatment of DR.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018 Jun 20;19(6):1816. doi:10.3390/ijms19061816.
- Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, Fort PE. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019 Sep;62(9):1539–49. doi:10.1007/s00125-019-4959-1.
- Busik JV. Lipid metabolism dysregulation in diabetic retinopathy. J Lipid Res. 2021 Jan 6;62:100017. doi:10.1194/jlr.TR120000981.
- Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res. 2010 Aug 20;107(4):495–500. doi:10.1161/CIRCRESAHA.110.221317.
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002 Oct;56(8):365–79. doi:10.1016/s0753-3322(02)00253-6.
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006 Jun;83(6Suppl):1467S–1476S. doi:10.1093/ajcn/83.6.1467S.
- Lupo G, Motta C, Giurdanella G, Anfuso CD, Alberghina M, Drago F, Salomone S, Bucolo C. Role of phospholipases A2 in diabetic retinopathy: in vitro and in vivo studies. Biochem Pharmacol. 2013 Dec 1;86(11):1603–13. doi:10.1016/j.bcp.2013.09.008.
- Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci. 2018 Oct 23;19(11):3285. doi:10.3390/ijms19113285.
- Gong Y, Fu Z, Liegl R, Chen J, Hellström A, Smith LE. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am J Clin Nutr. 2017 Jul;106(1):16–26. doi:10.3945/ajcn.117.153825.
- Funk CD. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta. 1996 Nov 11;1304(1):65–84. doi:10.1016/s0005-2760(96)00107-5.
- Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015 Dec;6:297–310. doi:10.1016/j.redox.2015.08.006.
- Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012 Jul 23;2(7):e36. doi:10.1038/nutd.2012.10.
- Tikhonenko M, Lydic TA, Opreanu M, Li Calzi S, Bozack S, McSorley KM, Sochacki AL, Faber MS, Hazra S, Duclos S. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One. 2013;8(1):e55177. doi:10.1371/journal.pone.0055177.
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007 Jul;13(7):868–73. doi:10.1038/nm1591.
- Sala-Vila A, Díaz-López A, Valls-Pedret C, Cofán M, García-Layana A, Lamuela-Raventós RM, Castañer O, Zanon-Moreno V, Martinez-Gonzalez MA, Toledo E et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol. 2016 Oct 1;134(10):1142–49. doi:10.1001/jamaophthalmol.2016.2906.
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci Transl Med. 2011 Feb 9;3(69):69ra12. doi:10.1126/scitranslmed.3001571.
- Yamaguchi A, Stanger L, Freedman CJ, Standley M, Hoang T, Adili R, Tsai WC, Van Hoorebeke C, Holman TR, Holinstat M. DHA 12-LOX-derived oxylipins regulate platelet activation and thrombus formation through a PKA-dependent signaling pathway. J Thromb Haemost. 2021 Mar;19(3):839–51. doi:10.1111/jth.15184.
- Morita I, Takahashi R, Ito H, Orimo H, Murota S. Increased arachidonic acid content in platelet phospholipids from diabetic patients. Prostaglandins Leukot Med. 1983 May;11(1):33–41. doi:10.1016/0262-1746(83)90106-3.
- Wang MH, Hsiao G, Al-Shabrawey M. Eicosanoids and oxidative stress in diabetic retinopathy. Antioxidants (Basel). 2020 Jun 12;9(6):520. doi:10.3390/antiox9060520.
- Dobrian AD, Morris MA, Taylor-Fishwick DA, Holman TR, Imai Y, Mirmira RG, Nadler JL. Role of the 12-lipoxygenase pathway in diabetes pathogenesis and complications. Pharmacol Ther. 2019 Mar;195:100–10. doi:10.1016/j.pharmthera.2018.10.010.
- Dong C, Liu S, Cui Y, Guo Q. 12-Lipoxygenase as a key pharmacological target in the pathogenesis of diabetic nephropathy. Eur J Pharmacol. 2020 Jul 15;879:173122. doi:10.1016/j.ejphar.2020.173122.
- Kühn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006 Jul;45(4):334–56. doi:10.1016/j.plipres.2006.02.003.
- Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:245–62. doi:10.1016/s0090-6980(02)00034-5.
- Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol. 1995 Jul;15(7):942–48. doi:10.1161/01.atv.15.7.942.
- Funk CD, Funk LB, FitzGerald GA, Samuelsson B. Characterization of human 12-lipoxygenase genes. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3962–66. doi:10.1073/pnas.89.9.3962.
- Nishiyama M, Watanabe T, Ueda N, Tsukamoto H, Watanabe K. Arachidonate 12-lipoxygenase is localized in neurons, glial cells, and endothelial cells of the canine brain. J Histochem Cytochem. 1993 Jan;41(1):111–17. doi:10.1177/41.1.8417106.
- Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:303–12. doi:10.1016/s0090-6980(02)00036-9.
- Haeggström JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011 Oct 12;111(10):5866–98. doi:10.1021/cr200246d.
- Porro B, Songia P, Squellerio I, Tremoli E, Cavalca V. Analysis, physiological and clinical significance of 12-HETE: a neglected platelet-derived 12-lipoxygenase product. J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Aug 1;964:26–40. doi:10.1016/j.jchromb.2014.03.015.
- Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta. 2015 Apr;1851(4):340–55. doi:10.1016/j.bbalip.2014.10.008.
- Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L, Nadler JL. Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia. 2002 Jan;45(1):125–33. doi:10.1007/s125-002-8253-x.
- Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004 Aug;11(8):875–84. doi:10.1038/sj.cdd.4401395.
- Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J et al. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011 Sep 30;286(39):33832–40. doi:10.1074/jbc.M110.216564.
- Napolitano M. The role of the 12(S)-HETE/GPR31/12-HETER axis in cancer and ischemia-reperfusion injury. Biochem Soc Trans. 2019 Apr 30;47(2):743–54. doi:10.1042/BST20180635.
- Bolick DT, Orr AW, Whetzel A, Srinivasan S, Hatley ME, Schwartz MA, Hedrick CC. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2005 Nov;25(11):2301–07. doi:10.1161/01.ATV.0000186181.19909.a6.
- Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-lipoxygenase products reduce insulin secretion and {beta}-cell viability in human islets. J Clin Endocrinol Metab. 2010 Feb;95(2):887–93. doi:10.1210/jc.2009-1102.
- Tersey SA, Bolanis E, Holman TR, Maloney DJ, Nadler JL, Mirmira RG. Minireview: 12-lipoxygenase and islet β-cell dysfunction in diabetes. Mol Endocrinol. 2015 Jun;29(6):791–800. doi:10.1210/me.2015-1041.
- Sasson S, Eckel J. Disparate effects of 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid in vascular endothelial and smooth muscle cells and in cardiomyocytes. Arch Physiol Biochem. 2006 Apr;112(2):119–29. doi:10.1080/13813450600712035.
- Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol. 1997 Nov;273(5):H2224–31. doi:10.1152/ajpheart.1997.273.5.H2224.
- Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z et al. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes. 2011 Feb;60(2):614–24. doi:10.2337/db10-0008.
- Othman A, Ahmad S, Megyerdi S, Mussell R, Choksi K, Maddipati KR, Elmarakby A, Rizk N, Al-Shabrawey M. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS One. 2013;8(2):e57254. doi:10.1371/journal.pone.0057254.
- Ibrahim AS, Elshafey S, Sellak H, Hussein KA, El-Sherbiny M, Abdelsaid M, Rizk N, Beasley S, Tawfik AM, Smith SB et al. A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase. J Lipid Res. 2015 Mar;56(3):599–611. doi:10.1194/jlr.M056069.
- Elmasry K, Ibrahim AS, Saleh H, Elsherbiny N, Elshafey S, Hussein KA, Al-Shabrawey M. Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy. Diabetologia. 2018 May;61(5):1220–32. doi:10.1007/s00125-018-4560-z.
- Ibrahim AS, Tawfik AM, Hussein KA, Elshafey S, Markand S, Rizk N, Duh EJ, Smith SB, Al-Shabrawey M. Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids. Biochim Biophys Acta. 2015 Mar;1851(3):290–98. doi:10.1016/j.bbalip.2014.12.017.
- Takayama H, Gimbrone MA Jr, Schafer AI. Vascular lipoxygenase activity: synthesis of 15-hydroxyeicosatetraenoic acid from arachidonic acid by blood vessels and cultured vascular endothelial cells. Thromb Res. 1987 Mar 15;45(6):803–16. doi:10.1016/0049-3848(87)90090-9.
- Goswami SK. Cellular redox, epigenetics and diseases. Subcell Biochem. 2013;61:527–42. doi:10.1007/978-94-007-4525-4_23.
- Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008 May;57(5):1387–93. doi:10.2337/db07-1217.
- Zheng Z, Li Y, Jin G, Huang T, Zou M, Duan S. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. Biomed Pharmacother. 2020 Sep;129:110354. doi:10.1016/j.biopha.2020.110354.
- Burdon KP, Rudock ME, Lehtinen AB, Langefeld CD, Bowden DW, Register TC, Liu Y, Freedman BI, Carr JJ, Hedrick CC. Human lipoxygenase pathway gene variation and association with markers of subclinical atherosclerosis in the diabetes heart study. Mediators Inflamm. 2010;2010:170153. doi:10.1155/2010/170153.
- Xuan Q, Ouyang Y, Wang Y, Wu L, Li H, Luo Y, Zhao X, Feng D, Qin W, Hu C et al. Multiplatform metabolomics reveals novel serum metabolite biomarkers in diabetic retinopathy subjects. Adv Sci (Weinh). 2020 Oct 1;7(22):2001714. doi:10.1002/advs.202001714.
- Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006 Jun 15;14(12):4295–301. doi:10.1016/j.bmc.2006.01.057.
- Pergola C, Jazzar B, Rossi A, Buehring U, Luderer S, Dehm F, Northoff H, Sautebin L, Werz O. Cinnamyl-3,4-dihydroxy-α-cyanocinnamate is a potent inhibitor of 5-lipoxygenase. J Pharmacol Exp Ther. 2011 Jul;338(1):205–13. doi:10.1124/jpet.111.180794.
- Rai G, Joshi N, Jung JE, Liu Y, Schultz L, Yasgar A, Perry S, Diaz G, Zhang Q, Kenyon V et al. Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies. J Med Chem. 2014 May 22;57(10):4035–48. doi:10.1021/jm401915r.
- Luci D, Jameson JB II, Yasgar A, Diaz G, Joshi N, Kantz A, Markham K, Perry S, Kuhn N, Yeung J. Discovery of ML355, a potent and selective inhibitor of human 12-lipoxygenase. In: Probe reports from the NIH molecular libraries program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US). 2013 Apr 12. updated 2014 Sep 18. https://www.ncbi.nlm.nih.gov/books/NBK47352/
- Luci DK, Jameson JB, Yasgar A, Diaz G, Joshi N, Kantz A, Markham K, Perry S, Kuhn N, Yeung J et al. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl) amino) benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J Med Chem. 2014 Jan 23;57(2):495–506. doi:10.1021/jm4016476.
- Ma K, Xiao A, Park SH, Glenn L, Jackson L, Barot T, Weaver JR, Taylor-Fishwick DA, Luci DK, Maloney DJ et al. 12-lipoxygenase inhibitor improves functions of cytokine-treated human islets and type 2 diabetic islets. J Clin Endocrinol Metab. 2017 Aug 1;102(8):2789–97. doi:10.1210/jc.2017-00267.
- Tourdot BE, Holinstat M. Targeting 12-lipoxygenase as a potential novel antiplatelet therapy. Trends Pharmacol Sci. 2017 Nov;38(11):1006–15. doi:10.1016/j.tips.2017.08.001.
- Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, Liu ZY, Shen LJ, Zhang P, Wang PX et al. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat Med. 2018 Jan;24(1):73–83. doi:10.1038/nm.4451.
- Yang F, Zhang Y, Ren H, Wang J, Shang L, Liu Y, Zhu W, Shi X. Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis. J Exp Clin Cancer Res. 2019 Dec 12;38(1):489. doi:10.1186/s13046-019-1480-9.
- Fehrenbacher N, Tojal da Silva I, Ramirez C, Zhou Y, Cho KJ, Kuchay S, Shi J, Thomas S, Pagano M, Hancock JF et al. The G protein-coupled receptor GPR31 promotes membrane association of KRAS. J Cell Biol. 2017 Aug 7;216(8):2329–38. doi:10.1083/jcb.201609096.
- Honn KV, Guo Y, Cai Y, Lee MJ, Dyson G, Zhang W, Tucker SC. 12-HETER1/GPR31, a high-affinity 12(S)-hydroxyeicosatetraenoic acid receptor, is significantly up-regulated in prostate cancer and plays a critical role in prostate cancer progression. FASEB J. 2016 Jun;30(6):2360–69. doi:10.1096/fj.201500076.
- Van Doren L, Nguyen N, Garzia C, Fletcher EK, Stevenson R, Jaramillo D, Kuliopulos A, Covic L. Lipid receptor GPR31 (G-protein-coupled receptor 31) regulates platelet reactivity and thrombosis without affecting hemostasis. Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):e33–e45. doi:10.1161/ATVBAHA.120.315154.