Abstract
Purpose
Corneal infection in humans caused by Pythium insidiosum can lead to blindness and the host ocular immune response to it is less studied. Herein, we investigate the expression of mediators of innate and adaptive immune responses in a rabbit model.
Methods
P. insidiosum zoospores were injected intracorneally in right eye of the nine New Zealand White rabbits while left eye was injected with 1XPBS. RT-qPCR and multiplex ELISA (mELISA) were used to study the expression of antimicrobial peptides (AMPs) and immune mediators in infected cornea on 3rd, 7th and 9th day of post-infection(PI). STRING-11.0 analysis was used to predict the interactions of immune mediators. mRNA expressions of pathogen recognition receptors (PRRs) were determined in human corneal epithelial cells (HCECs) stimulated with P. insidiosum zoospores. Data was analyzed using one-way ANOVA with Post-Hoc Tukey HSD test and p-value <0.05 was considered significant.
Results
mRNA expression assay for IL-1β, IL-6, IL-8 and Cathelicidin antimicrobial peptide (CAP)-18 showed significant upregulation (p-value < 0.05) on 7th day post-infection (PI) compared to 3rd and 9th day while Leukocyte protein (LeukoP) was elevated significantly on 3rd day followed by 7th and 9th day PI . Only IL-17A among other adaptive immune cytokines showed significant upregulation on 7th day compared to 9th day PI. Expressed in pg/mL, mELISA showed significant higher levels (p-value < 0.05) of IL-1β, IL-8 in infected tissue in each of the time points compared to control. STRING analysis revealed co-expression of IL-1β, IL-8 and IL-6. Among PRRs, Dectin 1 and TLR4 showed significant upregulation in HCECs at 12 hrs compared to 6 hrs.
Conclusion
In the rabbit P. insidiosum keratitis model, innate immune mediators: IL-1β, IL-8, IL-6, AMPs: LeukoP and CAP-18 are strongly associated in early to mid-stage of corneal infection. Dectin 1 and TLR4 were observed to be associated with recognition of P. insidiosum.
Introduction
Pythium insidiosum is an etiologic agent of severe life threatening infectious pythiosis in animals and humans.Citation1 It is a parafungus, a member of oomycete that causes various forms of infection in mammals, usually vascular, ocular, cutaneous, subcutaneous and gastrointestinal. Among these there were 33% cases of ocular infections.Citation2 P. insidiosum triggers cellular immune response by activating Th (CD4+) cells cytokines.Citation3,Citation4 The current scenario of pythiosis immunotherapy relies on inducing switch from Th2 to Th1 immune response with probable mechanism of intracellular killing of P. insidiosum. In brief, Pythium antigen processed by antigen presenting cells (APCs) activates naïve Th cells to proliferate and differentiate into Th1 subset. This cell subset produces IFN-γ and IL-2 that trigger cytotoxic T lymphocytes (CTL) and mononuclear cell response, which was considered for direct killing of Pythium.Citation3 In 2018, Ledure et al, reported the activation of Th17 along with Th1 cells when dendritic cells were induced with β-glucans antigen of PythiumCitation5 while vascular infection by Pythium zoospores in BALB/c mice induced to release of Th1 (IL-2, IFN-γ) and Th17 (IL-17A) cytokines which were associated with self-tissue healing.Citation1 The outcome of pythiosis immunotherapy was reported as successful in up to 70% in equines and 55% in humansCitation5 while it was inconclusive in 18 patients with vascular and ocular pythiosis.Citation6 In 2020, Wongprompitak et alCitation7 reported the role of TLR-2 mediated NF-κβ activation that induced IL-6 and IL-8 expression in human corneal epithelial cell line challenged with P. insidiosum. Evidenced by literature search, investigators have studied the cell-mediated immunity and innate immune mediators for pythiosis in cell lines, mice and rabbits in their different organs but there is lack of information related to antimicrobial peptides, innate and adaptive immune mediators of P. insidiosum in cornea in vivo. An in vivo study will determine their role in corneal infection by P. insidiosum. There are several innate immune cells that play important role in host defense mechanisms against microbial infections. Innate immune response is a non-specific response that plays a role within hours while adaptive immune response is highly specific and occurs within days, which shows rapid and effective immune defense against pathogens. The immune cells of cornea include epithelial cells, keratocytes, neutrophils, eosinophils, macrophages, natural killer cells and Langerhans cells.Citation8 These cells produce cytokines, chemokines and growth factors that play critical role in innate and adaptive immunity of cornea. Host immune response to Candida albicans shows C-type lectin receptor (CLR), a type of pattern recognitions receptor (PRR) of innate immune cells that recognizes β (1,3) and β (1,6) glucan of C. albicans and induces downstream signaling that activates the NF-κβ and other signaling pathway to produce pro-inflammatory cytokines.Citation9 P. insidiosum possesses β (1,3) and β (1,6) glucansCitation10 which may induce similar pathway to produce pro-inflammatory cytokines. In our earlier study the corneal tissue infected by P. insidiosum in rabbit showed the key inflammatory cells to be eosinophils, polymorphonuclear leukocytes and chronic lymphonuclear cells.Citation11 Eosinophils degranulate around the Pythium hyphaeCitation3 while neutrophils (polymorphonuclear leukocyte) produce cytokines (IL-1β, IL-6), chemokine (IL-8) and other growth factors that play crucial role in host defense.Citation12 Corneal epithelial cells produce antimicrobial peptides: defensins and LL37 (also known as CAP-18)Citation13 that showed host corneal protection from Pseudomonas aeruginosaCitation14 while the other antimicrobial peptide leukocyte protein p15 (15kda) produced as part of innate immune response of rabbit that showed antibacterial activity against E. coli.Citation15 Several investigators have used rabbit model to study host defense and regulation of inflammatory response to various infections, especially intestinal infections.Citation16,Citation17 Rabbits are the animals of intermediate size between rodents and primatesCitation18 and they possess closer phylogenetic similarities to primates than rodents.Citation17 Therefore, it enables to analyze the clinical features and grading their clinical scores in infection of rabbit eye. The various aspects of the development of rabbit model for ocular pythiosis along with the clinical scores have been reported previously.Citation11 The size of rabbit corneal tissue is similar to human cornea which provides adequate sample for the investigation of several facets including histopathological evaluation, mRNA expression of cytokines (RT-qPCR) and their validation by multiplex ELISA using the same tissue infected with P. insidiosum. In a rabbit model we investigated the host innate and adaptive immune response to P. insidiosum by RT-qPCR using previously studied rabbit gene primers (in rabbit ileal loop model infected with Shigella flexneri)Citation17 and multiplex ELISA. To determine the role of pathogen recognition receptors (PRRs) that involve in innate immune response to fungi,Citation19–23 we challenged the human corneal epithelial cells (HCECs) with zoospores of P. insidiosum and analyzed their mRNA expression using RT-qPCR.
Materials and methods
Animals and ethics approval
The present study was approved (ethics approval number: SLL/PCT/IAEC/23-17) by the committee for the purpose of control and supervision of experiments on animals (CPCSEA). Nine New Zealand White (NZW) rabbits with body weight ranging from 1.5–2.5 Kgs and with the average age of 14 weeks were used in this study. Rabbits were kept individually in sterile stainless steel cages at temperature 21 ± 3 °C, relative humidity 30%–70% and 12 hours of light and dark cycle of photoperiod. They were provided RO (reverse osmosis) purified drinking water and pellet feed ad libitum.
Inoculum and dose of infection
Clinical isolate of P. insidiosum (L-3258/17) was obtained from corneal scrapings of a patient with keratitis and used as inoculating agent to establish infection in rabbit cornea for this study. Identification of this isolate was established by its morphological traits and confirmed by sequencing ITS region of its DNA (accession no. MK966741). The procedure used in zoosporogenesis of Pythium has been described previouslyCitation24 and concentration of zoospores (107 zoospores/100 μl) was measured by heamocytometer.Citation11 Prior to the infection, the rabbits received intramuscular anesthesia of ketamine (40 mg/kg body wt.) - xylazine (5 mg/kg body wt.) mixture and topical ocular anesthesia of 0.5% proparacaine hydrochloride. The 100 μl inoculum containing zoospores in PBS (phosphate buffer saline) was injected intracorneally using 1 mL tuberculin syringe under the surgical microscope in right eye while left eye (control) received equal volume of PBS (pH 7.4) via same route.
Sample collection
Before harvesting of cornea, just immediately after sacrificing the rabbits by injecting intravenous thiopental sodium (100 mg/kg body wt.), in order to rule out any contamination, corneal scrapings were collected using #15 surgical sterile blades and cultured on blood agar (BA), followed by incubation at 37 °C for 48hrs. Both right (infected) and left corneas (control) were harvested from three rabbits at each pre-determined time point (3rd, 7th and 9th day post infection). One fourth part of harvested cornea was preserved in micro-centrifuge tube containing 400 µL RNAlaterTM solution (Invitrogen, Thermo Fisher Scientific) at -80 °C until RNA extraction was done for RT-qPCR (Real time quantitative-polymerase chain reaction) and one half was kept in tissue processing cassette (Best Scientific Co., Ambala) and immediately transferred into Liquid Nitrogen (LN2) followed by preservation at −80 °C for mELISA (multiplex enzyme-linked immunosorbent assay). Remaining one fourth of the cornea was preserved in 10% buffered formalin for histopathology studies results of which have been reported in our earlier communication.Citation11
mRNA expression assay by RT-qPCR
The study of cytokines expression was initiated by RNA extraction (TRIzol method, Life technologies) from infected and uninfected corneal tissue. Prior to synthesis of cDNA [PrimeScript™ 1st strand cDNA Synthesis Kit (TakaraBio)], RNA was subjected to DNase treatment (DNase I recombinant RNase free, Roche, Sigma-Aldrich) followed by RNA quantification (Qubit 3.0 Fluorometer - Qubit™ RNA HS Assay Kit - Thermo Fisher Scientific) and RT-qPCR [QuantStudio 3.0 (Applied Biosystem)] for antimicrobial peptides (AMPs), cytokines and chemokines using previously described procedure for rabbit genes.Citation17 RT-qPCR was performed in total 10 µL volume of reaction mixture containing, 10 ng of cDNA(1 µL), 5 µL(1x) [2x-TB Green® Premix Ex Taq™ II (Tli RNase H Plus), ROX Plus] and 0.05 µM of primers. Reactions (Triplicate or duplicate) were run on QuantStudio 3.0 (Applied Biosystem) with PCR thermal cycler conditions: one cycle of cDNA denaturation and primers annealing for 10 min at 95 °C and 2 min at 60 °C, 40 cycles of cDNA polymerization for 15 sec at 95 °C and 60 sec at 60 °C, dissociation curve for 15 sec at 95 °C, 15 sec at 60 °C and 15 sec at 95 °C. Post RT-qPCR, Ct (Cycle threshold) values were recorded in Microsoft Excel sheet and fold change of gene expression was calculated by double delta Ct method (2–ΔΔCt method).
Protein expression assay by mELISA
The RT-qPCR result was further validated by mELISA. As very few cytokines were available which are specific to rabbit, only IL-8, IL-1β, MIP-1β and IL-17A expression were validated. Corneal tissue preserved at -80 °C were thawed on ice, washed with 1xPBS twice in petridish and 500 µL 1x lysis buffer [RIPA Buffer (10X), Cell Signaling Technology plus cOmplete™, EDTA-free Protease Inhibitor Cocktail, Roche (1 tablet in 10 ml of 1x RIPA buffer] was added and chopped using number #15 surgical sterile blade. The lysate was transferred into 1.5 ml eppendorf tube followed by 5 min incubation on ice and centrifugation (for 10 min at 14,000 × g, at 4 °C). Supernatant was collected in fresh tube without disturbing pellets and stored at −20 °C until use. Prior to performing mELISA for IL-1β, IL-8, IL-17A and Macrophage inflammatory protein (MIP)-1β using Quantibody® Rabbit Cytokine Array Q1(4), (RayBiotech protocol), protein concentrations were determined by BCA method [Bicinchoninic Acid (BCA) Protein Assay, G-Biosciences] and were adjusted to 1000 µg/mL with 1x RIPA buffer followed by two fold dilution with MiliQ-double autoclaved water. Microarray scanner G2565CA (Agilent Technologies) with Cy3 fluorescence detector was used to read all slides. Data was extracted using RayBiotech software and analyzed on Microsoft Excel spreadsheet.
STRING network analysis for cytokine interactions
In order to predict the association of these antimicrobial peptides, cytokines and chemokines in the immune response of Oryctolagus cuniculus (rabbits) to infection, the network analysis was carried out using web based tool: STRING network database (Ver 11.0).Citation25
Determining the expression profile of PRRs in HCECs infected with P. insidiosum
The HCECs were seeded at the density of 0.5 × 106 cells/well in a 6 well dish using complete Dulbecco's Modified Eagle Medium [DMEM (gibco, thermofisher scientific) supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum)] for 2 days with an MOI of 0.005 zoospores of P. insidiosum as described previously by Wongprompitak et al, 2020).Citation7 The clinical isolate of P. insidiosum (L-3574/2021) was obtained from corneal scrapings of a patient diagnosed with Pythium keratitis. Prior to infection, cells were incubated with incomplete DMEM medium for 1 hour. Control cells were incubated with equal volume of 1x PBS (Sigma Aldrich). Post stimulation with zoospores of P. insidiosum for 6 and 12 hours, the cells were lysed in TRIzol reagent (Invitrogen, ThermoScintific) for RNA extraction. The RNA estimation (Nanodrop 2000c, ThermoScintific), cDNA synthesis (Verso cDNA synthesis Kit) and RT-qPCR (DyNAmo ColorFlash SYBR Green qPCR Kit using QuantStudio3, Applied biosystem) were performed for investigation of mRNA expression of PRRs using previously described primers (Dectin 1, Toll like receptors (TLRs)-2, 3, 4, 6 and NOD-like receptor, pyrin domain containing 3 (NLRP3) (Roy S et al, 2015).Citation26 GAPDH gene was used to normalize the mRNA expression of PRRs. The calculation of relative gene expressions in fold change was carried out using the delta-delta Ct method (2–ΔΔCt method).
Data analysis
The expression of immune mediators in infected tissues was compared with control uninfected tissue (from nine rabbits). The graphs were constructed and analyzed by GraphPrism (ver-5.0). The significance level of cytokines expression by both the methods was determined by one way analysis of variance (ANOVA) with Post-Hoc Tukey HSD test and the p-value ≤0.05 was considered significant. The data were reported in fold change or pg/mL with standard deviation (SD) for RT-qPCR and for mELISA respectively.
Results
The rabbits which were inoculated with intracorneal injection of P. insidiosum zoospores in right eye developed infection on next day. Clinical features like total corneal involvement (total infiltrate: short thin arrow), ring (long thin arrow) or patchy infiltrate (short thick arrow) were induced in infected cornea (). The left eye of rabbits which received intracorneal injection of phosphate buffer saline (PBS-pH-7.4) left only injection marks for approximately 3 days and they were clinically normal. Culture of corneal scrapings from infected eye of all rabbits on blood agar showed pure growth of P. insidiosum, there was no contamination ().
Figure 1 Clinical, pictorial representation of P. insidiosum infected (intracorneally) rabbit right eye [3rd, 7th and 9th day post infection (PI)] and uninfected left eye. Pictures a, b, c, d, e, f, g, h and i are infected right eye of rabbit (R)3, R7, R29, R9, R10, R36, R11, R12 and R30 respectively with total (short thin arrow), ring (long thin arrow) or patchy infiltrate (short thick arrow) while j, k and l are the representative pictures of uninfected control eye with normal clinical characteristics (received only PBS, pH-7.4).
![Figure 1 Clinical, pictorial representation of P. insidiosum infected (intracorneally) rabbit right eye [3rd, 7th and 9th day post infection (PI)] and uninfected left eye. Pictures a, b, c, d, e, f, g, h and i are infected right eye of rabbit (R)3, R7, R29, R9, R10, R36, R11, R12 and R30 respectively with total (short thin arrow), ring (long thin arrow) or patchy infiltrate (short thick arrow) while j, k and l are the representative pictures of uninfected control eye with normal clinical characteristics (received only PBS, pH-7.4).](/cms/asset/75d6235a-4f7a-4a3f-a912-a5c3e9df8d59/icey_a_2023192_f0001_c.jpg)
Figure 2. Representative pictures a, b and c showing the blood agar (BA) culture of corneal scrapings obtained from P. insidiosum infected right eye of rabbits R29, R36 and R30 respectively before harvesting of cornea. (R: rabbit).
mRNA expression assay
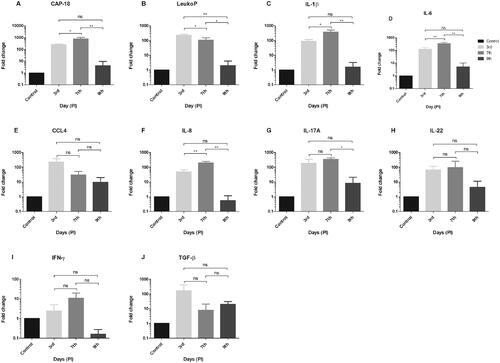
The RT-qPCR which was used to determine the association of innate and adaptive immune mediators in rabbit cornea to P. insidiosum showed significant upregulation of various immune mediators on 7th day compared to 3rd and 9th day post infection (DPI) (p < 0.05) over control () including antimicrobial protein CAP-18 [3rd vs. 7th vs. 9th DPI (245 vs. 768 vs. 4.8-fold)], pro-inflammatory cytokines IL-1β (90 vs. 366 vs. 1.5-fold) and IL-6 (125 vs. 353 vs. 5-fold) and Chemokine IL-8 (48 vs. 194 vs. 0.5-fold). Additionally, another antimicrobial protein, the LeukoP was significantly increased on 3rd day compared to 7th and 9th DPI (230 vs. 106 vs. 2-fold, p < 0.05). The expressions of these cytokines were also higher on 3rd day compared to 9th day post infection. Apart from the above innate immune mediators, there was no significant difference observed for CCL4, it was higher at early stage compared to later stages. Among the other adaptive immune cytokines (IFN-γ, IL-22, and TGF-β), only IL-17A was significantly higher (p-value = 0.028) on 7th day (339 fold) compared to 9th day DPI (8-fold). Although the expression of TGF-β was not statistically different, it was higher on 3rd (162-fold) and 9th day (19-fold) compared to 7th day DPI (7-fold).
Figure 3. Cytokines expression by RT-qPCR showing fold change (relative expression) over uninfected control. GAPDH was used as housekeeping gene. This figure showing the expression of AMPs (A) CAP-18 and (B) LeukoP, pro-inflammatory cytokines (C) IL-1β and (D) IL-6, chemokines (E) CCL4 and (F) IL-8 and the adaptive immune cytokines (G) IL-17A, (H) IL-22, (I) IFN-γ and (J) TGF-β on 3rd, 7th and 9th day PI respectively. The AMPs: CAP-18, LeukoP and innate immune mediators: IL-1β, IL-6, and IL-8 are significantly upregulated on 7th compared to 3rd and 9th day except LeukoP which was significantly higher on 3rd day followed by 7th and 9th day PI. The expression of these cytokines are also higher on 3rd day compared 9th day PI. Among the other adaptive immune cytokines, only IL-17A showed significantly higher expression on 7th day compared to 9th day PI. Abbreviations: PI: Post infection; ns: not significant. Statistical significant p-value ≤0.05*, 0.005** (one way ANNOVA, Post-Hoc Tukey HSD test).
Protein expression assay
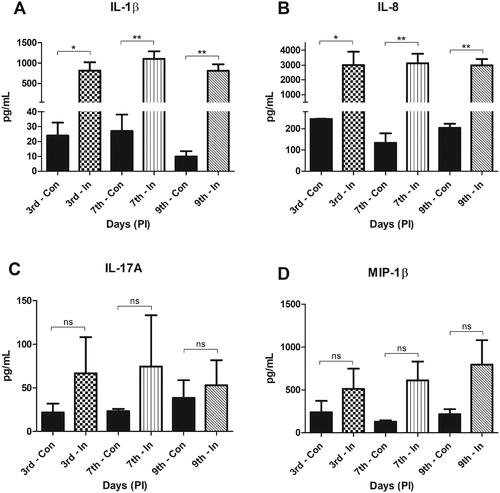
To validate if the innate immune mediators are significantly associated with P. insidiosum infection in rabbit cornea, multiplex ELISA was performed for the available cytokines. The P. insidiosum challenged cornea of rabbit showed significantly elevated levels of pro-inflammatory cytokine IL-1β [3rd, 7th and 9th DPI (814, 1104 and 809 pg/mL, p-value <0.05) and chemokine IL-8 (3007, 3112, and 2974 pg/mL, p-value <0.05) compared to uninfected control in all of the time points (). The expression levels of IL-17A and MIP-1β were not significantly different in comparison to control.
Figure 4. Multiplex-ELISA showing the expression level (pg/mL) for (A) IL-1β, (B) IL-8, (C) IL-17A and (D) MIP-1β/CCL4 in control and infected tissue. IL-β and IL-8 showed significant elevated expression in infected tissue in all the time points of post infection compared to its respective control. Abbreviations: Con: Control; In: infected; PI: Post infection; ns: not significant. Statistical significant p-value ≤0.05*, 0.005**(one way ANNOVA, Post-Hoc Tukey HSD test).
STRING network analysis
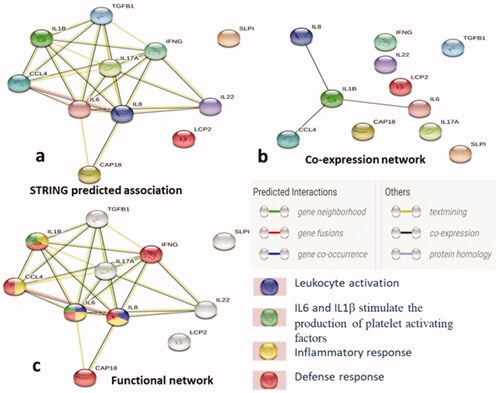
STRING (ver-11.0) analysis was carried out for studying the interactions of antimicrobial peptides, cytokines and chemokines in immune response of rabbit (Oryctolagus cuniculus) to microbial infection. The STRING network demonstrated the predicted association between these molecules with an average local clustering coefficient of 0.807. The network showed significantly more interaction than expected with the PPI (protein-protein interaction) enrichment p-value ≤1.0e − 16. The STRING predicted association (at the top right of ) was the general interaction between the antimicrobial peptide, cytokine and chemokine, while the network with black colored edges, at the top left of this figure showed the strong co-expression of pro-inflammatory cytokines IL-1β, IL-6, CCL4 and chemokine IL-8. Along with the crucial involvement of IL-6, IL-8, CCL4 and IL-1β in inflammatory response (yellow colored nodes) seen in the functional network (right bottom of the figure), CAP-18 (not involved in inflammatory response) was also involved in host defense response (red colored nodes). IL-6 and IL-8 are also associated with leukocyte activation (blue colored nodes), which might produce the leukocyte antimicrobial proteins (LeukoP). This functional network also showed the role of IL-6 and IL-1β in the production of platelets activating factors (green colored nodes), which interact physically and functionally with circulating leukocytes.
Figure 5. The networks showing evidence mode interactions (STRING Database Ver 11.0) of the antimicrobial peptides, cytokines and chemokine in host immune response of rabbit. (a) Simple network that shows the predicted association of immune mediators, (b) Network indicating the co-expression of IL-8, IL-1β, IL-6 and CCl4, and (c) The functional association manifesting the role of IL-6 and IL-8 in leukocyte activation (blue colored node), IL-6, IL-8, IL-1β and CCl4 in inflammatory response (yellow colored node) and along with these IFN-γ and CAP-18 are involved in host defence response of the host (red colored node) while IL-6 and IL-1β involved in the production of platelets activating factors (green colored nodes) that physically and functionally associates with leukocytes. Nodes: Proteins, edges: interactions, bottom left panel showing the type of interactions between the immune molecules.
Expression profile of PRRs in HCECs challenged with P. insidiosum
In order to further validate the involvement of innate immune response to P. insidiosum in acute phase of infection, we stimulated the HCECs with zoospores of P. insidiosum for 6 hrs and 12 hrs. The HCECs which were stimulated with zoospores showed the germination of branched, aseptate filaments of Pythium at the aforementioned time points (). The infected HCECs showed significant elevated levels of Dectin 1 (upregulated by 3.4 fold, p-value <0.05) and TLR4 (upregulated by 2.5 fold, p-value <0.005) among other PRRs (TLR2, TLR3, TLR6 and NLRP3) at 12 hrs. However, we did not observe any significant difference at 6 hrs over control ().
Figure 6. Stimulation of human corneal epithelial cells (HCECs) with zoospores (MOI: 0.005) of P. insidiosum for 6 and 12 hours. The yellow arrows indicate branched filaments of P. insidiosum germinated from its zoospores.
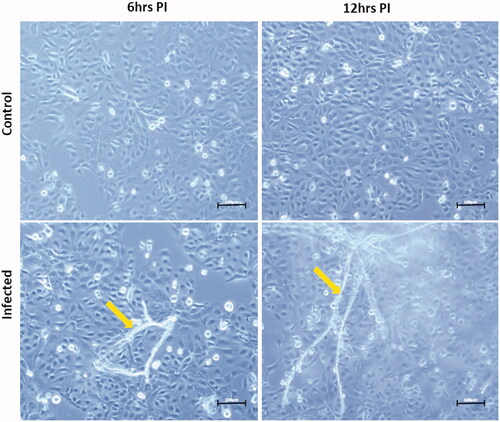
Figure 7. Expression profile of various pathogen recognition receptors [PRRs: Dectin 1 (A), Toll like receptor (TLR)-2 (B), TLR3 (C), TLR4 (D), TLR6 (E) and NLRP3 (F)] in human corneal epithelial cells (HCECs) induced with zoospores of (MOI: 0.005) P. insidiosum over time (6 and 12 hours respectively). GAPDH was used as housekeeping gene for normalization of PRRs expression. The PRRs: Dectin 1 and TLR4 showed statistically significant upregulation at 12 hrs in comparison to 6 hrs post stimulation. Abbreviations: ns: not significant. Significant p-value ≤0.05*, 0.005** (one way ANNOVA, Post-Hoc Tukey HSD test).
![Figure 7. Expression profile of various pathogen recognition receptors [PRRs: Dectin 1 (A), Toll like receptor (TLR)-2 (B), TLR3 (C), TLR4 (D), TLR6 (E) and NLRP3 (F)] in human corneal epithelial cells (HCECs) induced with zoospores of (MOI: 0.005) P. insidiosum over time (6 and 12 hours respectively). GAPDH was used as housekeeping gene for normalization of PRRs expression. The PRRs: Dectin 1 and TLR4 showed statistically significant upregulation at 12 hrs in comparison to 6 hrs post stimulation. Abbreviations: ns: not significant. Significant p-value ≤0.05*, 0.005** (one way ANNOVA, Post-Hoc Tukey HSD test).](/cms/asset/25e7f361-e98e-40dc-9850-a3c9bbccb319/icey_a_2023192_f0007_b.jpg)
Discussion
The corneal infection in humans caused by P. insidiosum is devastating and frequently it is required surgical removal of infected tissues for its therapeutic management.Citation27 There are limited studies related to the pathogenesis of P. insidiosum and immune response of host cornea. For the evaluation of mRNA expression of various cytokines and chemokines, this study has employed qPCR method and wherever possible the results were validated by protein estimation by mELISA.
Our data demonstrate that antimicrobial peptide: Leukocyte protein (LeukoP)-p15 was significantly upregulated on 3rd compared to 7th and 9th day post infection with the significant difference observed among all time points by RT-qPCR. In addition to the expression of LeukoP, CAP-18 also showed elevated level on 3rd and 7th day post infection by RT-qPCR. The cathelicidin showed bactericidal activity by inducing morphological changes in cell membrane while leukocyte proteins-P15 (15-kda protein) which was expressed in rabbits had strong antimicrobial activity against Escherichia coli.Citation15 As the AMPs are the important component of innate immune response, it could be involved in inhibiting or killing of Pythium. Both LeukoP and CAP-18 (cationic antimicrobial peptides) are the members of cathelicidin family produced by granulocytes. These proteins contain N-terminal conserved regions while C-terminal domains are variable which possess antimicrobial activity.Citation28 The human cathelicidin antimicrobial protein LL-37 with 16 kDa molecular mass is also known as hCAP18. In humans, it is designated as hCAP18 because it showed close similarity to rabbit CAP18 which had molecular mass of 18 kDa.Citation29 The inactive precursor of CAP18/LL-37 undergoes proteolytic cleavage for its activity. The expression of this protein is associated with various cell types including neutrophils, monocytes, macrophages, epithelial cells, natural killer cells and B cells.Citation30 At physiologic conditions this antimicrobial protein has net positive charge that evokes direct electrostatic interaction with anionic charged plasma membrane of bacteria for their elimination.Citation29 It has been reported previously that this protein showed microbicidal effects on bacteria, fungi and viruses via similar actions. These cathelicidin proteins have also been found in various species such as sheep, horses, chickens, cattle and certain species of fish.Citation31 Another antimicrobial protein LeukoP (15 kDa protein) which was expressed by various lymphocytes shares similar features with other cathelicidin proteins. This 15 kDa protein does not undergo the proteolytic processing for release of its active peptides.Citation32 There are few reports which showed the synergistic effect of 15 kDa leukocyte protein with bactericidal/permeability increasing proteins (BPI, 50 kDa molecule).Citation32,Citation33 This BPI (antimicrobial protein) showed its inhibitory action against Escherichia coli and its effective concentration was reduced by 50 fold in presence of 15 kDa protein.Citation33
Furthermore, the potent pro inflammatory cytokine IL-1β and chemokine IL-8 studied by qPCR and validated by mELISA induced a strong immune response in rabbit cornea to P. insidiosum. The pro-inflammatory cytokine IL-1β, which was significantly expressed by both methods, exerts protective role against pathogens. Higher level of IL-1β is associated with fungal ocular infection in patients.Citation32 Another pro-inflammatory cytokine IL-6, which showed significant increased expression on 7th day in comparison to 3rd and 9th day post infection also have protective role against pathogen. Human corneal epithelial cells (hCECs) challenged with P. insidiosum were induced to increase the level of IL-6 pro-inflammatory cytokine.Citation7 It is a multifunctional pro-inflammatory cytokine, which is crucial for efficient host defense against pathogenic bacteria. The human corneal epithelial cells (hCECs) produced antimicrobial peptide LL-37 (known as CAP-18) in response to flagellin protein of P. aeruginosa,Citation13 also associated with IL-6 and IL-8 cytokines production induced by P. insidiosum.Citation7 The association of these immune cells with infection caused by P. insidiosum supports our findings of cytokine expression, which showed the significant higher levels of innate immune mediators on 3rd and 7th day post infection.
The chemokine IL-8, which was significantly higher on 7th day compared to 3rd and 9th day post infection studied by RT-qPCR also showed significantly higher expression in infected tissue compared to control by mELISA on three of these time points. It is one of the chemokines, which has specificity to activate and recruit neutrophils to the local site of inflammatory region.Citation34 As it is well known for chemotaxis, it also plays a crucial role as potent pro-inflammatory immune mediator.Citation35 Although not statistically significant, the expression of other chemokine CCL4/MIP-1β demonstrated by RT-qPCR and mELISA was higher on 3rd followed by 7th and 9th day post infection studied by RT-qPCR. It was higher in infected tissue (mELISA) in comparison to control in all of the time points. It is a chemotactic immune mediator which induces recruitment of eosinophils at the site of inflammation. The eosinophils play important role in innate and adaptive immunity and maintain tissue homeostasis in human mucosal surface.Citation36 The histopathology of corneal tissue infected with P. insidiosum of rabbits included in this study also showed predominant infiltration with eosinophils and other polymorphonuclear cells.Citation11
As the corneal epithelium lacks the lymphatic vessels, it mainly responds to pathogens via innate immune system. The innate immunity of the host involves the pathogen recognition receptors (PRRs) that recognize the pathogen associated molecular patterns (PAMPs) of the pathogens to trigger various signaling pathway to produce pro inflammatory cytokines.Citation37 Among several PRRs, Dectin 1, TLR2, TLR3, TLR4, TLR6 and NLRP3 have been associated with recognition of PAMPs molecules of fungi. Our study showed the association of Dectin 1 and TLR4 with recognition of P. insidiosum. The blockade of TLR2 in HCECs did not alter the expression of IL-6 and IL-8 mRNA7 that suggest the association of other PRRs as well with Pythium recognition in HCECs. Several fungal species were recognized via TLR4 and Dectin 1 receptors that produce inflammatory response.Citation19–23 The stimulation of HEK cells with P. insidiosum zoospores revealed the association TLR2 in its recognition.Citation7 Dectin 1 interacts with β-glucan while TLR2/TLR6 recognize α1,4-glucans, zymosan and Glucuronoxylomannans. Toll like receptor-3 recognizes the double-stranded RNA molecules while TLR4 identify and interacts with zymosan and mannan lipopolysaccharides.Citation38 Another PRR, the NLRP3 recognizes the β1,3-glucans (NLRP3)22 of fungi to regulate the cytokines secretion. The dectin 1 receptor activates NF-κβ activation through CARD9 while TLRs depends importantly on MyD88 proteins to secret inflammatory cytokines, chemokines and antimicrobial peptides.Citation22,Citation39 Though, this study addresses involvement of PRRs in the innate immune response of HCECs to P. insidiosum investigated by RT-qPCR, owing to lack of RNA, we could not evaluated the expression of these PRRs in the rabbit corneal tissues infected with P. insidiosum.
Unfortunately, the effective virulence factors of P. insidiosum have not been elucidated yet. Thus, it is difficult to investigate the specific mechanism of pathogenesis. Although, immune response studies are limited to P. insidiosum, there are few reports which tried to evaluate the association of innate immune mediators. In 2020, Wongprompitak et alCitation7 showed the involvement of TLR2 receptor mediated NF-kβ activation in human kidney cells (HEK). The stimulation of HCECs with Pythium zoospores induced the production of the pro-inflammatory cytokine IL-6 and chemokine IL-8 which regulate the activation and migration of inflammatory cells at the site of infection. The pro inflammatory cytokines IL-6 also involved in healing and tissue repair mechanism.Citation40 There are various cell types of innate immune system including neutrophils and eosinophils that involve in secretion of pro-inflammatory markers in response to infection. The neutrophils produce pro-inflammatory cytokines: IL-1β, IL-6 and chemokine: IL-8 to eliminate intruders.Citation12 The cells of eosinophil degranulate around the hyphae of P. insidiosum during natural infection which produced IL-6, IL-8, Macrophage inflammatory protein-1 and other cytokines.Citation36
The expressions of other adaptive immune cytokines (IFN-γ and IL-22) were not statistically significant except IL-17A on 7th day compared to 9th by RT-qPCR, it was higher in infected tissue compared to control (mELISA) in each of these temporal periods. It is a pro-inflammatory cytokine which is produced by Th17 cells subset. These cells are reportedly induced by P. insidiosum zoospores to produce IL-17A in disseminated pythiosis of BALB/c mice.Citation1 The key reason of not finding significant expression of other adaptive immune cytokines (IFN-γ and IL-22) in this study could be the upregulation of TGF-β in early and late stage of infection. TGF-β is a regulatory cytokine,Citation41 in order to balance the functions of the immune system it can inhibits the differentiation of Th1, CTL (cytotoxic T lymphocytes), NKT (natural killer T cells) and promote the differentiation of Th17 cells (IL-17A) subsets.Citation42 Therefore, the cytokine IL-22 produced by NKT cell subsetsCitation43 and IFN-γ produced by Th1 cells were not statistically different in corneal tissue infected with P. insidiosum. In addition to this, the less success rate (∼55% in human) of Pythium immunization outcome in various studiesCitation4 could also be a possible explanation of not finding significant changes in adaptive immune cytokines expression. In addition to the merits of the study, it also has limitation. As the expression of antimicrobial peptides (CAP18 and LeukoP), pro-inflammatory cytokines (IL-6, IL-22, IFN-γ) and TGF-1 were studied by RT-qPCR, due to unavailability of rabbit specific cytokines, the results of these immune mediators could not be validated by mELISA.
Unexpectedly, it was observed that levels of IL-1β, IL-17A, IL-8, and CCL4 mRNA expressions were diminished on 9th day post infection (PI) investigated using RT-qPCR but the levels were higher on the same day demonstrated by multiplex ELISA. The discrepancy in the levels of these mRNA and proteins could be due alteration in the steady state, short-term adaptation and long-term state changes under certain conditions.Citation44 There is substantial evidence that suggest the role of regulatory processes in post-transcriptional modification of mRNA, translational changes in proteins and its degradation regulation. All of these can control abundances of protein and mRNA in cells.Citation45 Sometimes, the rate of peptide synthesis and degradation varies significantly that could lead the mismatch in expression of mRNA and proteins.Citation44 In 1992, Bar A et al found that the surface protein expression of CD15 was high, while the CD15 mRNA expression was low in myeloid-derived suppressor cells.Citation46
In order to check the interaction of these antimicrobial peptides, cytokines and chemokines and their association with rabbit immune response to pathogens we carried out the STRING network analysis. The network revealed the role of cytokines in immune response of the host defense and showed the co-expression of IL-1β, IL-6 and IL-8 that indicates the strong association of pro-inflammatory cytokines and chemokines with microbial infection in rabbits.
The rabbit corneas were infected with zoospores of P. insidiosum which germinates within few hours in infected tissues. Our study shows the host immune response on 3rd, 7th and 9th day post infection. Therefore, it is the immune response generated by hyphae of P. insidiosum in rabbit cornea. A report by Wongprompitak et al (2020)Citation7 showed that both hyphae and zoospore of P. insidiosum stimulated NF-κB activation via TLR2 in HEK-Blue™-hTLR2.
Conclusions
This study suggests that the innate immune mediators: IL-1β, IL-8, IL-6 and antimicrobial peptides: LeukoP and CAP-18 are significantly associated with host corneal immune response towards P. insidiosum infection in rabbits. Additionally, our data showed significantly elevated expression of these innate immune mediators in early to mid-stage of infection. The PRRs: Dectin 1 and TLR4 are involved in the recognition of P. insidiosum by human corneal epithelial cells (HCECs). Adaptive immune response on the other hand seems to be poor for the period of study. As the cytokines possess protective role, the efficacy of innate cytokine therapy for Pythium keratitis using especially IL-1β, IL-8 and IL-6 should be evaluated in a rabbit model followed by a clinical trial in the patients. We believe that targeting the innate immune mediators could play a crucial role in managing pythiosis.
Supplemental Material
Download Zip (353.3 KB)Acknowledgement
The authors are thankful to Dr. Pavan Kalra for his help with clinical examination of the rabbit’s cornea and to Dr. Sanhita Roy (L V Prasad Eye Institute, Hyderabad, India) for generously providing us the human corneal epithelial cells (HCECs) and primers of pathogen recognition receptors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data supporting the results or analysis is presented as attached Supplementary files (S1, S2 and S3) separately.
Additional information
Funding
References
- Tondolo JSM, Loreto ÉS, Ledur PC, Jesus FPK, Silva TM, Kommers GD, Alves SH, Santurio JM. Chemically induced disseminated pythiosis in BALB/c mice: a new experimental model for Pythium insidiosum infection. PLoS One. 2017;12:e0177868. doi:10.1371/journal.pone.0177868.
- Krajaejun T, Sathapatayavongs B, Pracharktam R, Nitiyanant P, Leelachaikul P, Wanachiwanawin W, Chaiprasert A, Assanasen P, Saipetch M, Mootsikapun P, et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin Infect Dis. 2006;43:569–576. doi:10.1086/506353.
- Mendoza L, Newton JC. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Med Mycol. 2005;43:477–486. doi:10.1080/13693780500279882.
- Loreto S, Tondolo JS, Zanette RA, Alves SH, Santurio JM. Update on pythiosis immunobiology and immunotherapy. WJI. 2014;4:88–97. doi:10.5411/wji.v4.i2.88.
- Ledur PC, Tondolo JSM, Jesus FPK, Verdi CM, Loreto ÉS, Alves SH, Santurio JM. Dendritic cells pulsed with Pythium insidiosum (1,3)(1,6)-β-glucan, heat-inactivated zoospores and immunotherapy prime naïve T cells to Th1 differentiation in vitro. Immunobiology. 2018;223:294–299. doi:10.1016/j.imbio.2017.10.033.
- Permpalung N, Worasilchai N, Plongla R, Upala S, Sanguankeo A, Paitoonpong L, Mendoza L, Chindamporn A. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: a retrospective study of 18 patients. J Antimicrob Chemother. 2015;70:1885–1892. doi:10.1093/jac/dkv008.
- Wongprompitak P, Pleewan N, Tantibhedhyangkul W, Chaiprasert A, Prabhasawat P, Inthasin N, Ekpo P. Involvement of toll-like receptor 2 on human corneal epithelium during an infection of Pythium insidiosum. Asian Pac J Allergy Immunol. 2020;38:129–138. doi:10.12932/AP-110518-0311.
- Abdelfattah NS, Amgad M, Zayed AA. Host immune cellular reactions in corneal neovascularization. Int J Ophthalmol. 2016;9:625–633. doi:10.18240/ijo.2016.04.25.
- Conti HR, Gaffen SL. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 2015 195:780–788. doi:10.4049/jimmunol.1500909.
- Tondolo JSM, Ledur PC, Loreto ÉS, Verdi CM, Bitencourt PER, de Jesus FPK, Rocha JP, Alves SH, Sassaki GL, Santurio JM.. Extraction, characterization and biological activity of a (1,3)(1,6)-β-d-glucan from the pathogenic oomycete Pythium insidiosum. Carbohydr Polym. 2017;157:719–727. doi:10.1016/j.carbpol.2016.10.053.
- Kalra P, Ahirwar LK, Mittal R, Ranjith K, Das S, Manjulatha K, Bagga B, Mohamed A, Joseph J, Sharma S. Clinical and histopathological evaluation of a rabbit model for Pythium insidiosum keratitis. Curr Eye Res. 2020;45:542–549. doi:10.1080/02713683.2019.1676911.
- Tamassia N, Bianchetto-Aguilera F, Arruda-Silva F, Gardiman E, Gasperini S, Calzetti F, Cassatella MA. Cytokine production by human neutrophils: revisiting the "dark side of the moon". Eur J Clin Invest. 2018;48 (Suppl 2):e12952. doi:10.1111/eci.12952.
- Kumar A, Yin J, Zhang J, Yu FS. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest Ophthalmol Vis Sci. 2007;48:4664–4670. doi:10.1167/iovs.07-0473.
- Sharma P, Guha S, Garg P, Roy S. Differential expression of antimicrobial peptides in corneal infection and regulation of antimicrobial peptides and reactive oxygen species by type III secretion system of Pseudomonas aeruginosa. Pathog Dis. 2018;76: fty001. doi:10.1093/femspd/fty001.
- Zarember K, Elsbach P, Shin-Kim K, Weiss J. p15s (15-kD antimicrobial proteins) are stored in the secondary granules of rabbit granulocytes: implications for antibacterial synergy with the bactericidal/permeability-increasing protein in inflammatory fluids. Blood. 1997;89:672–679.
- Shang Y, Ren F, Song Z, Li Q, Zhou X, Wang X, Xu Z, Bao G, Wan T, Lei T, et al. Insights into Campylobacter jejuni colonization and enteritis using a novel infant rabbit model. Sci Rep. 2016;6:28737. doi:10.1038/srep28737.
- Schnupf P, Sansonetti PJ. Quantitative RT-PCR profiling of the rabbit immune response: assessment of acute Shigella flexneri infection. PLoS One. 2012;7:e36446. doi:10.1371/journal.pone.0036446.
- Esteves PJ, Abrantes J, Baldauf HM, BenMohamed L, Chen Y, Christensen N, González-Gallego J, Giacani L, Hu J, Kaplan G, et al. The wide utility of rabbits as models of human diseases. Exp Mol Med. 2018;50:1–10. doi:10.1038/s12276-018-0094-1.
- Jin X, Qin Q, Tu L, Zhou X, Lin Y, Qu J. Toll-like receptors (TLRs) expression and function in response to inactivate hyphae of Fusarium solani in immortalized human corneal epithelial cells. Mol Vis. 2007;13:1953–1961.
- Guo H, Wu X. 2009. Innate responses of corneal epithelial cells against Aspergillus fumigatus challenge. FEMS Immunol Med Microbiol. 56:88–93. doi:10.1111/j.1574-695X.2009.00551.x.
- Hua X, Yuan X, Li Z, Coursey TG, Pflugfelder SC, Li DQ. A novel innate response of human corneal epithelium to heat-killed Candida albicans by producing peptidoglycan recognition proteins. PLoS One. 2015;10:e0128039. doi:10.1371/journal.pone.0128039.
- Figueiredo RT, Carneiro LA, Bozza MT. Fungal surface and innate immune recognition of filamentous fungi. Front Microbiol. 2011;2:248. doi:10.3389/fmicb.2011.00248.
- Li C, Zhao GQ, Che CY, Li N, Lin J, Xu Q, Wang Q, Liu Y, Qiu S. Expression of dectin-1 during fungus infection in human corneal epithelial cells. Int J Ophthalmol. 2014;7:34–37. doi:10.3980/j.issn.2222-3959.2014.01.06.
- Mendoza L, Prendas J. A method to obtain rapid zoosporogenesis of Pythium insidiosum. Mycopathologia. 1988;104:59–62. doi:10.1007/BF00437925.
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi:10.1093/nar/gky1131.
- Roy S, Marla S, Praneetha DC. Recognition of Corynebacterium pseudodiphtheriticum by toll-like receptors and up-regulation of antimicrobial peptides in human corneal epithelial cells. Virulence. 2015;6:716–721. doi:10.1080/21505594.2015.1066063.
- Hasika R, Lalitha P, Radhakrishnan N, Rameshkumar G, Prajna NV, Srinivasan M. Pythium keratitis in South India: incidence, clinical profile, management, and treatment recommendation. Indian J Ophthalmol. 2019;67:42–47. doi:10.4103/ijo.IJO_445_18.
- Zarember KA, Katz SS, Tack BF, Doukhan L, Weiss J, Elsbach P. Host defense functions of proteolytically processed and parent (unprocessed) cathelicidins of rabbit granulocytes. Infect Immun. 2002;70:569–576. doi:10.1128/IAI.70.2.569-576.2002.
- Kuroda K, Okumura K, Isogai H, Isogai E. The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs. Front Oncol. 2015;5:144. doi:10.3389/fonc.2015.00144.
- Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi:10.1016/j.bbamem.2006.03.030.
- Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E. Cathelicidins: family of antimicrobial peptides. A review. Mol Biol Rep. 2012;39:10957–10970. doi:10.1007/s11033-012-1997-x.
- Zhang Y, Liang Q, Liu Y, Pan Z, Baudouin C, Labbé A, Lu Q. Expression of cytokines in aqueous humor from fungal keratitis patients. BMC Ophthalmol. 2018;18:105. doi:10.1186/s12886-018-0754-x.
- Levy O, Ooi CE, Weiss J, Lehrer RI, Elsbach P. 1994. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Invest. 94:672–682. doi:10.1172/JCI117384.
- Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64:456–460.
- Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, Giedlin MA, Mullenbach G, Tekamp-Olson P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995;155:1428–1433.
- Moqbel R, Levi-Schaffer F, Kay AB. Cytokine generation by eosinophils. J Allergy Clin Immunol. 1994;94(6 Pt 2):1183–1188. doi:10.1016/0091-6749(94)90330-1.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi:10.1016/j.cell.2010.01.022.
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi:10.3389/fimmu.2014.00461.
- Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, Korting HC. Toll-like receptors as key mediators in innate antifungal immunity. Med Mycol. 2004;42:485–498. doi:10.1080/13693780400011112.
- Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020;11:232. doi:10.1186/s13287-020-01755-y.
- Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi:10.1016/j.immuni.2019.03.024.
- Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9:a022236. doi:10.1101/cshperspect.a022236.
- Parks OB, Pociask DA, Hodzic Z, Kolls JK, Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front Cell Dev Biol. 2015;3:85. doi:10.3389/fcell.2015.00085.
- Li J, Zhang Y, Yang C, Rong R. Discrepant mRNA and protein expression in immune cells. Curr Genomics. 2020;21:560–563. doi:10.2174/1389202921999200716103758.
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi:10.1038/nrg3185.
- Bar A, Striem S, Vax E, Talpaz H, Hurwitz S. Regulation of calbindin mRNA and calbindin turnover in intestine and shell gland of the chicken. Am J Physiol. 1992;262:R800–5. doi:10.1152/ajpregu.1992.262.5.R800.
