Abstract
Purpose
To evaluate a novel hydrophobic, non-diffractive, extended depth of focus (EDOF) intraocular lens (IOL) design in comparison to two monofocal aspheric lenses.
Methods
Inclusion criteria for this prospective, monocentric cohort study were opacification of the crystalline lens and patients’ wishes for surgery. In the case of the EDOF IOL, patients asked for a presbyopia correction. All patients received surgery on both eyes. Corrected and uncorrected distance visual acuity (CDVA, UCDVA), uncorrected and distance corrected intermediate visual acuity (UIVA, DCIVA) and defocus curves (all monocular and binocular) were compared three months postoperatively.
Results
Fifty-six eyes were implanted with an EDOF IOL (LuxSmartTM, Bausch & Lomb GmbH, Berlin, Germany), 50 eyes with a monofocal aspheric IOL: 32 eyes with a clear IOL (Polylens® AS 61, Polytech Domilens, Roßdorf, Germany), 16 eyes with a yellow IOL (iSert® 251, Hoya Surgical Optics GmbH, Frankfurt, Germany). Three months postoperatively, UCDVA was comparable with the EDOF IOL, versus the monofocal IOL (P > 0.9). Binocular DCIVA in the EDOF IOL was significantly higher than in the monofocal IOL (P = 0.001). Monocular DCIVA better than 20/23 Snellen was achieved in 10% with the monofocal IOL and in 68% (P < 0.0001) with the EDOF IOL. Defocus curves showed a depth of focus at 20/23 Snellen of 1.6 vs. 0.83 diopters (D) in the EDOF IOL, vs. the monofocal IOL. No patient reported halos or starbursts in non-standardized questioning.
Conclusion
This non-diffractive EDOF IOL provided comparably high UCDVA and significantly higher DCIVA than the mono-focal lenses, causing only mild optical phenomena.
Introduction
Extended depth of focus (EDOF) lenses are designed to correct presbyopia by providing spectacle independence over a wider range, than classic monofocal intraocular lenses (IOL),Citation1,Citation2 while minimizing optical phenomena like glare or halos, which are common to diffractive bifocal or trifocal lenses, or refractive IOL with higher near addition as +3.0 diopters (D).Citation2–5
Currently, there are various optical designs in use to achieve an extended depth of focus. As published by Kohnen et al., EDOF lenses can be subdivided into four categories: Small aperture lenses use a pinhole effect to extend the range of visual acuity. Bioanalogic lenses mimic the pre-presbyopic crystalline lens in their optical characteristics and demonstrate refractive power, largest in the centre and lesser in the periphery of the IOL. Diffractive optics use concentric blazed gratings, similar to trifocal lenses. The fourth group uses a non-diffractive design and comprises lenses with usually a small zone of greater refractive power in the centre and decreasing refractive power in the periphery.Citation2 Because of the large number of lenses claiming EDOF capabilities, the American Academy of Ophthalmology (AAO) released a consensus statement with requirements for the evaluation of EDOF lenses.Citation6,Citation7
The LuxSmartTM (Bausch & Lomb GmbH, Berlin, Germany) is a non-diffractive, aspheric hydrophobic one-piece acrylic IOL with a 6 mm diameter optical zone, four fixation haptics and 11 mm diameter in total. Benard et al. found the combination of polynomial terms of 4th and 6th order to increase the depth of field when the weighting of both terms shows opposite signs.Citation8,Citation9 The LuxSmartTM utilizes this principle, to enhance the depth of focus. These higher-order aberrations are localized in the central 2 mm zone of the optic with a continuous transition zone leading to a monofocal distance corrected periphery. In this study, we evaluated the capabilities of this newly introduced, non-diffractive EDOF IOL in a standardized real-life setting and report the first clinical data of this IOL.
Patients and methods
This prospective monocentric cohort study compared consecutive patients, who received routine cataract surgery with yellow and clear acrylic aspheric monofocal IOL (clear hydrophilic 3-piece IOL: Polylens® AS 61, Polytech Domilens, Roßdorf, Germany, yellow hydrophobic 1-piece IOL: iSert® 251, Hoya Surgical Optics GmbH, Frankfurt, Germany) to patients, who received routine cataract surgery with the wish for presbyopia correction via EDOF IOL (LuxSmartTM, Bausch & Lomb GmbH, Berlin, Germany) in the time between 08/2020 and 12/2020. After informed consent, these patients preferred the EDOF IOL over the trifocal IOL, because of possible optical phenomena. The study adhered to the tenets set forth in the declaration of Helsinki and was approved by the Ethics Committee of the University of Cologne. All patients included in the analysis, gave informed consent, following information about the nature of the study and had the need for surgery, because of vision-impairing bilateral crystalline lens opacification. This was either defined as a decreased visual acuity to below 20/32 (0.2 logMAR) or typical complaints related to lens turbidity (e.g. glare, decreased night vision). Patients with preoperative astigmatism of >−1.5 D were excluded from analysis since no toric variant was used. Significant ocular morbidity is defined as a contraindication for multifocal lenses, including EDOF lenses. Therefore, the EDOF lens in our study was only offered to patients with no, or only non-significant ocular comorbidity (for example medically controlled glaucoma without visual field defects or age-related macular degeneration without foveal changes and without need for therapy). Additionally, there were out-of-pocket fees, associated with the EDOF IOL, like medical insurance in Germany does not compensate for multifocal lenses including EDOF lenses, meaning an unwillingness to pay for this lens was an exclusion criterion for this study.
All patients received surgery on both eyes on separate days (usually 21 days apart). Two experienced surgeons (both over 5000 cataract surgeries) performed the procedures, using ultrasound phacoemulsification and implantation of an IOL in the capsular bag with the same equipment in the same surgical environment. A clear corneal incision of 2.8 mm was always placed temporally in 9 and 3 o’clock positions, respectively. Paracenteses were placed in 12 and 6 o’clock positions. The estimated size of the capsulorhexis, by Utrata forceps, was 4 mm. All patients received capsular polishing, following phacoemulsification.
Preoperative examinations comprised subjective refraction and best-corrected distance visual acuity (BCDVA), intraocular pressure (Goldmann applanation tonometry), slit-lamp examination including diagnostic mydriasis, biometry (IOL-Master 700, Carl Zeiss Meditec AG, Jena, Germany), corneal tomography (Pentacam, Oculus, Wetzlar, Germany) and retinal Spectral-Domain Optical-Coherence-Tomography (SD-OCT) (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany).
Postoperative examinations for evaluation of IOL capacities were carried out 3 months after surgery of the second eye. They comprised subjective refraction with corrected distant visual acuity (CDVA), uncorrected distant visual acuity (UCDVA), uncorrected intermediate visual acuity at 80 cm (UIVA) and distance-corrected intermediate visual acuity (DCIVA), as well as slit-lamp examination and SD-OCT.
Contrast sensitivity was tested with the Mesotest® II (Oculus, Wetzlar, Germany). In this test an optotype (Landoldt ring) for visual acuity level 20/200 (0.1 Snellen decimal) is presented at varying levels of luminance against a background of 0.1 cd/m2. Results are given as Aulhorn/Harms-fractions by the machine and converted into logCSWeber for statistical analysis in this manuscript.Citation10
Monocular and binocular defocus curves were tested, using best-corrected distance correction and measuring visual acuity with 0.5 D defocus steps from +1.5 D to −2.5 D). The depth of focus was defined as the range of lens powers with a mean visual acuity ≥0.2 logMAR.
Statistical analysis was performed by commercially available software Prism Version 9 (GraphPad, La Jolla, CA, USA). Comparison of visual acuity was performed by non-parametric Kruskal-Wallis Test, with Dunn’s correction for multiple comparisons. Direct comparisons were performed by non-parametric Mann-Whitney-Test. Correlation analysis of corneal parameters with visual acuity was performed with non-parametric Spearman correlation.
Results
Baseline characteristics
The study included 53 patients, who received surgery on both eyes. Twenty-eight (56 eyes) asked for presbyopia correction and received the EDOF IOL. Twenty-five patients (50 eyes) received aspheric mono-focal lenses, of which 16 patients (32 eyes) received a clear monofocal IOL, and 9 patients (18 eyes) received a yellow monofocal IOL. The mean age was 72 ± 7 years (52–84 years). Forty-four patients had no known ocular conditions, other than cataracts. Four patients (2 with monofocal lenses, 2 with EDOF lenses) suffered from a pre-existing primary open-angle glaucoma, all without visual field defects. One patient had mild Fuchs corneal dystrophy (with monofocal IOL). Four patients had early age-related macular degeneration (all with monofocal lenses). None of the patients with pre-existing ocular disorders, other than cataracts had a postoperative CDVA of less than 0.1 logMAR 3 months post-surgery. Mean preoperative CDVA at baseline was 20/40 Snellen (20/25–20/80) (0.28 ± 0.14 logMAR (0.1–0.6)) and 20/40 Snellen (20/20–20/100) (0.28 ± 0.16 logMAR (0.0–0.7)) for patients receiving monofocal and EDOF lenses, respectively (P = 1.00). All patients were followed for 3 months after surgery.
Predictability
A UCDVA of 20/25 or better was achieved in 78% and 82% of all monofocal and EDOF eyes respectively (). The difference between UCDVA and CDVA was the same in 34% of all monofocal and EDOF eyes and within one Snellen line in 72% and 83% of all monofocal and EDOF eyes, respectively (). A UIVA of 20/25 was achieved in 12% and 46% of all monofocal and EDOF eyes, respectively (). The difference between UIVA and CDIVA was the same, or better in 90% and 88% of all monofocal and EDOF eyes, respectively. UIVA was within one line in 90% and 93% of all monofocal and EDOF eyes, respectively (). Postoperative spherical equivalent was within ±0.5 D in 82% and 96% of all monofocal and EDOF eyes, respectively (). The postoperative refractive cylinder was 0 D in 74% and 75% of all monofocal and EDOF eyes respectively (). In eyes, with EDOF lenses postoperative cylinder was with the rule in 4 cases (7%), against the rule in 1 case (2%) oblique in 8 cases (14%) and non-existent in 43 cases (77%). In eyes with monofocal lenses it was with the rule in 5 cases (10%), against the rule in 1 case (2%) oblique in 7 cases (14%) and non-existent in 37 cases (74%). The main incision was placed temporally in all cases. The flattening effect of the incision was 0.93 ± 0.86 (−4.5–0.5) D for monofocal eyes and 0.95 ± 0.64 (−2.75–0.0) for EDOF eyes ().
Figure 1. Efficacy, predictability and refractive cylinder analysis of the lenses used. (A) Efficacy: Histogram of postoperative UCDVA and CDVA. (B) Efficacy: Histogram of postoperative UIVA and DCIVA. (C) Efficacy: Histogram of difference between postoperative UCDVA and CDVA. (D) Efficacy: Histogram of difference between postoperative UIVA and DCIVA. (E) Predictability: Histogram of postoperative spherical equivalent refraction to Plano target refraction. (F) Refractive cylinder: Histogram of the postoperative refractive cylinder. (G + H) Refractive cylinder: Histogram of pre- and postoperative refractive cylinder in EDOF (G) and monofocal (H) lenses. CDVA: corrected distant visual acuity; DCIVA: Distance corrected intermediate visual acuity; EDOF: extended depth of focus; SEQ: spherical equivalent; UCDVA: uncorrected distant visual acuity; UIVA: uncorrected intermediate visual acuity.
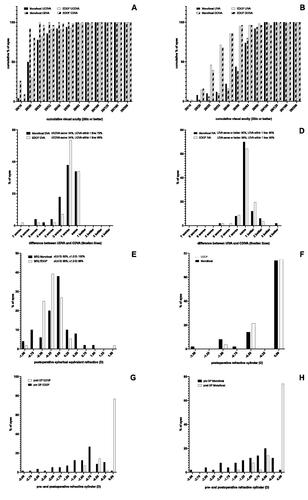
To exclude the influence of the two different types of monofocal lenses, used in this study, we performed a direct comparison of UCDVA, CDVA, UIVA and DCIVA of the two monofocal groups: There was no difference in monocular or binocular visual acuity at any distance, neither corrected nor uncorrected (P > 0.999).
Postoperative visual acuity
Three months after surgery, monocular and binocular CDVA and UCDVA were comparable with monofocal and EDOF lenses (P both > 0.999). Monocular, as well as binocular UIVA and DCIVA, were significantly higher with the EDOF IOL (P < 0.001 and P = 0.001, respectively) (). A monocular DCIVA ≥ 20/32 Snellen (0.2 logMAR) 3 months after surgery was achieved in 5 out of 50 eyes (10%) with monofocal lenses and in 38 out of 56 eyes (68%) with EDOF lenses (P < 0.001) ( lists all visual acuity scores.).
Figure 2. (A) Binocular visual performance of the EDOF and monofocal IOL. Distant visual acuity of the EDOF and monofocal IOL is comparable, while intermediate visual acuity of the EDOF IOL yields superior results (***P < 0.001). (B) Monocular defocus curves of the EDOF and monofocal IOL. The depth of focus at 0.2 logMAR of the EDOF versus monofocal IOL was 2.1 versus 1.5 D respectively. The EDOF IOL provides −0.6 D more pseudo accommodation than the monofocal IOL, supporting the intermediate vision.
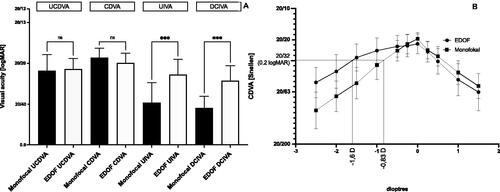
Table 1. Visual Acuity 3 months after surgery.
A binocular UCDVA and UIVA ≥ 20/32 Snellen (0.2 logMAR) was achieved in 23 of 25 patients (92%) and 6 of 25 patients (24%), respectively with monofocal lenses. With EDOF lenses, a binocular UCDVA and UIVA ≥ 20/32 Snellen (0.2 logMAR) was achieved in 28 of 28 patients (100%) and in 27 of 28 patients (96%).
Defocus curves
The monocular defocus curves 3 months after surgery differed significantly between EDOF lenses and mono-focal lenses with a fogging of −1.0 D and more (P < 0.001 each), indicating visual superiority from a distance of 100 cm and less. At 20/32 Snellen (0.2 logMAR) the depth of focus of the EDOF IOL was 1.6 D. The depth of focus of the monofocal IOL was 0.83 D. The EDOF IOL provides 0.77 D larger pseudophakic pseudo accommodation supporting intermediate vision (). Intermediate visual acuity at −1.43 D (70 cm) and −1.52 (66 cm), as derived from the defocus curve was 20/29 (0.16 logMAR) and 20/30 (0.18 logMAR).
Contrast sensitivity (CS)
The CS, measured with glare lighting conditions was 0.18 ± 0.1 (0.02–0.3) logCSWeber with the monofocal lenses, and thus slightly lower compared to 0.25 ± 0.09 (0.02–0.3) logCSWeber with the EDOF lenses (P = 0.01). Of the four patients with early macular degeneration, one had a CS of 0.3 logCSWeber, two had a CS of 0.1 logCSWeber and one had a CS of 0.02 logCSWeber. The one patient with Fuchs’ corneal dystrophy had a CS of 0.1. Thus, the accompanying ocular disorders were mild enough, not to influence the CS. In non-standardized questioning, patients reported neither starbursts nor halos with monofocal and EDOF lenses.
Corneal aberrations and pupil diameter
We determined preoperative corneal aberrations (corneal astigmatism, total corrected spherical aberration (TCSA) and totally corrected irregular astigmatism (TCIA) with IOL-Master700 and Pentacam, respectively. Mesopic pupil diameter we determined with IOL-Master 700. Mean corneal astigmatism in EDOF and monofocal eyes was −0.73 ± 0.44 D (−1.42–0) and −0.75 ± 0.43 D (−1.5–0), respectively. Mean pupil diameter for EDOF and monofocal eyes was 4.1 ± 1.1 mm (2.1–4.7) and 4.1 ± 0.9 mm (2.5–5.6), respectively. Mean TCSA in EDOF and monofocal eyes was 0.442 ± 0.116 µm (0.257–0.719) and 0.462 ± 0.142 µm (0.144–0.736), respectively. Mean TCIA in EDOF and monofocal eyes was 0.228 ± 0.089 µm (0.102–0.501) and 0.229 ± 0.084 µm (0.102–0.414), respectively. To evaluate the influence of these factors on postoperative visual acuity, we performed a correlation analysis. Preoperative corneal astigmatism had a strong correlation with postoperative UCDVA in EDOF, as well as in monofocal eyes (EDOF: r = −0.547, P < 0.001; monofocal: r = −0.483, P < 0.001). The correlation of corneal astigmatism with UIVA, CDVA and DCIVA in EDOF eyes was significant but weaker: (EDOF: UIVA: r = −0.326, P = 0.014, CDVA: r = −0.397, P = 0.002, DCIVA: −0.299, P = 0.025). In monofocal eyes, intermediate visual acuity showed no correlation with corneal astigmatism but only with distant visual acuity: (monofocal: UIVA: r = −0.075, P = 0.603, CDVA: r = −0.315, P = 0.026, DCIVA: r = −0.043, P = 0.765).
Because of the correlation of preoperative corneal astigmatism with postoperative visual acuity, we performed a subgroup analysis of eyes with preoperative corneal astigmatism >0.75 D versus ≤0.75 D. Interestingly, none of the comparisons reached a significance level at any distance, when visual acuity of eyes with astigmatism >0.75 D was compared to eyes ≤0.75 D. However, eyes with EDOF lenses had highly superior intermediate visual acuity, compared to eyes with monofocal lenses, in the group with >0.75 D, as well as ≤0.75 D astigmatism. Distant visual acuity was comparable in both groups. All values are displayed in Supplementary Table 1 (online supplemental material). In EDOF, as well as in monofocal eyes, neither pupil diameter, nor TCSA or TCIA showed a correlation with postoperative visual acuity (r all < ± 0.175, P all > 0.194).
Discussion
Extended depth of focus technology is an emerging field of presbyopia-correcting lenses with several optical designs developed recently, to achieve spectacle independence over a wider range of object distances.Citation2 This multitude of available lens designs calls for standardized evaluation of the lenses, to allow ophthalmologists to select the best possible IOL for the individual patient’s needs. The AAO consensus statement for the evaluation of EDOF lenses aims for the best possible comparability between studies, but may not always be realizable in routine clinical settingsCitation6 In this study we aimed to evaluate a newly developed non-diffractive EDOF IOL in a clinical setting, reporting the first clinical results of this IOL.
The LuxSmartTM EDOF IOL achieves high values for UCDVA and CDVA, which are comparable to the monofocal reference lenses, used in this study. Both, corrected and uncorrected distant VA values are also comparable to previously reported values for other EDOF lenses.Citation1,Citation2,Citation11–14 Thus, the LuxSmartTM does not show lower performance, compared to existing EDOF lenses, regarding distant VA. These results are also in accordance with a recently published report on 12 patients, who received the LuxSmartTM EDOF IOL.Citation15 The predictability, regarding distant VA, is also comparable to the monofocal IOL (). Although the spherical equivalent (SEQ) of the EDOF IOL is slightly more myopic than in the monofocal IOL, there is less variance in the SEQ of the EDOF IOL (). The difference between CDVA and UCDVA in our study even favours the EDOF IOL slightly ().
Postoperative astigmatism is able to induce increased depth of focus and improve near vision.Citation16,Citation17 The orientation of the axis seems to be of influence on this finding, however, the published data is somewhat contradictory, reporting a beneficial effect with the rule astigmatismCitation16, as well against the rule astigmatism on near vision.Citation17 To rule out a possible bias of astigmatism on the depth of focus in one of the groups in our study, we analyzed postoperative astigmatism, as well as the orientation of the axis. We found that both, the amount of postoperative astigmatism, as well as the orientation of the axis were equally distributed in both groups. Thus, a bias on the depth of focus, caused by postoperative astigmatism, seems rather unlikely.
At an intermediate range of object distances (tested at 80 cm), DCIVA and UIVA of the LuxSmartTM were highly superior to the monofocal lenses and achieved values of 20/23 Snellen (0.08 logMAR) (UIVA) and 20/25 Snellen (0.12 logMAR) (DCIVA) versus 20/39 Snellen (0.32 logMAR) (UIVA) and 20/44 Snellen (0.36 logMAR) (DCIVA) (P = 0.0005 and P = 0.001, respectively). Here the difference between DCIVA and UIVA was even smaller than that of the CDVA versus UCDVA (). In comparison to our study, the aforementioned report describes similar values of UIVA for the monofocal IOL but slightly inferior values for the EDOF IOL.Citation15 It has, however, to be noted that in this report intermediate vision was tested at 66 cmCitation15, whereas we tested at 80 cm, as recommended by the manufacturer. This is very likely the reason for the slightly lower values of UIVA. In contrast to this report, we here also analysed the DCIVA, to address the possible bias of a postoperative myopic spherical equivalent. However, in our study, the DCIVA was only slightly inferior to the UIVA. According to the manufacturer’s recommendations, we chose 80 cm for testing intermediate vision. This is a limitation to our study, since many reports choose 66 or 70 cm for intermediate vision testing, as recommended by the AAO. Intermediate vision, derived from the defocus curve at 70 cm and 66 cm was 20/29 (0.16 logMAR) and 20/30 (0.18 logMAR) and thus comparable to the aforementioned report.Citation15
Patients, who received the EDOF IOL, achieved an uncorrected binocular visual acuity of at least 20/32 Snellen (0.2 logMAR) in 100% for UCDVA and in 96% for UIVA. Patients with monofocal lenses achieved this visual acuity only in 92% (UCDVA) and 24% (UIVA) of all cases. This may reflect a high percentage of spectacle independence. Although we did not perform a structured spectacle-independence questionnaire, the report of Campos et al. also found high percentages of spectacle independence in daily life with this EDOF IOL.Citation15
This is also comparable to values reported for other EDOF lenses with non-diffractive optics, derived from clinical settings.Citation13,Citation14 Diffractive optics EDOF lenses seem to achieve slightly higher values of UIVA and DCIVACitation1,Citation11,Citation12, but are also susceptible to higher rates of disturbing optical effects (ranging between 5 and 60%).Citation1,Citation11,Citation12,Citation18,Citation19 Very recently, data from premarket trials of the United States Food and Drug Administration were published in a comparative studyCitation20, reporting data on several diffractive EDOF and trifocal lenses and a novel non-diffractive EDOF IOL (Acrysof Vivity, Alcon Laboratories Inc.). Understudy conditions, this respective non-diffractive IOL achieved very high values of UIVA and DCIVA. While this IOL caused almost no severe photic phenomena, patients complained about haloes and starbursts in up to 18% with mild symptoms and in about 9% with moderate symptoms.Citation20 In our study in non-standardized questioning, none of the patients complained about haloes or starbursts and the frequency of mild to moderate glare was comparable in EDOF and monofocal lenses. This is in accordance with the aforementioned recent report, where a structured questionnaire also did not detect optic phenomena more frequently, than in the monofocal group.Citation15 In our study, we did not perform a quality-of-life or spectacle-independence questionnaire. This is a limitation of our study and should be performed in further studies on this EDOF lens.
Corneal astigmatism correlates quite strongly with decreased postoperative visual acuity. Although the direct comparison of eyes with astigmatism >0.75 D to ≤0.75 D showed no significant difference in uncorrected visual acuity, the goal of any surgery is to achieve the best possible visual acuity for the patient. We, therefore, suggest using a toric variant, at least of the EDOF IOL in eyes with corneal astigmatism >0.75 D. This may be due to a certain flattening effect of the corneal incision. Corneal aberrations, other than corneal astigmatism do not seem to have a strong influence on postoperative visual acuity at any measured distance, neither in EDOF nor in monofocal eyes. However, it would be interesting to correlate the amount of higher corneal aberrations to overall patient satisfaction in a further study. The same applies to pupil diameter: It showed no correlation with visual acuity, but further investigation of the influence on patient satisfaction could be interesting.
In this study, we allowed the inclusion of mild and controlled ocular comorbidities other than cataracts. One could argue that this may have influenced postoperative visual acuity and contrast sensitivity. However, none of the patients with pre-existing ocular disorders, described in the results, had a postoperative CDVA of less than 20/25 Snellen (0.1 logMAR) 3 months post-surgery. This reflects the fact that the disorders were only mild.
In conclusion, the LuxSmartTM is a non-diffractive optics EDOF IOL with very limited optical side effects such as haloes or glare, providing spectacle independent vision from far to intermediate object distances. Diffractive optics EDOF lenses tend to achieve better intermediate and near visual acuity but at cost of a higher rate of disturbing optical phenomena. Further studies with larger numbers of participants should be performed with new adjustments to the formula constants to support our findings.
Supplemental Material
Download PDF (104.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Cochener B, Concerto SG. Clinical outcomes of a new extended range of vision intraocular lens: international multicenter concerto study. J Cataract Refract Surg. 2016;42(9):1268–1275. doi:https://doi.org/10.1016/j.jcrs.2016.06.033.
- Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. 2020;46(2):298–304. doi:https://doi.org/10.1097/j.jcrs.0000000000000109.
- de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12(12):CD003169. doi:https://doi.org/10.1002/14651858.CD003169.pub4.
- Mendicute J, Kapp A, Levy P, Krommes G, Arias-Puente A, Tomalla M, Barraquer E, Rozot P, Bouchut P. Evaluation of visual outcomes and patient satisfaction after implantation of a diffractive trifocal intraocular lens. J Cataract Refract Surg. 2016;42(2):203–210. doi:https://doi.org/10.1016/j.jcrs.2015.11.037.
- Rodov L, Reitblat O, Levy A, Assia EI, Kleinmann G. Visual outcomes and patient satisfaction for trifocal, extended depth of focus and monofocal intraocular lenses. J Refract Surg. 2019;35(7):434–440. doi:https://doi.org/10.3928/1081597X-20190618-01.
- MacRae S, Holladay JT, Glasser A, Calogero D, Hilmantel G, Masket S, Stark W, Tarver ME, Nguyen T, Eydelman M. Special report: American academy of ophthalmology task force consensus statement for extended depth of focus intraocular lenses. Ophthalmology. 2017;124(1):139–141. doi:https://doi.org/10.1016/j.ophtha.2016.09.039.
- Rampat R, Gatinel D. Multifocal and extended depth-of-focus intraocular lenses in 2020. Ophthalmology. 2021;128(11):e164–e185. doi:https://doi.org/10.1016/j.ophtha.2020.09.026.
- Benard Y, Lopez-Gil N, Legras R. Subjective depth of field in presence of 4th-order and 6th-order Zernike spherical aberration using adaptive optics technology. J Cataract Refract Surg. 2010;36(12):2129–2138. doi:https://doi.org/10.1016/j.jcrs.2010.07.022.
- Benard Y, Lopez-Gil N, Legras R. Optimizing the subjective depth-of-focus with combinations of fourth- and sixth-order spherical aberration. Vision Res. 2011;51(23–24):2471–2477. doi:https://doi.org/10.1016/j.visres.2011.10.003.
- Wilhelm H, Peters T, Durst W, Roelcke S, Quast R, Hutten M, Wilhelm B. [Assessment of mesopic and contrast vision for driving licences: which cut-off values, which methods are appropriate?]. Klin Monbl Augenheilkd. 2013;230(11):1106–1113. doi:https://doi.org/10.1055/s-0033-1351030.
- Ganesh S, Brar S, Pawar A, Relekar KJ. Visual and refractive outcomes following bilateral implantation of extended range of vision intraocular lens with micromonovision. J Ophthalmol. 2018;2018:7321794. doi:https://doi.org/10.1155/2018/7321794.
- Kohnen T, Bohm M, Hemkeppler E, Schonbrunn S, DeLorenzo N, Petermann K, Herzog M. Visual performance of an extended depth of focus intraocular lens for treatment selection. Eye. 2019;33(10):1556–1563. doi:https://doi.org/10.1038/s41433-019-0443-x.
- Savini G, Balducci N, Carbonara C, Rossi S, Altieri M, Frugis N, Zappulla E, Bellucci R, Alessio G. Functional assessment of a new extended depth-of-focus intraocular lens. Eye. 2019;33(3):404–410. doi:https://doi.org/10.1038/s41433-018-0221-1.
- Savini G, Schiano-Lomoriello D, Balducci N, Barboni P. Visual performance of a new extended depth-of-focus intraocular lens compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg. 2018;34(4):228–235. doi:https://doi.org/10.3928/1081597X-20180125-01.
- Campos N, Loureiro T, Rodrigues-Barros S, Rita Carreira A, Moraes F, Carreira P, Machado I. Preliminary clinical outcomes of a new enhanced depth of focus intraocular lens. OPTH. 2021;15:4801–4807. doi:https://doi.org/10.2147/OPTH.S344379.
- Leube A, Ohlendorf A, Wahl S. The influence of induced astigmatism on the depth of focus. Optom Vis Sci. 2016;93(10):1228–1234. doi:https://doi.org/10.1097/OPX.0000000000000961.
- Serra P, Chisholm C, Sanchez Trancon A, Cox M. Distance and near visual performance in pseudophakic eyes with simulated spherical and astigmatic blur. Clin Exp Optom. 2016;99(2):127–134. doi:https://doi.org/10.1111/cxo.12350.
- Ang RE, Picache GCS, Rivera MCR, Lopez LRL, Cruz EM. A comparative evaluation of visual, refractive, and patient-reported outcomes of three extended depth of focus (edof) intraocular lenses. Clin Ophthalmol. 2020;14:2339–2351. doi:https://doi.org/10.2147/OPTH.S255285.
- Lubiński W, Podborączyńska-Jodko K, Kirkiewicz M, Mularczyk M, Post M. Comparison of visual outcomes after implantation of atlisa tri 839 mp and ymphony intraocular lenses. Int Ophthalmol. 2020;40(10):2553–2562. doi:https://doi.org/10.1007/s10792-020-01435-z.
- Schallhorn JM. Multifocal and extended depth of focus intraocular lenses: a comparison of data from the united states food and drug administration premarket approval trials. J Refract Surg. 2021;37(2):98–104. doi:https://doi.org/10.3928/1081597X-20201111-02.
