Abstract
Purpose
To provide a complete nerve architecture and main sensory neuropeptide distribution in the chicken cornea.
Methods
Adult chickens aged 6 months and 4 years were used. The whole cornea was stained with protein gene product (PGP) 9.5 antibody-a pan marker for nerve fibers, calcitonin gene-related peptide (CGRP), and substance P (SP) antibodies; whole-mount images were acquired to build an entire view of corneal innervation. Relative corneal epithelial nerve fiber densities, including subbasal bundles and superficial terminals, were assessed by computer-assisted analysis.
Results
An average of about 76.3 ± 5.7 (n = 8 corneas, 4 M/4F) stromal nerve trunks enter the cornea radially and are evenly distributed around the limbus with no significant difference between male and female chickens. The subbasal nerve bundles do not extend in a given direction and, as a result, do not form a vortex in the center of the cornea. Furthermore, the chicken cornea contains more SP-positive nerves than CGRP-positive nerves. It is also shown that aging significantly reduces corneal epithelial nerve density in chickens.
Conclusions
This is the first study to provide a complete map of the entire corneal nerves and CGRP and SP sensory neuropeptide distribution in the adult chicken cornea. The findings show chicken corneal innervation has many differences to human and mammal cornea.
Introduction
As the window of the eye, the cornea is one of the most sensitive tissues in the human body, with a densely innervated epithelium. Corneal nerves are important to maintain the integrity and transparency of the tissue.Citation1–4 Decreasing corneal innervation results in altered epithelial morphology and function, poor tear film, and delayed wound healing.Citation4 Many ocular or systemic diseases and ophthalmic surgeries can damage the corneal nerves, leading to conditions ranging from mild dry eye to severe neurotrophic keratitis with corneal blindness.
Chickens are one of the popular models used to study corneal neurobiology, and embryonic chickens have been widely employed to study the development of corneal innervation.Citation5–7 Several studies have reported that chicken corneas are superior models to rodents because they are larger than rodents, thus making them more suitable for experimental procedures, such as refractive surgery and myopia research.Citation8 Importantly, chicken corneas have a Bowman’s layer similar to humans,Citation7,Citation9 and several studies have employed chicken eyes to investigate the developmental factors of refractive error and wound healing response after refractive surgery.Citation8,Citation10–13
Embryonic chicken corneas have been widely used to study the development of innervation,Citation14–16 but little is known about the neural structure of the adult cornea. Using an improved technique of immunofluorescence and imaging developed in our laboratory, we have provided a complete map of the corneal nerve structure in humans and in several experimental animal models, including mice, rats, and cats.Citation17–22 In the current study, we used this technique to disclose the entire nerve architecture of adult chickens as well as the relative content of the sensory neuropeptides calcitonin gene-related protein (CGRP) and substance P (SP). We also studied the effect of aging on corneal innervation.
Materials and methods
Animals
Twelve white leghorn chickens were used in this study. The chickens were grown on the property of one of the authors (J. He) and used for his own consumption. Chickens were free to roam in the 60 × 80 sq. ft. backyard during the day, and at night, they were returned to the hencoop in the garage, which was maintained at 20°–26 °C. The birds had access to clean tap water and special pelleted food for chickens (Petco, New Orleans). Of these chickens, eight (male/female, M/F = 4/4) were 6 months old, and the remaining four were 4-year-old females.
Immunofluorescence
After the birds were killed by decapitation, the corneas were removed along the corneoscleral rim and immediately fixed in freshly prepared 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 hours at room temperature (RT). Corneas were transported to the laboratory and washed thoroughly with PBS (four times for 15 minutes each), and tissues were incubated with rabbit monoclonal anti-protein gene product 9.5 (PGP9.5, EPR4118) antibody (1:1000, Abcam Inc., Cambridge, MA) in PBS containing 5% goat normal serum plus 0.5% Triton X-100 for 72 hours at RT with constant shaking. After washings with PBS (four times for 15 minutes each), the corneas were incubated with the secondary antibody Alexa Fluor 488 goat anti-rabbit IgG [(H + L), [1:1000, Thermo Fisher Scientific, Waltham, MA]] for 24 hours at RT and washed thoroughly with PBS. To exclude non-specific labeling, the primary antibody was replaced by rabbit normal serum. Images were recorded with a fluorescent microscope (Olympus IX71; Olympus Corp., Tokyo, Japan). Entire whole-mount views of corneal nerves were built at different layers, including superficial terminals, subbasal bundles, and stromal nerve trunks. For better contrast, the color images were switched to black and white in some figures with a black background and then inverted to a white background as previously described.Citation20–23
For double immunofluorescence, the whole cornea was primarily incubated with PGP9.5 (1:1000) plus mouse monoclonal anti-CGRP (1:500, Abcam, ab81887) or rat monoclonal (NC1/34HL) anti-SP (1:100, Santa Cruz, Dallas, Texas, USA) for 48 hours at room temperature, and followed by the corresponding secondary antibodies: Alexa fluor 488 goat anti-rabbit IgG (H + L), Alexa fluor 594 goat anti-mouse IgG (H + L) and Alexa fluor 594 goat anti-rat IgG (H + L). The dilution of all secondary antibodies was 1:1000. After thorough washings with PBS, the images were recorded as described above. For transected images of corneal nerves, 15 μm cryostat cross-sections were prepared from the samples after finishing the whole mount examination. Images were recorded as described for whole mounts.
Data analysis
To investigate the distribution of epithelial nerves, we divided the cornea into central and peripheral zones. Adult chicken corneas have a radius of approximately 5 mm (flat mount, average of 10 corneas). The central zone was defined by a radius of 2 mm starting at the apex, and the peripheral zone with a radius of 2 mm beginning at the limbus, leaving 1 mm of space between the two zones uncounted to avoid overlap. To compare the densities of the epithelial nerves, four images for each zone were randomly chosen from each cornea (1 image/quadrant). Twenty-four images (recorded with a 10× objective lens) for each zone from six corneas (3 M/3F) were averaged. Nerve terminals in the superficial epithelia within the central and peripheral zones were calculated by directly counting the number of terminals in each image. Twenty images per zone from six corneas (males = females) were analyzed. The terminal numbers in each image were counted using the Image J software (NIH). Since each image comprised an area of 0.15 mm2, the terminal numbers per square millimeter were calculated.
In order to check the relative content of CGRP and SP neuropeptides in epithelial nerves, the corneas of 8 chickens that had been stained with PGP9.5 were relabeled with CGRP or SP. For each neuropeptide, 8 corneas from 4 chickens (M = F) were used; 16 whole-mount images from the central zone (2 images/cornea) were recorded, and then the same numbers of images were taken for PGP9.5. In the same visual field, the percentage of PGP9.5 equaled that of the total nerve area, and the ratio of the peptide-positive nerve area against PGP9.5 represented the relative content.
Comparison of central and peripheral corneal nerve densities, terminal numbers, and the relative content of neuropeptides in the central cornea or between young and old hens were expressed as mean ± SD, and a t-test was performed. P < 0.05 was considered as a statistical difference between the groups.
Results
Stromal innervation
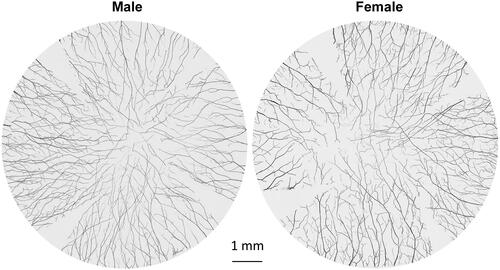
An average of 76.3 ± 5.7 (n = 8 corneas, 4 M/4F) stromal nerve trunks enter the cornea radially and are evenly distributed around the limbus, with no significant difference between male and female chickens (). These stromal nerve trunks are divided into branches that extend toward the center of the cornea. Unlike in humans or rodents in which the stroma branches interconnect to form a visible stromal neural network,Citation17,Citation20–22 no such connections or neural fusions between nerve branches were detected in chicken corneas during the course.
Figure 1. Representative images of male and female whole mount chicken corneal stromal nerves from 6-month-old chickens. The whole cornea was labeled with anti-PGP9.5 antibody, and images were recorded using a fluorescent microscope with a 10× objective lens focusing on the middle stroma. Each reconstructed cornea consisted of about 150 images.
Epithelial innervation
Epithelial nerves derive from the tips of stromal nerve branches. These tips penetrate the Bowman's membrane into the subbasal layer, where they produce bundles of subbasal nerves that extend in all directions. The subbasal bundles split into smaller nerve fibers, which connect to each other to form a neural network in the subbasal layer. Fine nerve endings germinate from the network to terminate in the epithelial layer. shows two quadrants of the corneal epithelial nerve structures recorded from a male and a female chicken, both 6 months old. Highlighted images (i-iv) acquired with a 10 x objective lens show the detailed nerve structures of superficial terminals and subbasal bundles in . Two complete maps of the corneal epithelial innervation, including the subbasal bundles and superficial terminals, obtained from two eyes of 6-month-old male and female chickens are provided in Supplementary Figures 1 and 2. In addition, two supplementary videos recorded from the centers of two eyes of 6-month-old male and female chickens displaying the detailed nerve structures at different corneal layers are supplied as Supplementary Video 1 (male) and Supplementary Video 2.
Figure 2. Epithelial nerve architecture of chicken cornea. (A) Two quadrants of corneal subbasal nerves were documented from a male and a female chicken of the same age (see Supplementary Figures 1 and 2 for entire images). (B) Highlighted images (10× lens) showing the detailed nerve structures at the corneal central (i, iii) and peripheral (ii, iv) zones. The images of the superficial terminals and the subbasal bundles were documented at the same points. (C) Cross-sectional view of chicken corneal nerve structure. Highlighted images show the detailed epithelial nerve distribution at the center and periphery. The image counterstained with DAPI shows the location of subbasal bundles (yellow arrows) and the shape of epithelial terminals (red arrows) in the central cornea.
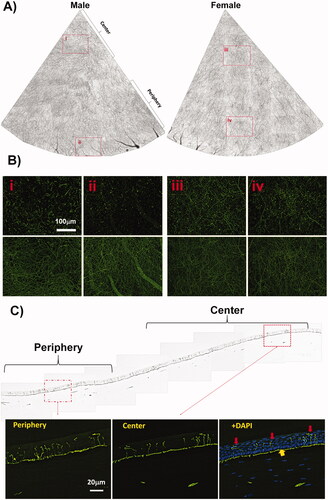
The subbasal nerve density, calculated from the whole-mount images (a total of 48 images, 24 images per zone) as the percentage of total area, was 20.3% ± 3.2% in the central area and 19.7% ± 2,2% in the peripheral area, with no significant difference of nerve density, (p = 0.15, n = 6 corneas, 3 M/3F). Epithelial nerve terminals calculated as the average number of nerve terminals/mm2 also showed no significant difference between the central area (1682 ± 369) and the peripheral area (1555 ± 323, p = 0.13).
The reconstructed corneal sagittal sections labeled with PGP9.5 antibody showed a view of the nerve structure in a quarter cornea (). The images highlighted from the center and periphery depict the detailed distribution of nerve endings in the epithelium, indicating that there was no significant difference in the density of nerve endings between the central and peripheral epithelium.
The influence of gender on epithelial nerve density
To investigate the effect of gender on epithelial innervation, we compared the density of the central epithelial nerves between 6-month-old roosters and hens. There was no significant difference in the density of central epithelial nerves between genders, including nerve endings and subbasal bundles. The subbasal nerve density in the roosters (n = 16 images, 4 corneas) was 20.9%±3.4%, and in the hens (n = 16 images, 4 corneas), it was 19.6%±2.9%, p = 0.23. The number of epithelial nerve endings of the rooster was 1635.8 ± 343.7 per square millimeter, while the hen was 1728.2 ± 398.8 per square millimeter (p = 0.49).
CGRP and SP content of young chicken corneal nerves
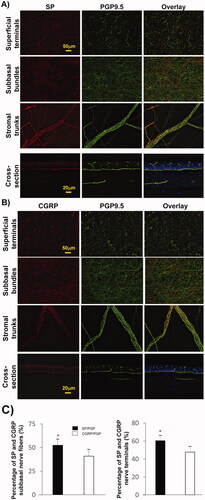
To investigate the distribution of CGRP and SP sensory nerves in the chicken cornea, PGP9.5-labeled corneas were double-labeled with anti-CGRP antibody (4 corneas) or anti-SP antibody (4 corneas). Representative images of corneal subbasal nerve bundles and terminals in the central area and stromal nerve trunks in the limbal area are shown in . SP-positive nerve fibers in the central area constituted 52.5% ± 6.4% of the total subbasal nerve content, while CGRP-positive nerves were 40.9%±6.8%, showing significant difference in the content of these two neuropeptides (n = 8 eyes; p < 0.001, ). Similarly, the percentage of SP-positive nerve terminals was higher (60.4% ± 5.9%) than the percentage of CGRP-positive terminals (47.8% ± 5.8%), p < 0.005.
Figure 3. Relative content of SP and CGRP sensory neuropeptides in chicken corneas. (A, B) Representative images showing the expression of SP- and CGRP-positive nerves in the central subbasal nerve bundles, terminals, and limbal stromal trunks. Cross-sections double-labeled with PGP + SP or CGRP and counterstained with DAPI showing the detailed nerve structure in a transverse view. (C) Percentage of positive SP or CGRP subbasal nerves with respect to total nerve area (PGP9.5 nerves) in each image recorded with a 20× objective lens. Sixty images for each neuropeptide and the same number of images for PGP9.5 were recorded from four corneas. Data expressed as average ± SD. *P< 0.001.
Influence of aging on epithelial nerve density
To test the effect of aging on corneal nerves, we compared the central epithelial nerve density of 6-month-old and 4-year-old hens. As shown in , there is a significant decrease in the density of central epithelial nerves in old chickens. An entire corneal epithelial innervation obtained from a 4-year-old hen and a video recorded from the central cornea are provided in Supplementary Figure 3 and Supplementary Video 3.
Figure 4. Effect of aging on chicken corneal epithelial nerve density. (A) Subbasal nerve density of chicken central corneas was calculated as the percentage of the total area in each image. The number of terminals was counted per mm2. Data expressed as average ± SD. *P< 0.05. (B) Representative images recorded with a 10× objective lens show the central epithelial nerve distribution in the cornea of young (6 months) and old (4 years) hens (see Supplementary Figure 3 for entire images).
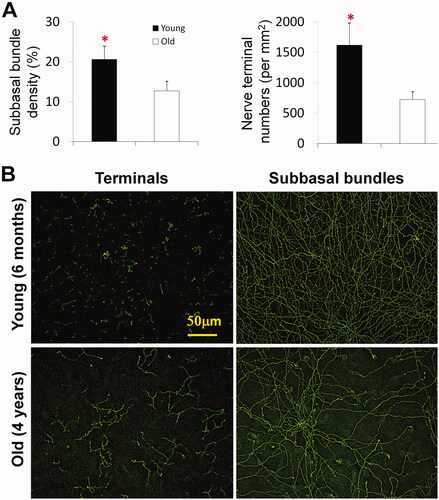
The subbasal nerve density of young hens was 20.2 ± 3.3% (n = 16 images, four corneas), and the old hens were 12.1 ± 2.1% (n = 16 images, four corneas), with a significant difference of p < 0.001. Similarly, nerve terminals, calculated from images recorded in the central area as the average number of terminals/mm2 were also greater in the young hens (1618 ± 360) than in the old hens (725 ± 112) p < 0.001, where the terminals were longer (. It is worth noting that newly regenerated fibers are often found in the surrounding area corneas of old chickens, and these fibers extend in all directions to fill the space left by the degenerated nerve bundle. A video recorded with a 10× lens showing newly-regenerating corneal nerves is provided in Supplementary Video 4.
Discussion
Avian corneal innervation in embryonic development has been well studied by immunochemical methods.Citation7,Citation14–16 In chicks, corneal innervation begins when the growth cone of the putative corneal nerve reaches the centrotemporal side of the developing eye between E4 and E5, at which point, the nerves extend both dorsally and ventrally, forming a pericorneal nerve ring around its entire circumference. Originating from the neural ring, the axons begin to branch to radially innervate the stroma at the periphery of the cornea, and the stromal nerves bifurcate repeatedly as they project toward the center of the cornea and the epithelium.Citation7 In this study, we used our improved technique of immunofluorescence and neuroimaging to show, for the first time, the entire corneal nerve structure of adult chickens, including the stromal nerve trunk, subbasal nerve bundles, and epithelial nerve endings. We also studied the distribution of the major sensory neuropeptides SP and CGRP and the influence of aging on corneal innervation.
As previously described in humans, rodents, including mice and rats, and cats,Citation17,Citation20–22 the corneal nerves of birds can be divided into stromal nerve trunks, subbasal nerve bundles, and superficial nerve endings according to their anatomical locations. These nerves are evenly distributed in the four quadrants of the cornea. There is no difference in nerve density between genders, but aging significantly reduces epithelial nerve density.
In addition to some of the above common features, the corneal nerve anatomy of the chicken has characteristics different from humans and mammals. There are two times more neural trunks entering the corneal stroma in chickens than in humansCitation17 and between three and six times higher than in mice, cats, and rats.Citation20–22 In addition, stromal neural trunks in the chicken cornea do not connect in the central area to form a stromal nerve network.
The epithelial nerve structure of the chicken cornea is also special. Unlike what we have seen in humans or mammals, the subbasal nerve bundles of birds do not grow centripetally but extend in all directions. These nerve bundles cross and connect to each other to form an epithelial neural network in the subbasal layer. As a result, they do not form a whirl-like structure or vortex in the center of the cornea, as we have seen in humans, mice, rats, and cats, and there is no difference in the density of epithelial nerves between the central and the peripheral cornea as seen in humans and other mammals.Citation17,Citation20–23
CGRP and SP are the two main sensory neuropeptides found in corneal nervesCitation1 that play an important role in wound healing as well as in inflammation and neuropathic pain.Citation2,Citation24–28 Their expression has been studied previously by both histochemical and immunofluorescence methods in a wide range of animal species, including chickens.Citation29 Using dual-labeled immunofluorescence, we found that the proportion of SP in the cornea of young chickens was significantly higher than that of CGRP-positive fibers, which is contrary to our previous findings in mammals, including mice, rats, cats, and rabbits.Citation20–22,Citation27 In an early study of dogs, both CGRP- and SP-positive nerve fibers constitute 99% of the total corneal innervation.Citation28 In chickens, SP-positive nerves accounted for about 52%, while CGRP-positive nerves accounted for 41% of the total innervation of corneal subbasal epithelium, which indicates that the difference in the content of these neuropeptides in the nerve fibers of different animals is related to the species. Nerve fibers positive to other neuropeptides are described in mammalsCitation1 but are unknown in the chicken cornea. Further studies with validated antibodies will be important to define the neurochemistry of chicken cornea.
Our previous studies have reported that in humans and rats, aging can greatly reduce the density of corneal epithelial nerves.Citation17,Citation21 In the current study, the density of corneal epithelial nerves, including the subbasal nerve fibers and the number of terminals, in aged hens is significantly reduced, and nerve endings appear longer and more branched, possibly to compensate for the loss of terminals and sensitivity. In humans, the decrease in corneal nerve density and changes in neuro-morphology by aging are considered key causes of the increase in the incidence of dry eye in the elderly. Thus, new regenerative nerves appear in the aging corneas to compensate (Supplementary Video 1).
In summary, we report that the anatomical structure of the chicken corneal nerves has some characteristics different from that of humans and mammals but shares some common features with humans: the presence of Bowman’s membrane, number of corneal epithelial layers,Citation9 and decrease of corneal epithelial nerve density in aging. These characteristics make the chicken cornea not only a good model for the study of embryonic innervation and development but also to be used to study the neural changes during adulthood and aging.
Supplemental Material
Download Zip (101.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi:10.1016/S0014-4835(03)00050-2.
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–525. doi:10.1016/j.exer.2003.09.023.
- Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–134. doi:10.1136/bjophthalmol-2014-306280.
- Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–285. doi:10.1016/j.survophthal.2013.09.002.
- Sundarraj N, Fite D, Belak R, Sundarraj S, Rada J, Okamoto S, Hassell J. Proteoglycan distribution during healing of corneal stromal wounds in chick. Exp Eye Res. 1998;67(4):433–442. doi:10.1006/exer.1998.0540.
- Ritchey ER, Code K, Zelinka CP, Scott MA, Fischer AJ. The chicken cornea as a model of wound healing and neuronal re-innervation. Mol Vis. 2011;17:2440–2454.
- Bee JA. The development and pattern of innervation of the avian cornea. Dev Biol. 1982;92(1):5–15. doi:10.1016/0012-1606(82)90145-2.
- Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98(6):507–517. doi:10.1111/cxo.12312.
- Fowler WC, Chang DH, Roberts BC, Zarovnaya EL, Proia AD. A new paradigm for corneal wound healing research: the white leghorn chicken (Gallus gallus domesticus). Curr Eye Res. 2004;28(4):241–250. doi:10.1076/ceyr.28.4.241.27837.
- Gómez S, Herreras JM, Merayo J, García M, Argüeso P, Cuevas J. Effect of hyaluronic acid on corneal haze in a photorefractive keratectomy experimental model. J Refract Surg. 2001;17(5):549–554. doi:10.3928/1081-597X-20010901-08.
- Merayo-Lloves J, Yáñez B, Mayo A, Martín R, Pastor JC. Experimental model of corneal haze in chickens. J Refract Surg. 2001;17(6):696–699. doi:10.3928/1081-597X-20011101-11.
- Martínez-García MC, Merayo-Llovés J, Blanco-Mezquita T, Mar-Sardaña S. Wound healing following refractive surgery in hens. Exp Eye Res. 2006;83(4):728–735. doi:10.1016/j.exer.2006.02.017.
- Javier JAD, Lee JB, Oliveira HB, Chang JH, Azar DT. Basement membrane and collagen deposition after laser subepithelial keratomileusis and photorefractive keratectomy in the leghorn chick eye. Arch Ophthalmol. 2006;124(5):703–709. doi:10.1001/archopht.124.5.703.
- Riley NC, Lwigale PY, Conrad GW. Specificity of corneal nerve positions during embryogenesis. Mol Vis. 2001;7:297–304.
- Kubilus JK, Linsenmayer TF. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(2):782–789. doi:10.1167/iovs.09-3942.
- Lwigale PY, Bronner-Fraser M. Semaphorin3A/neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development. Dev Biol. 2009;336(2):257–265. doi:10.1016/j.ydbio.2009.10.008.
- He J, Bazan NG, Bazan HEP. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91(4):513–523. doi:10.1016/j.exer.2010.07.007.
- He J, Bazan HEP. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 2012;119(5):956–964. doi:10.1016/j.ophtha.2011.10.036.
- He J, Bazan HEP. Corneal nerve architecture in a donor with unilateral epithelial basement membrane dystrophy. Ophthalmic Res. 2013;49(4):185–191. doi:10.1159/000345766.
- He J, Bazan HEP. Neuroanatomy and neurochemistry of mouse cornea. Invest Ophthalmol Vis Sci. 2016;57(2):664–674. doi:10.1167/iovs.15-18019.
- He J, Pham TL, Bazan HEP. Neuroanatomy and neurochemistry of rat cornea: changes with age. Ocul Surf. 2021;20:86–94. doi:10.1016/j.jtos.2020.11.005.
- He J, Pham TL, Bazan HEP. Mapping the entire nerve architecture of the cat cornea. Vet Ophthalmol. 2019;22(3):345–352. doi:10.1111/vop.12600.
- Pham TL, Kakazu A, He J, Bazan HEP. Mouse strains and sexual divergence in corneal innervation and nerve regeneration. Faseb J. 2019;33(3):4598–4609. doi:10.1096/fj.201801957R.
- Suvas S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol. 2017;199(5):1543–1552. doi:10.4049/jimmunol.1601751.
- He J, Pham TL, Kakazu AH, Bazan HEP. Remodeling of substance P sensory nerves and transient receptor potential melastatin 8 (TRPM8) cold receptors after corneal experimental surgery. Invest Ophthalmol Vis Sci. 2019;60(7):2449–2460. doi:10.1167/iovs.18-26384.
- Hegarty DM, Hermes SM, Morgan MM, Aicher SA. Acute hyperalgesia and delayed dry eye after corneal abrasion injury. Pain Rep. 2018;3(4):e664. doi:10.1097/PR9.0000000000000664.
- Cortina MS, He J, Li N, Bazan NG, Bazan HEP. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012;130(1):76–83. doi:10.1001/archophthalmol.2011.287.
- Marfurt CF, Murphy CJ, Florczak JL. Morphology and neurochemistry of canine corneal innervation. Invest Ophthalmol Vis Sci. 2001;42(10):2242–2251.
- Corvetti G, Pignocchino P, Sisto Daneo L. Distribution and development of substance P immunoreactive axons in the chick cornea and uvea. Basic Appl Histochem. 1988;32(1):187–192.
