Abstract
Purpose
To compare the angle of retinal arteries and macular vessel density and foveal avascular zone (FAZ) in early stage familial exudative vitreoretinopathy (FEVR) patients with inner retinal layer (IRL) persistence with FEVR patients without IRL persistence and normal people.
Methods
This study enrolled 113 early stage FEVR patients and 55 age-matched normal subjects. FEVR patients were divided into IRL group and non-IRL group based on the presence or absence of IRL in fovea. The angle of superior temporal and inferior temporal branch retinal arteries on ultra-wide-field fundus images were measured. Superficial and deep vessel density of whole image, fovea and parafovea, the area and perimeter of FAZ, A-circularity index (AI, perimeter/standard circle perimeter with equal area) and vessel density around the 300-μm width of the FAZ (FD), central macular thickness (CMT) on 3 mm × 3mm OCTA were measured.
Results
30 FEVR patients in IRL group, 83 FEVR patients in non-IRL group, 55 normal people in control group were evaluated. BCVA were worst in IRL group (p < .001). The angle of retinal arteries was smaller in FEVR groups (p < .001) and were smallest in IRL group (p < .001). Superficial and deep vessel density of whole and parafovea area in FEVR patients were significantly lower than that in normal people (p < .05), AI were biggest (p = .01) and FD were smallest in IRL group (p < .001). CMT in IRL group were thicker than non-IRL group and control group (p < .05).
Conclusion
Worse BCVA, smaller angle of retinal arteries (more vessels traction), lower macular vessel density, smaller and more irregular FAZ and thicker CMT were observed in FEVR patients with IRL persistence even in early stage.
Familial exudative vitreoretinopathy (FEVR) is a hereditary disorder which characterized by peripheral retinal avascular zone and abnormal vessels, macular dragging vitreoretinal traction, tractional or exudative retinal detachments.Citation1 FEVR is accompanied with vascular abnormalities.
Using spectral-domain optical coherence tomography (SD-OCT), Zhang T reported 29/60 FEVR eyes with macular abnormalities including epimacular membrane, macular ectopia, vitreomacular traction and perifoveal posterior vitreous detachment.Citation2 Tanenbaum et al. reported a case of macular retinal pigment epithelial clumping in FEVR.Citation3 Early stage FEVR generally appears as avascular retinal periphery without other characteristic features and patients usually have no obvious clinical symptoms.Citation4 Yonekawa et al.Citation5 found macular abnormalities such as vitreous traction, diminished foveal contour, persistent fetal foveal architecture, cystoid macular edema et al. persist in all stage 2 or great and a part of stage 1 FEVR patients in their study. Chen et al.Citation6 found 48.78% FEVR eyes had a persistence of IRL, the thickness of IRL and the area of foveal avascular zone (FAZ) were negatively correlated.
Peripheral retinal avascularity was the most important feature in FEVR which clinicians focus more on the peripheral retina. Actually, the traction of peripheral retina may often impact macula. Optical coherence tomography angiography (OCTA) can demonstrate microvascular architecture intuitively. Chen et al.Citation7 first conducted OCTA in FEVR children and observed fine vascular abnormalities. Koulisis et al. found FEVR patients had lower vessel density and fewer vessel branches and larger caliber vessels on OCTA.Citation8 They considered macular microvascular parameters on OCTA may serve as biomarkers of changes in the retinal periphery. Hsu et al.Citation9 found density alternations, dilation, straightening, heterogeneous of macular vessels in stage 2 or great FEVR patients by OCTA. Chen et al.Citation6 found smaller FAZ, decreased vascular density in superficial and deep layers of parafoveal area in early stage FEVR patients by OCTA but they didn’t explored the correlation between IRL persistence and vessel density.
Nagura et al. found vascular traction was a common manifestation which lead to smaller angle of retinal arteries and they measured the retinal artery angle to assess the traction of idiopathic epiretinal membrane.Citation10 This is a quantitative index to know the degree of traction which may be apply to FEVR.
To date, there are a few previous researches explore macular vessels in FEVR by OCTA especially about IRL persistence. Our study determined to compare the angle of retinal arteries and more parameters in vessel density and FAZ in FEVR patients (with and without IRL persistence) and normal people by OCTA to know the features of macular alternations in early stage FEVR with IRL.
Materials and methods
This study enrolled 113 persons diagnosed as stage 1 FEVR by fluorescein angiography (FA) and 55 age-matched normal people as control group at the affiliated eye hospital of Wenzhou Medical University and Zhejiang Provincial People’s Hospital. All subjects underwent examinations of best corrected visual acuity (BCVA), slit lamp microscope, ultra-wide-field fundus imaging (Optos 200Tx) and OCTA (RTVue-XR Avanti, Optovue Inc, Fremont, CA, USA). FEVR patients were divided into two groups based on the presence or absence of the inner retinal layer (IRL) in fovea according to OCT images and defined as IRL group and non-IRL group. FEVR patients with other serious macular abnormalities such as cystoid macular edema were excluded. FEVR staging was performed according to 2014 FEVR classification standard.Citation11
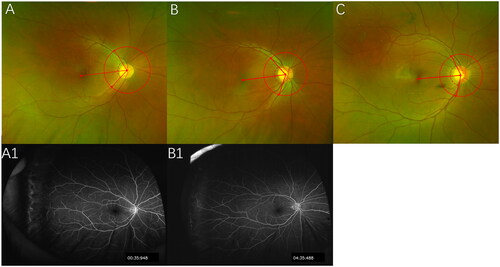
We selected the posterior pole ultra-wide-field fundus images of FEVR patients. The measurement of retinal arteries angle referred to Nagura et al.’s research.Citation10 Drew a circle with optic disk at center, the diameter was the distance between optic disk and macula. Drew two lines between optic disk and the intersection points of the drawn circle and super and inferior temporal branch retinal arteries, respectively. The angle of these two lines was measured (shown in ).
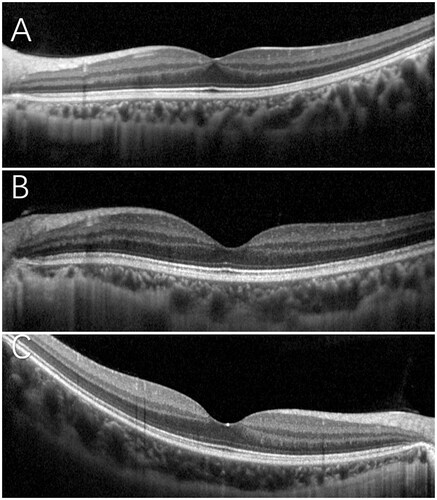
Figure 1. The angle measurement on ultra-wide-field images in IRL group 107.3°(A), non-IRL group 117.2°(B), and control group 139.6°(C). Ultra-wide-field FA images in IRL group (A1) and in non-group (B1).
Age, gender, logMAR BCVA and FEVR stage were collected. Superficial and deep vessel density of whole image, fovea and parafovea, the area of FAZ, perimeter of FAZ, A-circularity index (AI, perimeter/standard circle perimeter with equal area) and vessel density around the 300-μm width of the FAZ (FD), central macular thickness (CMT) on 3 mm × 3mm OCTA were measured. All the scan quality of OCTA was no less than 6.
SPSS 19.0 (Inc, Chicago, IL, USA) was used in statistical analyses. Kolmogorov-Smirnov test was used for normality testing. The data according with normal distribution were expressed as mean ± standard deviation, independent-samples T test was used to compare the parameters of two groups and one-way ANOVA was used to compare the parameters of three groups. LSD-t test was used in pairwise comparison. The data not according with normal distribution were expressed as median (interquartile range) and Kruskal–Wallis test was used to compare. A p value of less than .05 was considered significant difference. The correlation of angle and the parameters of OCTA was performed by pearson correlation analysis.
Results
Basic data
30 FEVR patients in IRL group, 83 FEVR patients in non-IRL group, 55 normal people in control group were evaluated. The mean age was 28.50 ± 11.90, 28.39 ± 9.79 and 31.40 ± 7.69 (F = 1.794, p > .05) years old in IRL group, in non-IRL group, in control group. There was no significant difference in age and gender among three groups ().
Table 1. Basic data.
BCVA in IRL group were worst
The mean BCVA were 0 (0.01), 0 (0) and 0 (0) (p < .05) in IRL group, in non-IRL group, in control group. There were significant differences between IRL group and the other two groups (p < .05).
Stage
There were 20 FEVR patients in IRL group and 64 FEVR patients in non-IRL group as stage 1 A (avascular periphery or anomalous intraretinal vascularization without exudate or leakage). There were 10 FEVR patients in IRL group and 19 FEVR patients in non-IRL group graded as stage 1B (avascular periphery or anomalous intraretinal vascularization with exudate or leakage).
The angle were smallest in IRL group (shown in )
The mean angle in FEVR group and normal group were 102.64 ± 16.60° and 121.13 ± 11.34°, respectively (t = 7.452, p < .05). The mean angle in three groups were 97.09 ± 13.52°, 104.65 ± 17.22°, and 121.13 ± 11.34° (F = 31.370, p < .05), respectively. There were significant differences among three groups (p < .05).
OCTA parameters
The OCTA parameters comparison of three groups was showed in . The OCT and OCTA images of three groups were showed in and , respectively.
Figure 3. OCTA superficial vessel density (1), deep vessel density (2), and FAZ (3) images of IRL group (A), non-IRL group (B), and control group (C).
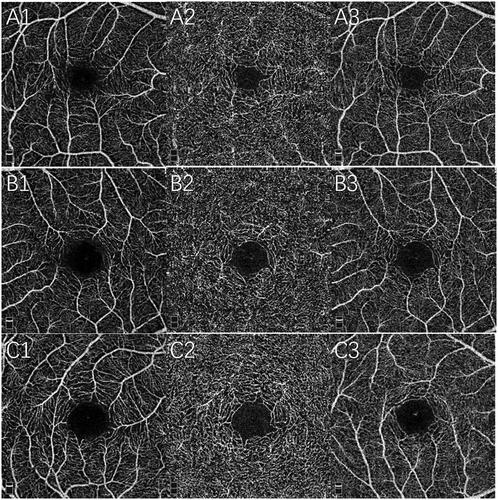
Table 2. OCTA parameters comparison of three groups.
Superficial vessel density was lower in FEVR groups
The mean whole superficial vessel density in three groups were 47.51 ± 1.94%, 47.60 ± 2.58%, and 49.16 ± 1.58% (F = 9.783, p < .05), respectively. The mean fovea superficial vessel density in three groups were 21.15 ± 7.55%, 18.28 ± 6.05%, and 20.45 ± 5.04% (F = 3.496, p < .05), respectively. The mean parafovea superficial vessel density in three groups were 49.89 ± 2.11%, 50.56 ± 2.68%, and 52.01 ± 1.79% (F = 10.013, p < .05), respectively. The mean whole superficial vessel density and the mean parafovea superficial vessel density of IRL group and non-IRL group were smaller than control group (p < .05).
Deep vessel density was lower in FEVR groups
The mean whole deep vessel density in three groups were 50.58 ± 3.34%, 51.43 ± 2.97%, and 52.87 ± 2.59% (F = 6.915, p < .05), respectively. The mean fovea deep vessel density in three groups were 34.22 ± 9.61%, 31.58 ± 7.87%, and 34.48 ± 6.53% (F = 2.722, p > .05), respectively. The mean parafovea deep vessel density in three groups were 52.60 ± 3.51%, 53.76 ± 3.06%, and 54.95 ± 2.67% (F = 6.145, p < .05), respectively. The mean whole deep vessel density and the mean parafoveal deep vessel density of IRL group and non-IRL group were smaller than control group (p < .05).
AI were biggest and FD were smallest in IRL group
The mean FAZ area in three groups were 0.275 ± 0.127 mm2, 0.327 ± 0.118mm2, and 0.290 ± 0.085 mm2 (F = 3.379, p < .05), respectively. There were significant differences between IRL group and non-IRL group (p < .05). The mean perimeter of FAZ in three groups were 2.07 ± 0.54 mm, 2.24 ± 0.41 mm, and 2.13 ± 0.33 mm (F = 2.380, p > .05), respectively. The mean AI in three groups were 1.15 ± 0.05, 1.12 ± 0.03, and 1.13 ± 0.03 (F = 4.731, p < .05), respectively. There were significant differences between non-IRL group and control group (p < .05). The mean FD in three groups were 49.28 ± 2.60%, 51.64 ± 2.70%, and 52.75 ± 3.08% (F = 14.802, p < .05), respectively. There were significant differences among three groups (p < .05).
CMT were thicker in IRL group
The mean CMT in three groups were 257.17 ± 16.92 μm, 248.76 ± 20.40 μm, and 243.58 ± 13.39 μm (F = 5.681, p < .05), respectively. The mean CMT of IRL group was significantly thicker than non-IRL group and control group (p < .05).
Correlation of angle and the parameters of OCTA
The angle and the correlation of angle and the parameters of OCTA was showed in . There was positive correlation between the angle and superficial vessel density, parafovea superficial vessel density, deep vessel density, fovea deep vessel density, parafovea deep vessel density, FD. There was negative correlation between the angle and AI (p < .05).
Table 3. The correlation of angle and the parameters of OCTA.
Discussion
Our study found IRL were observed even in early stage FEVR (26.54%). FEVR with IRL has worse BCVA, smaller angle of retinal arteries, lower superficial and deep vessel density of whole and parafovea area, thicker CMT.
We also found superficial and deep vessel density of whole and parafovea area in FEVR patients were significantly lower than that in normal people. The OCTA results in our study were similar to Chen et al.’sCitation6 research. Hasegawa et al.Citation12 found there was no significant difference of superficial and deep vessel density of fovea area between FEVR and normal people which were similar to our study, but they also found parafoveal deep vessel density in FEVR was larger which probably because of the different OCT model. Zhang et al. found parafoveal vascular density decreased with increasing FEVR stages.Citation13 FEVR is an inherited vitreoretinopathy. Mutant genes were mainly responsible for the abnormity in Wnt and Norrin signaling pathway. The Wnt pathway upregulated the endothelial cell growth and Norrin was involved in capillary development in the eyes. LRP5 was involved in maturation and lumen formation of capillaries connecting inner and outer plexiform layers in knockout mice.Citation14 Decreased macular vessel density in FEVR patients reflected the pathological changes.
Although macula abnormity such as diminished foveal contour, persistent IRL, cystoid macular edema et al. was commonly observed even in early stage FEVR.Citation15 Our study excluded these serious macula abnormities which may affect the accuracy of measurement on OCTA, focused on the persistent IRL. Previous studies reported the ratio of persistent IRL in FEVR patients. Yonekawa et al.Citation5 observed 20% IRL persistent in early stage FEVR patients while Hasegawa et al. reported 27.8%.Citation12 Chen et al. reported a high rate of 48.78% in early and advanced stage FEVR.Citation6 In the process of normal retina development, foveal region is composed of five layers with a thick ganglion cell layer and a thin outer nuclear layer at mid-gestation, inner plexiform layer and inner nuclear layer displaced outward subsequently which lead to the deeper and wider pit and the thinner IRL in foveal region, IRL disappeared at about 12 months after birth.Citation16 Müller cell provide essential tensile strength of the inner retinal tissue displacement. Persistent IRL was both observed in FEVR and ROP.Citation17 ROP were both associated with Wnt and Norrin signaling changes.Citation18,Citation19 Some mutation genes associated with FEVR such as FZD4, TSPAN12 and LRP5 were also observed in ROP.Citation20,Citation21 Persistent IRL was observed in ROP especially in threshold ROP with worse VA.Citation22 But retarded IRL displacement was also observed in adult preterms without ROP.Citation23 Whether the IRL persistence in FEVR was associated with genes mutation need further research.
We also observed whole and parafovea superficial and deep vessel density in IRL group were lower than non-IRL group though there was no significant difference, it may relate to the small sample in IRL group. However, fovea superficial vessel density in IRL group were significantly higher than non-IRL group and control group. The measured layer of superficial density was from inner limiting membrane to the outside of inner plexiform layer. The persistence of IRL may affect the fovea superficial vessel density. But there was no significant difference in fovea deep vessel density among three groups. FD was lower in FEVR groups, especially in IRL group. Chatzistergiou et al. found superficial fovea vessel density was higher in subjects with fovea plana explained by lack of FAZ whereas deep vessel density of the whole image was lower in that though without significant difference.Citation24
In our study, FAZ in IRL group were significantly smaller than non-IRL group. Hasegawa et al. found surface FAZ area and deep FAZ area were smaller in the FEVR and considered it may relate to IRL or inconspicuous foveal bulge on the OCT images.Citation12 We also found AI in IRL group were significantly bigger than that in non-IRL group and control group which meant macular in IRL group were more abnormal (small and irregular). It explained that BCVA in IRL group were significantly worse than non-IRL group and control group although all the FEVR patients were stage 1. In addition, Puell et al.Citation25 found increased macular IRL thickness was associated with worse low-contrast VA in the young persons. VA was associated negatively with IRL thickness in ROP.Citation26 IRL persistence may lead to decreased density in macula and increased density in FAZ, these two factors may both be unfavorable for VA. Zhang et al. thought lower superficial and deep capillary plexus density may be associated with the visual loss in FEVR.Citation13 In our study, FD were lowest in IRL group indeed.
Besides, the angle of retinal arteries was smaller in FEVR groups. IRL group had smallest angle in three groups we speculate that the traction in IRL group was more serious. Our study also observed the positive correlation between angle and vessel density, that meant vessel traction may cause the decreasing in vessel density. Macular ganglion cell-inner plexiform layer decreased significantly with narrowing of the peripapillary retinal artery angle in healthy eyes, macular ganglion cell-inner plexiform layer thickness was weakly but positively associated with standard automated perimetry sensitivity.Citation27,Citation28
Conclusion
Even in early stage FEVR, lower retina vessel density and vessels traction were observed in our study, especially in IRL group with worse BCVA and more obvious abnormality macula. Expanding the sample in IRL group in the future may explore the change better.
Ethics statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the examinations were performed on individuals after obtaining their informed consents. This study was approved by the Institutional Review Board of the Affiliated Eye Hospital of Wenzhou Medical University (ID:2020-062-G-08-01).
Author contributions
Yirun Shao and Jianbo Mao contributed to the conception and design of the study, data collection, analysis and interpretation of data, writing the article, final approval of the article. Yuyan Fang, Yijing Chen, Zhengxi Zhang, and Ziyi Xiang contributed to data collection and final approval of the article. Lijun Shen contributed to the conception and design of the study and final approval of the article.
Disclosure statement
No potential conflict of interest was repoted by the author(s).
Data availability statement
The data used to support the findings of this study were supplied by Lijun Shen under license and so cannot be made freely available. Requests for access to these data should be made to Lijun Shen ([email protected]).
Additional information
Funding
References
- Gilmour D. Familial exudative vitreoretinopathy and related retinopathies. Eye. 2015;29(1):1–14. doi:10.1038/eye.2014.70.
- Zhang T, Wang Z, Sun L, Li S, Huang L, Liu C, Chen C, Luo X, Yu B, Ding X. Ultra-wide-field scanning laser ophthalmoscopy and optical coherence tomography in FEVR: findings and its diagnostic ability. Br J Ophthalmol. 2021;105(7):995–1001. doi:10.1136/bjophthalmol-2020-316226.
- Tanenbaum R, Acon D, Rodriguez A, Negron C, Berrocal A. Macular retinal pigment epithelial clumping leading to a diagnosis of FEVR. Ophthalmic Surg Lasers Imaging Retina. 2021;52(9):505–508. doi:10.3928/23258160-20210819-01.
- Lyu J, Zhang Q, Wang S, Chen Y, Xu Y, Zhao P. Ultra-wide-field scanning laser ophthalmoscopy assists in the clinical detection and evaluation of asymptomatic early-stage familial exudative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(1):39–47. doi:10.1007/s00417-016-3415-x.
- Yonekawa Y, Thomas BJ, Drenser KA, Trese MT, Capone A Jr. Familial exudative vitreoretinopathy: spectral-domain optical coherence tomography of the vitreoretinal interface, retina, and choroid. Ophthalmology. 2015;122(11):2270–2277. doi:10.1016/j.ophtha.2015.07.024.
- Chen C, Liu C, Wang Z, Sun L, Zhao X, Li S, Luo X, Zhang A, Chong V, Lu L, et al. Optical coherence tomography angiography in familial exudative vitreoretinopathy: clinical features and phenotype-genotype correlation. Invest Ophthalmol Vis Sci. 2018;59(15):5726–5734. doi:10.1167/iovs.18-25377.
- Chen X, Viehland C, Carrasco-Zevallos OM, Keller B, Vajzovic L, Izatt JA, Toth CA. Microscope-integrated optical coherence tomography angiography in the operating room in young children with retinal vascular disease. JAMA Ophthalmol. 2017;135(5):483–486. doi:10.1001/jamaophthalmol.2017.0422.
- Koulisis N, Moysidis S, Yonekawa Y, Dai Y, Burkemper B, Wood E, Lertjirachai I, Todorich B, Khundkar T, Chu Z, et al. Correlating changes in the macular microvasculature and capillary network to peripheral vascular pathologic features in familial exudative vitreoretinopathy. Ophthalmol Retina. 2019;3(7):597–606. doi:10.1016/j.oret.2019.02.013.
- Hsu ST, Finn AP, Chen X, Ngo HT, House RJ, Toth CA, Vajzovic L. Macular microvascular findings in familial exudative vitreoretinopathy on optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):322–329. doi:10.3928/23258160-20190503-11.
- Nagura K, Inoue T, Zhou H, Obata R, Asaoka R, Arasaki R, Sato A, Nakamura K, Takeuchi M, Tanaka S, et al. Association between retinal artery angle and visual function in eyes with idiopathic epiretinal membrane. Transl Vis Sci Technol. 2021;10(9):35. doi:10.1167/tvst.10.9.35.
- Kashani A, Brown K, Chang E, Drenser K, Capone A, Trese M. Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(11):2220–2227. doi:10.1016/j.ophtha.2014.05.029.
- Hasegawa T, Hirato M, Kobashi C, Yamaguchi A, Takagi R, Tanaka Y, Kaburaki T, Kakehashi A. Evaluation of the foveal avascular zone in familial exudative vitreoretinopathy using optical coherence tomography angiography. Clin Ophthalmol. 2021;15:1913–1920. doi:10.2147/OPTH.S305520.
- Zhang J, Jiang C, Ruan L, Yang Q, Chang Q, Huang X. Macular capillary dropout in familial exudative vitreoretinopathy and its relationship with visual acuity and disease progression. Retina. 2020;40(6):1140–1147. doi:10.1097/IAE.0000000000002490.
- Chun-Hong X, Haiquan L, Debra C, Meng W, Catherine C, Xin D, Bo C, Bruce B. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet. 2008;17(11):1605–1612.
- Tauqeer Z, Yonekawa Y. Familial exudative vitreoretinopathy: pathophysiology, diagnosis, and management. Asia Pac J Ophthalmol. 2018;7(3):176–182.
- Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol. 2012;154(5):767 e2–778 e2. doi:10.1016/j.ajo.2012.05.007.
- Chen P, Kang E, Chen K, Ling X, Chang Y, Wang N, Liu L, Chen Y, Hwang Y, Lai C, et al. Foveal hypoplasia and characteristics of optical components in patients with familial exudative vitreoretinopathy and retinopathy of prematurity. Sci Rep. 2022;12(1):7694. doi:10.1038/s41598-022-11455-7.
- Rathi S, Jalali S, Musada G, Patnaik S, Balakrishnan D, Hussain A, Kaur I. NDPMutation spectrum of, and genes in Indian patients with retinopathy of prematurity. Br J Ophthalmol. 2018;102(2):276–281. doi:10.1136/bjophthalmol-2017-310958.
- Wang Z, Liu C, Huang S, Chen J. Wnt signaling in vascular eye diseases. Prog Retin Eye Res. 2019;70:110–133. doi:10.1016/j.preteyeres.2018.11.008.
- Zhang T, Sun X, Han J, Han M. Genetic variants of TSPAN12 gene in patients with retinopathy of prematurity. J Cell Biochem. 2019;120(9):14544–14551. doi:10.1002/jcb.28715.
- Kondo H, Kusaka S, Yoshinaga A, Uchio E, Tawara A, Tahira T. Genetic variants of FZD4 and LRP5 genes in patients with advanced retinopathy of prematurity. Mol Vision. 2013;19:476–485.
- Wu W, Lin R, Shih C, Wang N, Chen Y, Chao A, Chen K, Chen T, Hwang Y, Lai C, et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119(9):1907–1916. doi:10.1016/j.ophtha.2012.02.040.
- Sjöstrand J, Popović Z. Structural consequences of arrested foveal development in preterms with persisting signs of immaturity. Eye. 2020;34(6):1077–1085. doi:10.1038/s41433-019-0627-4.
- Chatzistergiou V, Cilliers H, Pournaras J, Ambresin A. Fovea plana on optical coherence tomography angiography: new perspectives. Retina. 2021;41(7):1541–1546. doi:10.1097/IAE.0000000000003046.
- Puell MC, Perez-Carrasco MJ, Palomo Alvarez C. Macular thickness and mesopic visual acuity in healthy older subjects. Curr Eye Res. 2019;44(1):82–88. doi:10.1080/02713683.2018.1522648.
- Chen Y, Chen Y, Chen S. Foveal microvascular anomalies on optical coherence tomography angiography and the correlation with foveal thickness and visual acuity in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2019;257(1):23–30. doi:10.1007/s00417-018-4162-y.
- Omoto T, Murata H, Fujino Y, Matsuura M, Fujishiro T, Hirasawa K, Yamashita T, Kanamoto T, Miki A, Ikeda Y, et al. Relationship between macular ganglion cell thickness and ocular elongation as measured by axial length and retinal artery position. Invest Ophthalmol Vis Sci. 2020;61(11):16. doi:10.1167/iovs.61.11.16.
- Araie M, Saito H, Tomidokoro A, Murata H, Iwase A. Relationship between macular inner retinal layer thickness and corresponding retinal sensitivity in normal eyes. Invest Ophthalmol Vis Sci. 2014;55(11):7199–7205. doi:10.1167/iovs.14-14964.