ABSTRACT
The Sparassodonta (Mammalia, Metatheria) are a group of carnivorous mammals that dominated the macropredatory guild of South America during the Cenozoic. Here, we describe a new sparassodont based on a single specimen from the middle Miocene Quebrada Honda local fauna of southern Bolivia. This specimen (UF 27881) does not clearly correspond to any major sparassodont group (e.g., Hathliacynidae, Borhyaenidae, etc.) and represents a morphotype previously unknown among the Sparassodonta. UF 27881 is distinguished from other sparassodonts by its short, broad, borhyaenid-like rostrum and small size, among other features. However, we decline to coin a new name for UF 27881 due to the fragmentary nature of this specimen and the absence of most of its dentition. This specimen suggests that the appearance of the Sparassocynidae and several hypercarnivorous didelphid taxa (including Thylophorops, Thylatheridium, Lutreolina, and Hyperdidelphys) represent an evolutionary response to the decline in small, predatory sparassodont taxa during the late Cenozoic. This study documents new morphological diversity among the Sparassodonta and highlights the value of fossils from traditionally undersampled parts of South America.
SUPPLEMENTAL DATA—Supplemental materials are available for this article for free at http://www.tandfonline.com/UJVP.
INTRODUCTION
Throughout much of the Cenozoic Era, South America was home to a unique guild of macropredatory carnivores. Whereas most of the macropredatory niches on other landmasses were occupied by eutherian carnivorans or creodonts, South America's carnivore guild was largely composed of metatherian mammals (Goin, Citation1995; Forasiepi, Citation2009), sebecosuchian crocodyliformes (Pol et al., Citation2012), giant boine and madtsoiid snakes (Head et al., Citation2009; Albino, Citation2011), and seriemas and their relatives, the aptly named phorusrhacid ‘terror birds’ (Alvarenga and Höfling, Citation2003; Noriega et al., Citation2009; Alvarenga et al., Citation2011). Little of this endemic carnivore diversity survives today, because South American ecosystems began to shift to a predatory guild dominated by eutherian carnivorans during the late Miocene (Huayquerian South American Land Mammal ‘Age’ or SALMA; Soibelzon Citation2011; Prevosti et al., Citation2013:). The most abundant group of predators in South America prior to this faunal shift was one of the few clades of carnivorous mammals to inhabit the continent, the Sparassodonta.
Members of the Sparassodonta are known from the late Paleocene or early Eocene (Nemolestes, Patene; Marshall, 1981; Forasiepi, Citation2009; Gelfo et al., Citation2009) to the mid-Pliocene (Thylacosmilus; Prevosti et al., 2013). In addition, two taxa from the early Paleocene of Tiupampa, Bolivia, Mayulestes ferox and Allqokirus australis, have been considered early members of this group by some authors (Marshall and Muizon, Citation1988; Muizon, Citation1998). However, recent phylogenetic studies have indicated that M. ferox is probably not closely related to sparassodonts (Rougier et al., Citation1998; Forasiepi, Citation2009). The evolutionary relationships of Allqokirus australis are less certain, because this species is only represented by a handful of posterior molars (Marshall and Muizon, Citation1988). Excluding these two taxa, the earliest definitive sparassodonts are Patene simpsoni (Marshall, Citation1981) and cf. Nemolestes sp. (Marshall, Citation1978) from the fissure-fill deposits at Itaboraí, Brazil, which may be late Paleocene or early Eocene in age (Gelfo et al., Citation2009). Following this early Cenozoic origin, sparassodonts diversified into a wide variety of forms by the latter half of the Eocene, including the robust ‘bear-like’ or ‘wolverine-like’ proborhyaenids Callistoe and Arminiheringia (Babot et al., Citation2002; Argot and Babot, Citation2011). The nested position of proborhyaenids within the Sparassodonta suggests that most of the major groups of sparassodonts had originated by this time (Babot et al., Citation2002; Forasiepi, Citation2009:fig. 56).
Although the Golden Age of marsupials in South America had ended by the late Eocene or early Oligocene (Goin et al., Citation2010), sparassodonts and other metatherians remained important components of South American mammal faunas for the rest of the Cenozoic. Among sparassodonts, the Miocene records the first appearance of true borhyaenids and the highly specialized, saber-toothed thylacosmilids (Marshall, Citation1978; Goin et al., Citation2007; Forasiepi, Citation2009). Much of the Miocene sparassodont fossil record is based on early Miocene fossils from southern Patagonia (Colhuehuapian and Santacrucian SALMAs) and late Miocene fossils from northern Argentina (Huayquerian SALMA). Early Miocene sites record terrestrial borhyaenids such as Arctodictis, Acrocyon, and Borhyaena (Argot, Citation2004a; Forasiepi, Citation2009; Ercoli et al., Citation2012; Prevosti et al., Citation2012), as well as more generalized basal borhyaenoids and hathliacynids such as Prothylacynus, Cladosictis, Lycopsis, Pseudonotictis, and Sipalocyon (Argot, Citation2004a; Ercoli et al., Citation2012; Prevosti et al., Citation2012). Although not as species-rich as early Miocene localities, late Miocene fossil sites still contain a diversity of sparassodont taxa, including the saber-toothed Thylacosmilus atrox (Marshall, Citation1976a; Goin and Pascual, Citation1987), the omnivorous basal sparassodont Stylocynus, the small hathliacynids Notictis and Borhyaenidium (Prevosti et al., Citation2013), and at least one indeterminate species of borhyaenid (Forasiepi et al., Citation2007).
In contrast to early and late Miocene taxa, middle Miocene sparassodonts are known from fewer localities and generally less complete specimens. Most well-preserved middle Miocene sparassodonts come from the locality of La Venta, Colombia, the best known of which is the basal borhyaenoid ‘Lycopsis’ longirostrus, which is based on a nearly complete, articulated juvenile skeleton with preserved gut contents (Marshall, Citation1977a). The La Venta Fauna also includes the enigmatic basal sparassodont Hondadelphys fieldsi (Marshall, Citation1976b; Marshall et al., Citation1990; Goin, Citation1997), the large-bodied borhyaenoid Dukecynus magnus, the thylacosmilid Anachlysictis gracilis, and poorly preserved remains that likely represent several additional species (Goin, Citation1997). Aside from the species from La Venta and the recently described Patagosmilus goini from the Collón Curá Formation of Argentina (Forasiepi and Carlini, Citation2010), well-preserved middle Miocene sparassodont remains have only been described from one other locality, Quebrada Honda, Bolivia.
Quebrada Honda is located in the Tarija Department of Bolivia, near the southernmost point of the country (). Fossils at this middle Miocene fossil site were first collected by Carlos Villarroel and Robert Hoffstetter in 1976, the latter of whom published the first formal report on this site's fauna in the following year (Hoffstetter, Citation1977). Quebrada Honda was later visited by a team from the University of Florida in 1978, who discovered a second, coeval site, Río Rosario, some 5 km from the first locality (MacFadden and Wolff, Citation1981). Although it is not possible to directly correlate the stratigraphy of Quebrada Honda and Río Rosario, the same mammal species have been collected at both localities; therefore, these sites are considered to represent a single fauna (MacFadden et al., Citation1990; Croft and Anaya, Citation2006; Croft, Citation2007). The Quebrada Honda mammal fauna is diverse, including at least 30 species of metatherians, xenarthrans, rodents, and endemic ungulates (see Croft, Citation2007, and references therein). Recent studies have described a new dwarf megatherioid (Pujos et al., Citation2011) and several species of caviomorph rodents (Croft et al., Citation2011) from this locality, and ongoing field studies have produced many new specimens including several new species that are currently under study (D. Croft, unpubl. data).
Until the present study, only one species of sparassodont had been identified at Quebrada Honda, Acyon myctoderos (Forasiepi et al., Citation2006). However, as detailed below, our examination of a previously undescribed specimen, UF 27881, indicates that this fossil represents an additional species of sparassodont at this locality. Although incompletely preserved, UF 27881 displays a unique combination of characters that suggest it represents a relatively basal sparassodont not closely related to Acyon. Moreover, UF 27881 clearly pertains to a species much smaller than A. myctoderos, comparable in size to some of the smallest known sparassodonts. In this paper, we describe this specimen as a small, hitherto unrecognized sparassodont and discuss its implications for the evolutionary history of South America's small mammalian predator guild.
MATERIALS AND METHODS
Measurements of this specimen were taken with digital calipers to the nearest 0.01 mm unless otherwise noted. Additional data on sparassodonts and other groups of metatherians (pucadelphids, didelphoids, etc.) were taken from the published literature or direct observations by the authors. A complete list of specimens and references used for comparative analysis can be found in Supplementary Data, Appendix S1.
This paper follows the system of South American Land Mammal Ages (SALMAs) as defined by Flynn and Swisher (Citation1995), as modified by Flynn et al. (Citation2002), Cione and Tonni (Citation2005), and Gelfo et al. (Citation2009). Like Flynn et al. (Citation2002), we consider the Friasian SALMA to represent a relatively short interval of time during the early middle Miocene, as opposed to the Friasian sensu lato of other authors (see discussion in Flynn and Swisher, Citation1995), which would include the Colloncuran, Laventan, and Mayoan SALMAs.
Anatomical Abbreviations
Upper and lower premolars are designated by P/p, whereas upper and lower molars are designated as M/m. Right and left are abbreviated R and L, respectively.
Institutional Abbreviations
CMNH, Cleveland Museum of Natural History, Cleveland, Ohio, U.S.A.; CORD-PZ, Museo de Paleontología, Facultad de Ciencias Exactas, Físicas y Naturales de la Universidad Nacional de Córdoba, Córdoba, Argentina; FMNH, The Field Museum, Chicago, Illinois, U.S.A.; IGM, Instituto Nacional de Investigaciones Geológico-Mineras, Bogotá, Colombia; MACN A, Ameghino collection, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires, Argentina; MACN Pv, Vertebrate paleontology collection, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires, Argentina; MLP, Museo de La Plata, La Plata, Argentina; MMP, Museo Municipal de Ciencias Naturales de Mar del Plata, Mar del Plata, Argentina; MNHN-Bol, Museo Nacional de Historia Natural, La Paz, Bolivia; PVL, Paleontología Vertebrados Lillo, Tucumán, Argentina; UCMP, University of California Museum of Paleontology, Berkeley, California, U.S.A.; UF, University of Florida/Florida Museum of Natural History, Gainesville, Florida, U.S.A.
SYSTEMATIC PALEONTOLOGY
MAMMALIA Linnaeus, 1758
METATHERIA Huxley, 1880
SPARASSODONTA Ameghino, 1894
gen. et sp. nov.
(–; , 2)
Specimen
UF 27881, a partial cranium preserving most of the rostrum, the anteriormost portion of the orbital region and braincase, and both M1s.
Locality
Unnamed formation of the Honda Group, Quebrada Honda local fauna, Bolivia. The exact horizon of UF 27881 is unknown as the specimen lacks additional stratigraphic data (R. Hulbert, pers. comm., February 2012)
Age
Late middle Miocene, Serravallian age, Laventan SALMA. MacFadden et al. (Citation1990) estimate the age of the fossil-bearing layers at this locality to be between 13.0 and 12.7 Ma, based on a combination of paleomagnetic and radioisotopic (40K/40Ar age determination) data.
Comments
UF 27881 can be referred to the Metatheria based on the alveoli of its postcanine dentition, which indicate the presence of three premolars and four molars. More specifically, UF 27881 can be assigned to the Sparassodonta based on the combination of several features, including the presence of a lacrimal tubercle, a single lacrimal foramen positioned within the orbit, contact between the nasal and lacrimal bones, and the absence of the maxillopalatine fenestrae and a single, well-developed major palatine foramen (see below). Although some of these features are also present in certain didelphoids (e.g., Hyperdidelphys, Sparassocynus), these characteristics only occur together in members of the Sparassodonta. UF 27881 exhibits a combination of features (discussed below) that prevent it from being assigned to either the Hathliacynidae or the Borhyaenoidea, the two major recognized subgroups of sparassodonts. This interpretation is supported by our phylogenetic analysis (discussed below), which also suggests that this specimen does not pertain to any currently recognized sparassodont clade. Although UF 27881 almost certainly represents a new species, we refrain from coining a new scientific name for this animal until more complete remains are discovered, because the two most phylogenetically informative regions of the cranium, the basicranium and dentition, are missing or poorly preserved.
DESCRIPTION
Rostrum
The rostrum of UF 27881 is triangular in dorsal view () and is constricted at the level of P1. Anterior to P1, the snout bulges conspicuously around the enlarged canine alveoli, similar to but less strongly developed than the condition observed in some other sparassodonts, such as Cladosictis centralis and Prothylacynus patagonicus. The rostrum also broadens posterior to the level of P1, with the ‘cheeks’ of the maxilla (sensu Forasiepi, Citation2009) flaring laterally behind the level of the infraorbital foramen in ventral view. One of the more unusual features of UF 27881 is its short snout relative to the rest of the skull. In most sparassodonts for which the rostrum is known, the preorbital length accounts for one-third to one-half of the entire length of the cranium (Figs. 2–3; see also Forasiepi, Citation2009). The only clear exceptions to this pattern are several genera of derived, large-bodied borhyaenoids (e.g., Borhyaena, Arctodictis, Pharsophorus, and Prothylacynus), in which the rostrum constitutes less than one-third of the total length of the skull. UF 27881 resembles these large, derived borhyaenoids in having a very short rostrum, probably less than one-third of total skull length (; ).
The anterior-most portion of the rostrum, including both premaxillae, is missing from UF 27881. However, enough of the snout is preserved in this specimen to show that the premaxillae did not possess a large ascending posterodorsal process, unlike many other sparassodonts. In these taxa, such as Cladosictis, Borhyaena, Arctodictis, Acyon, and Callistoe, as well as some non-sparassodont marsupials such as the thylacine (Thylacinus cynocephalus) and Tasmanian devil (Sarcophilus harrissi), a portion of the premaxilla extends between the maxilla and nasal bones to form part of the dorsal surface of the rostrum (D–G), often extending posterior to the canine. In contrast, the nasal and maxilla of UF 27881 are in close contact with one another and extend to the preserved anterior end of the cranium (this contact cannot be seen in because the anterior part of the nasomaxillary contact is obscured by matrix in dorsal view). The anterior edge of the right maxilla curves medially towards the midline of the skull and shows no evidence of an anterolateral process. In most marsupials other than sparassodonts, the paracanine fossa is formed by both the premaxillae and an anterolaterally extending process of the maxilla (Forasiepi, Citation2009). Therefore, as in most other sparassodonts, it is likely that the paracanine fossa in UF 27881 would have been formed solely by the premaxillae.
Most of the sutures of UF 27881 have been obscured by damage and postmortem deformation, particularly in the region of the nasofrontal suture. On the left side of the skull, two structures could represent the nasofrontal suture, of which the more anterior (see ) is more likely the true nasofrontal suture, based on comparison with other sparassodonts. In either case, the nasals of UF 27881 extend posteriorly as far as the orbits. The nasals are relatively narrow and uniform in width anterior to the infraorbital foramen but widen considerably posterior to this structure, resembling the condition in Borhyaena, Cladosictis, Arctodictis, and Acyon.
Parts of the rostrum of UF 27881, particularly the surface of the enlarged canine alveolus, are covered in many small foramina (). These foramina are most closely packed together near the anterior end of the maxilla, giving this area a pockmarked, ‘spongy’ texture. Among sparassodonts, similar foramina have been reported in the thylacosmilids Thylacosmilus and Patagosmilus (Forasiepi and Carlini, Citation2010), as well as a specimen tentatively referred to the proborhyaenid Arminiheringia (MLP 82-V-1-1; Forasiepi, pers. comm., August 2012). Small maxillary foramina surrounding the canine alveolus have also been observed in the non-mammalian cynodont Thrinaxodon (Estes, Citation1961) and therocephalian synapsids (Huttenlocker et al., Citation2011). Because such foramina are only present in sparassodonts with enlarged canines (Thylacosmilus, Patagosmilus, Arminiheringia, and UF 27881; see below), it is possible that these structures represent an adaptation related to the large canines of these taxa (see Discussion).
Palate
The palate is the best-preserved region of UF 27881, with the sutures between the palatines and maxillae clearly visible in ventral view (). The palatines extend anteriorly to the anterior root of M2, reaching the level of the maxillary foramen, and extend posteriorly beyond M4. The posterior end of the palatines shows that the region underlying the choanae was concave posteriorly, as in other sparassodonts (Forasiepi, Citation2009).
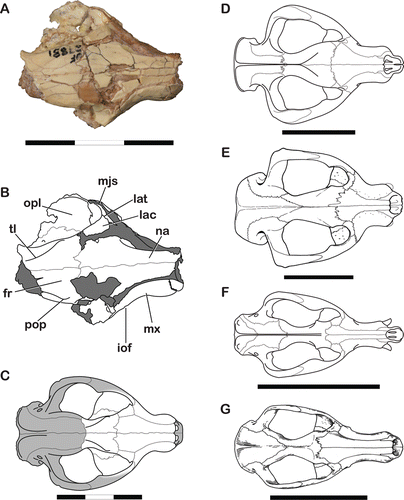
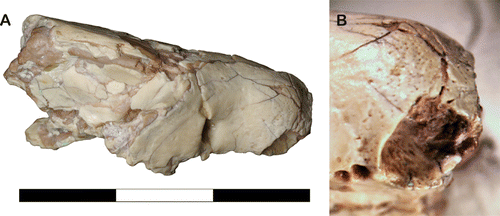
Table 1 Selected cranial measurements of UF 27881 (in mm).
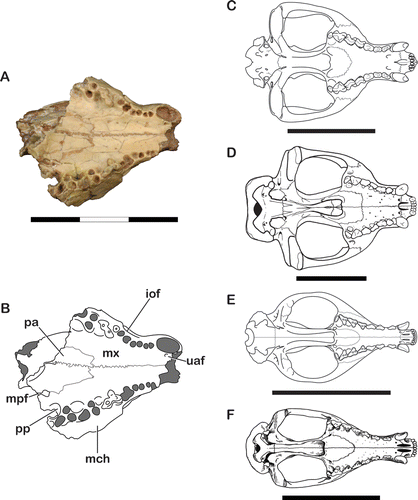
Like other sparassodonts (Forasiepi, Citation2009; C, D) and some didelphoids (e.g., Sparassocynus and caluromyids; see Reig and Simpson, Citation1972; Voss and Jansa, Citation2009), UF 27881 lacks any trace of the maxillopalatine fenestrae. Additionally, the palate of UF 27881 bears many tiny foramina instead of a single, large major palatine foramen. This condition has been noted in other sparassodonts (Forasiepi et al., Citation2006; Forasiepi, Citation2009) and contrasts with that seen in didelphoids and other marsupials. The largest of these foramina is located just medial to canine alveolus and opens anteriorly. This unnamed foramen is present in several other sparassodonts, including Arctodictis, Acyon, Cladosictis, Thylacosmilus, Borhyaena, and possibly Callistoe. Remnants of the minor palatine foramen are present on the left side of the palate of UF 27881, at the posterior border of the maxilla and palatine bones. This foramen is similar in size to that of living didelphoids and resembles the minor palatine foramina of most sparassodonts (except Arctodictis sinclairi; see Forasiepi, Citation2009). The posterior edges of the palate around the minor palatine foramen are not inflected inwards to form ‘corners’ as they are in didelphids and sparassocynids.
The palate of UF 27881 has three pairs of large, conspicuous palatal pits, located slightly lingual to the posterior roots of the first three pairs of molars. Although similar pits have been identified in several carnivorous marsupials, including many species of sparassodonts as well as the sparassocynid Hesperocynus, the presence of three palatal pits in UF 27881 is unusual. Most sparassodonts have only one or two pairs of palatal pits, the exact number of which is subject to ontogeny and individual variation. Further study has shown that in addition to UF 27881, three palatal pits are present in at least one specimen of Sipalocyon gracilis (MACN A 692) and Pseudonotictis pusillus (MLP 11-26). The only other carnivorous marsupial known to have a similar number of palatal pits is the sparassocynid Hesperocynus dolgopolae, which has three palatal pits located lingual to the posterior roots of the first three molars, as well as a fourth pit between P3 and M1 (Forasiepi et al., Citation2009). Because three or more pairs of palatal pits have only been observed in small-bodied taxa, this feature may be a result of allometric scaling.
Orbital Region and Anterior Braincase
The lacrimal of UF 27881 clearly identifies this specimen as a member of the Sparassodonta, because it possesses several features considered characteristic of this group (). Part of the lacrimal extends onto the rostrum to the level of M1, contacting the nasals on the dorsal surface of the snout and preventing contact between the maxilla and frontal bones. Previous studies have considered this feature to be characteristic of sparassodonts (e.g., Marshall, Citation1978; Goin, Citation1997), although this feature also occurs in some other taxa (such as deltatheroideans; Rougier et al., Citation1998). In contrast, strongly developed nasolacrimal contact has only been reported in one species of didelphoid, Hyperdidelphys dimartinoi (Goin and Pardiñas, Citation1996; Voss and Jansa, Citation2009). Like other members of the Sparassodonta, UF 27881 has a single lacrimal foramen that opens inside the orbit. The lateral margin of the lacrimal bears a large lacrimal tubercle that obscures the lacrimal foramen in lateral view. These features have been considered characteristic of the Sparassodonta (Marshall, Citation1976a, 1978, 1981), although they also occur in other marsupial groups (Forasiepi, Citation2009). The only other Neogene marsupial to have both a lacrimal tubercle and a single lacrimal foramen positioned inside the orbit is the sparassocynid Sparassocynus.
The anterior margin of the orbit of UF 27881 is formed by the frontal, lacrimal, and maxilla. Although the jugal is not preserved in this specimen, the shape of the maxilla-jugal suture suggests that this bone would have also contributed to the orbital rim. The ventral-most point of the maxillojugal suture in UF 27881 is very low, almost extending to the level of the palate. Compared with other sparassodonts (e.g., Borhyaena; see Sinclair, Citation1906), UF 27881 has a broad orbital platform, extending posteriorly to the level of the postorbital constriction. Much of this platform is composed of the orbital process of the maxilla, but at least some of the orbital platform is also composed of the palatine, as indicated by the sphenopalatine foramen visible on the orbital floor. The sphenorbital foramen appears to open near the palatomaxillary suture and is extremely small. This contrasts with the large maxillary foramen in this specimen, which is positioned near the floor of the orbit. The orbits appear relatively large compared with the rest of skull, but whether the large orbits of UF 27881 are abnormally large (possibly indicative of nocturnal or crepuscular behavior) or are simply the result of allometric scaling is a question that is beyond the scope of this study, particularly due to the incomplete nature of the specimen.
Table 2 Relative size of the infraorbital foramen (IOF) in UF 27881, didelphoids, and two sparassodonts.
The infraorbital foramen of UF 27881 is large () and positioned dorsal to P3. On the right side of the cranium, which is better preserved, the infraorbital foramen is positioned above the anterior root of the P3, whereas on left side of the skull, the infraorbital foramen is positioned dorsal to the posterior root of P3. Compared with living didelphoids, UF 27881 has a proportionally larger infraorbital foramen (), but is most similar in terms of relative size to the hypercarnivorous Lutreolina crassicaudata and the semiaquatic Chironectes minimus. Among sparassodonts, UF 27881 could only be directly compared with two juvenile specimens assigned to ‘Lycopsis’ longirostrus and Prothylacynus patagonicus. The infraorbital foramen of UF 27881 is relatively larger than that of ‘L.’ longirostrus, but was found to be either relatively larger or smaller than the foramen of P. patagonicus depending on what scaling metric was used. The infraorbital foramen of UF 27881 is nearly circular, in contrast to ‘Lycopsis’ longirostrus and Prothylacynus patagonicus, which have mediolaterally compressed infraorbital foramina (Marshall, Citation1977a, 1979; Forasiepi, Citation2009).
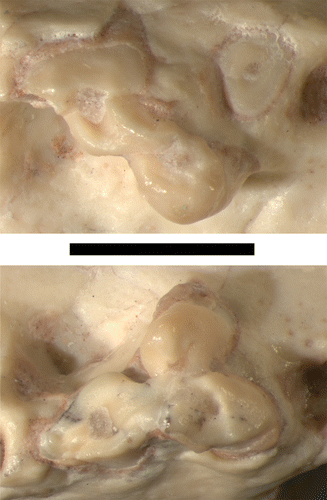
Table 3 Comparison of upper canine proportions in sparassodonts, the thylacinid Thylacinus cynocephalus, and the dasyurid Sarcophilus harrisii.
Most of the braincase is missing from UF 27881, but a small portion of the frontal bone is preserved, including both postorbital processes. These structures are little more than nubs on each side of the specimen. Posterior to these processes are the temporal lines, which are poorly developed and difficult to distinguish. Remarkably, there is no sign of a sagittal crest on the dorsal surface of UF 27881. In most sparassodonts, the sagittal crest is quite pronounced and extends onto the frontal bone in many species (e.g., Acyon and Cladosictis).
Dentition
Unfortunately, most of the dentition is not preserved in UF 27881. The only teeth that are present in this specimen are the left and right M1, both of which are broken and/or worn (, 5). However, the right canine alveolus and most of the postcanine alveoli are preserved in this specimen, except for LM4 (which is incomplete), RM3, and RM4. Thus, UF 27881 clearly had three upper premolars and four molars, which is plesiomorphic for metatherians and characteristic of most sparassodonts and didelphoids (Forasiepi, Citation2009; Voss and Jansa, Citation2009).
Despite its small size, several features of the dentition of UF 27881 indicate that this individual is most likely an adult. There is no sign of a replacement tooth below the alveolus of P3, indicating that the permanent adult premolar had already erupted by the time this animal had died. Additionally, the alveoli of M4, which are partially preserved on the left side of the skull, are well developed and fully formed, indicating that this tooth was also in place before being lost to postmortem damage. In most marsupials, including sparassodonts (Marshall, Citation1976c, 1977a), P3 and M4 are the last upper teeth to erupt, and their presence is usually taken to indicate a specimen is an adult (e.g., Tribe, Citation1990; Astúa and Leiner, Citation2008).
The canine alveolus of UF 27881 is relatively large and deep (), indicating a hypertrophied upper canine. Additionally, the orientation of this alveolus suggests that the canines were slightly procumbent in life. These features, along with the short snout of UF 27881, would have caused the skull of this animal to resemble a borhyaenid or proborhyaenid sparassodont. The canine alveolus of UF 27881 is nearly circular in cross-section (), and based on data from Christiansen (Citation2008:c) and Van Valkenburgh and Ruff (Citation1987:), the inferred canine proportions of UF 27881 most closely resemble those of several extant species of conical-toothed cats.
The alveoli of P1 and P2 indicate that these teeth were double-rooted and roughly subequal in size (). In contrast, the alveolus of P3 is larger than that of either P1 or P2, and the anterior root is larger than the posterior one. The right P3 is double-rooted, but the alveolus of LP3 suggests that the anterior root may have been partially subdivided in this tooth. The premolar alveoli, especially P1 and P2, are much smaller than those of the molars, in contrast to Acyon, Cladosictis, ‘Lycopsis’ longirostrus, Prothylacynus (though this species has a small P1), and Hondadelphys. UF 27881 is unusual among short-snouted sparassodonts in that P1 is oriented parallel to the tooth row. In contrast, all other short-snouted sparassodonts (which are all members of the Borhyaenoidea) have a P/p1 that is obliquely or even transversely oriented to the tooth row (Goin, Citation1997; Forasiepi, Citation2009). Another unusual feature of this specimen is the lack of gaps between the premolar alveoli. Most sparassodonts typically have prominent diastemata between their premolars, whereas closed dentitions are otherwise found only in large-bodied, short-snouted borhyaenoids such as borhyaenids and proborhyaenids (Forasiepi, Citation2009).
The left M1 is slightly more complete than the right M1, which is missing most of the paracone (). These teeth lack any sign of a cingulum or additional cusps or ridges, with each tooth apparently composed solely of the metacone, protocone, and paracone. The paraconule and metaconule are absent and there is no preprotocrista or postprotocrista, a feature shared with derived borhyaenoids (Prothylacynus, Borhyaena, Arctodictis, Thylacosmilus), and the hathliacynid Notogale. Compared with most other sparassodonts, the protocone of UF 27881 is relatively large and is not reduced to a swelling or absent as in many other short-snouted taxa (proborhyaenids, borhyaenids, thylacosmilids, and Pharsophorus). The apex of this cusp is deflected labially towards the metacone and paracone. Most marsupials have an extensive stylar shelf that displaces the metacone and paracone towards the midline of the tooth (Ungar, Citation2010). This condition is also present in many sparassodonts, including Patene, Hondadelphys, Sipalocyon, ‘Lycopsis’ longirostrus, and Lycopsis torresi. However, in UF 27881, the preserved bases of the metacone and paracone on M1 are positioned labially, almost to the edge of the maxilla, suggesting that the stylar shelf was not extensive on this tooth. Despite being relatively poorly preserved, it is apparent that the stylar shelf on M1 of UF 27881 is reduced compared with that of Sipalocyon or Patene. The first three molars of this specimen have three roots (), whereas M4 has at least two roots, possibly three. The alveoli of M1–3 are subequal in size and indicate that the molars were much larger than the premolars ().
Table 4 Selected dental measurements of UF 27881 (in mm).
PHYLOGENETIC ANALYSIS
In order to determine the position of UF 27881 within the Sparassodonta, we performed a maximum parsimony analysis in PAUP* version 4.0b10 (Swofford, Citation2002) based on a matrix of 39 ingroup taxa and 307 characters (Supplementary Data, file ECMatrix.nex and Appendix S2) compiled in Mesquite (Maddison and Maddison, Citation2011). Characters and codings in this analysis were taken from Forasiepi (Citation2009), with some modifications based on recent studies and personal observations. All characters were weighted equally. In addition to UF 27881, the early Eocene proborhyaenid Callistoe vincei and the early middle Miocene thylacosmilid Patagosmilus goini were also added to this analysis. The dasyuromorphian Thylacinus cynocephalus was excluded from this study due to its high degree of morphological convergence with sparassodonts (see Forasiepi, Citation2009, for more information). A complete list of changes from Forasiepi (Citation2009) can be found in Supplementary Data, Appendix S2.
Our analysis recovered eight most parsimonious trees (MPTs), each with a length of 994 steps. These trees had a consistency index (CI) of 0.379, retention index (RI) of 0.651, rescaled consistency index (RC) of 0.247, and homoplasy index (HI) of 0.621. Support measures for the consensus tree (Bremer support and bootstrap values via an analysis of 100 heuristic search bootstrap replicates) were computed in TNT (Goloboff et al., Citation2008). A combined version of the eight MPTs is presented in .
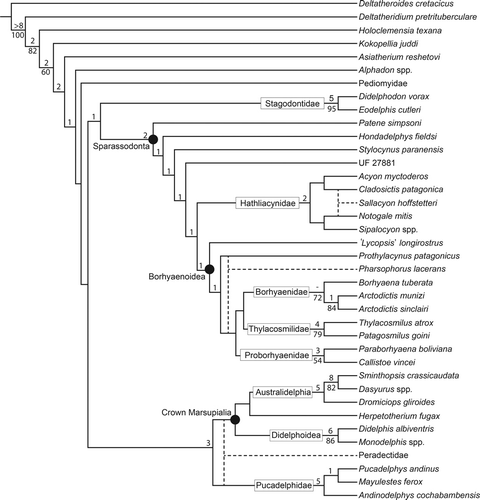
In all eight MPTs, UF 27881 is positioned as a basal sparassodont, sister to the clade formed by Hathliacynidae + Borhyaenoidea. The MPTs only differ in the position of a few taxa such as the hathliacynid Sallacyon hoffstetteri (). In some trees, Sallacyon hoffstetteri and Notogale mitis form a ‘Salla clade’ that sister to Sipalocyon, whereas in others Sallacyon groups with the hathliacynids Cladosictis and Acyon. Regardless of the position of Sallacyon, Cladosictis and Acyon were found to be more closely related to one another than either was to Notogale or Sipalocyon, and vice versa (see presentation of Hathliacynidae in ). Another unstable taxon in this analysis was the borhyaenoid Pharsophorus, which was either recovered as the sister taxon of Prothylacynus or as sister to the clade formed by the Proborhyaenidae, Borhyaenidae, and Thylacosmilidae. Like Forasiepi (Citation2009), we did not recover a close relationship between Pharsophorus and borhyaenids (Borhyaena and Arctodictis), contrary to traditional conception of the family Borhyaenidae (Marshall, Citation1978; Goin et al., Citation2010).
Both Patagosmilus goini and Callistoe vincei grouped with their assumed closest relatives in this analysis, Thylacosmilus atrox and Paraborhyaena boliviana, respectively. However, unlike some studies, we did not recover a monophyletic clade of Proborhyaenidae + Thylacosmilidae (Babot et al., Citation2002), nor a paraphyletic Proborhyaenidae (Babot, Citation2005, in Argot and Babot, Citation2011). Rather, in contrast to Forasiepi (Citation2009), we recovered thylacosmilids as the sister group to borhyaenids. This suggests that the relationships among these three clades of derived borhyaenoids are still unclear, and that more complete remains of this group are needed to clarify their interrelationships.
The Stagodontidae, represented by Didelphodon and Eodelphis, were recovered as the sister taxon to the Sparassodonta in this analysis. Stagodontids have previously been considered to be closely related to sparassodonts (e.g., Marshall et al., Citation1990), but this idea has been strongly criticized as based on homoplastic features related to a carnivorous diet (Fox and Naylor, Citation1995; Rougier et al., Citation1998). The topology recovered here may be due to such homoplastic features, but would support a hypothesized Cretaceous origin of the Sparassodonta (Forasiepi, Citation2009).
With regard to crown-group Marsupialia, the topology recovered here is similar to that of Forasiepi (Citation2009). However, Herpetotherium was recovered within crown-group Marsupialia, basal to the Australidelphia, rather than as a sister taxon to the group. This contrasts with the results of several other studies of marsupial phylogenetics (Sánchez-Villagra et al., Citation2007; Forasiepi, Citation2009; Horovitz et al., Citation2009), but agrees with that of Beck (Citation2012). In contrast to Horovitz et al. (Citation2009), we did not recover a close association between the Peradectidae and the Didelphoidea. Instead, the Peradectidae were recovered as the sister taxon to either crown-group Marsupialia or the Pucadelphidae.
The association of Mayulestes with Andinodelphys and Pucadelphys was one of the most stable arrangements in this analysis, appearing in nearly every experimental tree examined. This arrangement is similar to the results obtained in other phylogenetic studies of marsupials, such as Horovitz and Sánchez-Villagra (Citation2003), Sánchez-Villagra et al. (Citation2007), Horovitz et al. (Citation2009), and Beck (Citation2012). However, in this study Mayulestes was recovered within the Pucadelphidae rather than as the sister taxon to this group. Like Forasiepi (Citation2009), we did not recover a close relationship between Mayulestes and the Sparassodonta. However, in contrast to this study, we found the Tiupampa taxa to occupy a more crownward position than sparassodonts, near crown-group marsupials. In this respect the cladogram resembles the analysis of Rougier et al. (Citation1998), which recovered the Tiupampa taxa and Sparassodonta as successive sister groups to crown-group Marsupialia. Constraining Mayulestes as a member of the Sparassodonta added 13 steps to the MPT. Our analysis does not support the idea that Mayulestes belongs to the Sparassodonta, as proposed by Muizon (Citation1994, Citation1998), indicating that the similarities between this taxon and sparassodonts most likely represent convergent specializations towards carnivory. This, in turn, suggests that Mayulestes should not be used to represent Sparassodonta in phylogenetic analyses. Rather, Patene or another definitive sparassodont should be used, although many basal members of this group are known from much less complete remains.
DISCUSSION
Sparassodont Canine Morphology
Based on the shape of the canine alveolus, UF 27881 has canines that are more circular in cross-section than most other sparassodonts (see ). The proportions of the canines in this specimen are comparable to several living pantherine felids (Van Valkenburgh and Ruff Citation1987; Christiansen, Citation2008) and the extant cougar, Puma concolor (Van Valkenburgh and Ruff, Citation1987). The canine alveoli of UF 27881 are also relatively large for a non-borhyaenoid sparassodont, second only to a specimen of Cladosictis patagonica (MACN A 5927). Aside from MACN A 5927 and a specimen of Acrocyon riggsi (FMNH P13433), all sparassodonts with proportionally larger canines than UF 27881 (i.e., proborhyaenids and Arctodictis) are species that have been noted to possess abnormally large canines relative to the rest of the group (Marshall, Citation1978; Babot et al., Citation2002).
Interestingly, the specimens with the next most circular canine cross-sections among the Sparassodonta, MACN A 5931 and UCMP 38061 (corresponding to the basal borhyaenoids Prothylacynus patagonicus and ‘Lycopsis’ longirostrus, respectively), are both juveniles (Marshall, Citation1976c; Forasiepi, Citation2009). This suggests that the relatively round canines of these specimens could be related to their immature status, especially because the canine proportions of adult specimens of Prothylacynus resemble other sparassodonts.
Table 5 Comparison of craniodental features between hathliacynid-like sparassodonts (hathliacynids, basal sparassodonts, and basal borhyaenoids such as ‘Lycopsis’ longirostrus) and borhyaenid-like sparassodonts (borhyaenids, proborhyaenids, thylacosmilids, and basal borhyaenoids such as Prothylacynus and Pharsophorus).
Aside from these juvenile specimens, the sparassodont with most similar canine length/width ratios to UF 27881 is the early Miocene (Colhuehuapian SALMA) Arctodictis sinclairi, which has canine proportions comparable to that of a modern lion (Panthera leo; see Christiansen, Citation2008). Several other features of Arctodictis sinclairi suggest that this sparassodont may have been a general ecological analogue of big cats such as the lion (Panthera leo) or jaguar (Panthera onca). These features include its proportionally large infraorbital foramen (see below), fused mandibular symphysis (in contrast to Borhyaena, in which it is sutured but unfused; Marshall, Citation1978), and several features of the postcranium. Forasiepi (Citation2009) considered Arctodictis sinclairi to be a generalized carnivore in terms of its postcranium, intermediate in morphology between Borhyaena and Prothylacynus. A later study by Ercoli et al. (Citation2012) found A. sinclairi to group with both scansorial and terrestrial taxa but did not recover a clear association with either group. Other studies of carnivorous mammals have noted that features seemingly indicative of a scansorial or arboreal mode of life may instead be adaptations for grappling with prey, and vice versa (Meachen-Samuels, Citation2012). In particular, Arctodictis sinclairi has been noted to possess a relatively flexible wrist joint and restricted range of motion at the elbow (Forasiepi, Citation2009; Ercoli et al., Citation2012). Such a combination of features would be consistent with a predominantly terrestrial animal that occasionally climbed trees and hunted by grappling prey with its forelimbs, like modern big cats. It is not certain if the other species of Arctodictis, A. munizi, may have exhibited a similar lifestyle, because this taxon has canine proportions within the normal range of sparassodonts.
Compared with the taxa mentioned above, most sparassodonts included in this analysis had narrower, somewhat mediolaterally compressed canines (i.e., canine length/width ratios >1.35). These proportions are similar to basal machairodontine felids such as Promegantereon and macropredatory canids (Van Valkenburgh and Ruff, Citation1987). Indeed, some sparassodonts, such as Borhyaena, Arminiheringia, and Cladosictis, have canine length/width ratios that are more similar to some saber-toothed carnivores or macropredatory dogs than any modern conical-toothed cat. In contrast, most postcranial studies of sparassodonts have suggested that these animals were more similar to conical-toothed cats than canids or saber-toothed predators in their habits (i.e., they were ambush predators that, with the exception of thylacosmilids, did not kill their prey with a specialized canine bite; Argot, Citation2004a, 2004b). Conical canines are highly advantageous for ambush predators, because they can more easily withstand the highly erratic bending and torsional forces created by struggling prey (Meachen-Samuels and Van Valkenburgh, Citation2009). Predators with mediolaterally compressed canines, on the other hand, tend to favor hunting strategies that involve a minimal amount of contact between the prey and canine teeth, such as a series of shallow, slashing bites while chasing down prey (like macropredatory hyenas and dogs; see Van Valkenburgh and Ruff, Citation1987) or a single, highly precise throat bite (like saber-tooth taxa; see Akersten, Citation1985; Andersson et al., Citation2011; Wheeler, Citation2011).
Lacking both the conical canines of modern ambush predators and the cursorial limbs of pursuit-hunting taxa, sparassodonts may have killed their prey in a different manner than any living carnivoran. The recently extinct thylacine (Thylacinus cynocephalus) might provide a more suitable analogue for sparassodont hunting behavior, because it possessed forelimbs adapted for grappling prey (Figueirido and Janis, Citation2011) as well as mediolaterally compressed canines (Jones, Citation1995). Anecdotal evidence suggests that thylacines killed their prey by crippling it with an initial bite and then ripping open the abdominal cavity and eating the wounded animal alive (Jones, Citation1995). This hunting style is partially reminiscent of both living felids and macropredatory dog-like predators, which may reflect the mix of dog-like and cat-like traits in thylacines (Jones and Stoddart, Citation1998; Figueirido and Janis, Citation2011). However, sparassodonts differ from T. cynocephalus in their larger and more frequently damaged canines (; Jones and Stoddart, Citation1998), implying that there may still have been differences in the behavior of these two groups of predatory mammals.
Surprisingly, the other basal sparassodont included in this analysis, Hondadelphys fieldsi, had a canine length/width ratio of 1.8, comparable to several species of nimravids and intermediate to the proportions seen in basal saber-toothed cats such as Promeganteron and more derived species such as Smilodon (Van Valkenburgh and Ruff, Citation1987; Christiansen, Citation2008). This suggests that, despite its plesiomorphic didelphid-like cheek tooth morphology, Hondadelphys may not have resembled similar-sized living didelphoids in its habits.
Paleobiology of UF 27881
Sparassodonts generally fall into one of two major morphotypes, a paraphyletic stem group composed of taxa with dolichocephalic skulls (including hathliacynids, some basal borhyaenoids such as ‘Lycopsis’ longirostrus, and basal sparassodonts), and a monophyletic group with short snouts and deep skulls (Borhyaenidae, Proborhyaenidae, Thylacosmilidae, and closely related genera of basal borhyaenoids such as Prothylacynus and Pharsophorus). These two morphotypes can be distinguished from one another by several features (), and features of one morphotype are generally not present in members of the other group. In this regard, UF 27881 is unusual, because it combines characters of both borhyaenid-like and hathliacynid-like sparassodonts, such as a short snout and P/p1 oriented parallel to the tooth row. Other features typical of borhyaenid-like sparassodonts cannot be identified in UF 27881, because these regions are missing from this specimen.
Perhaps the most distinctive features of UF 27881 are the suite of characteristics associated with its short snout and hypertrophied canines. Short, broad snouts are a common feature of both hypercarnivorous placental (Van Valkenburgh, Citation2007; Meachen-Samuels and Van Valkenburgh, Citation2009; Figueirido et al., Citation2011) and marsupial (Wroe and Milne, Citation2007) taxa, because this arrangement helps to increase bite force, resist torsional forces from struggling prey (Goswami et al., Citation2011), and compensate for the trade-off between bite force and gape in taxa with long canines (Slater and Van Valkenburgh, Citation2009).
Other morphological features of UF 27881 may be related to the large canines of this taxon. For example, the small foramina covering the surface of the canine alveolus might be evidence of an extensive mucoperiosteum and enlarged gingivae, similar to what has been proposed for nimravids (Wheeler, Citation2011) and the machairodontine felid Smilodon (Riviere and Wheeler, Citation2005). This interpretation is supported by the presence of small maxillary foramina in the sparassodonts Thylacosmilus, Patagosmilus, and Arminiheringia, which also have hypertrophied canines (see above). Some authors have argued that the presence of these foramina in other synapsids (such as the cynodont Thrinaxodon) indicates that these taxa possessed enlarged vibrissae (see discussion in Estes, Citation1961). However, vibrissae do not leave osteological traces on the surface of the cranium in living mammals, even in taxa with highly developed vibrissae such as pinnipeds (Marshall et al., Citation2006; Muchlinski, Citation2010). Additionally, similar foramina are present in animals that lack vibrissae (such as the modern tegu Tupinambis), suggesting that these pits more likely transmitted arteries and/or nerves (Estes, Citation1961).
The inference of well-developed gingivae in sparassodonts is based on different osteological correlates than in eutherian carnivores (Smilodon and the Nimravidae), but this might result from differences in ontogeny. In contrast to eutherians, sparassodont canines are either open-rooted (e.g., proborhyaenids and thylacosmilids; Babot et al., Citation2002) or form roots late in life (e.g., hathliacynids and borhyaenids; Marshall, Citation1976c). Riviere and Wheeler (Citation2005) suggested that the presence of enlarged gum tissue in Smilodon represents an adaptation towards greater precision when biting, which would be essential for a mammal with hypertrophied canines that would be vulnerable to torsion forces created by struggling prey (Akersten, Citation1985; Van Valkenburgh and Ruff, Citation1987; Salesa et al., Citation2005). Other carnivorous mammals with enlarged canines, such as living felids, require similar sensory adaptations to avoid damaging their elongated canine teeth and to increase precision and efficiency when going for the killing bite (Turner and Antón, Citation1997). These animals often kill their prey via a precise bite to the neck, using their canines to sever the connections between the spinal column (Leyhausen, Citation1979). Hence, it seems likely that sparassodonts with enlarged canines evolved adaptations for achieving a precise killing bite similar to those proposed for placental saber-tooths.
In addition to well-developed gingivae, UF 27881 may have possessed well-developed vibrissae as indicated by its proportionally large infraorbital foramen. With some exceptions, the size of the infraorbital foramen is closely correlated with the size of the infraorbital nerve, which innervates the vibrissae (Muchlinski, Citation2010). Therefore, the size of the infraorbital foramen can be used to infer the development of the vibrissae. Muchlinski (Citation2010) found that cat-like ambush carnivorans (such as the fossa Cryptoprocta ferox, the marten Martes flavigula, and the felids Puma concolor, Panthera leo, and Panthera tigris) have relatively large infraorbital foramina, even compared with other terrestrial, short-snouted hypercarnivores (e.g., hyaenids).
In terms of general cranial morphology, UF 27881 is most similar to certain species of mustelids (e.g., Martes), dasyurids (e.g., Dasyurus maculatus), and, to a lesser extent, felids. Members of the genus Martes are small-bodied (mean total skull length of 75 mm according to Elbroch, Citation2006) mesocarnivorous to hypercarnivorous mustelids (Ben-David et al., Citation1997; Hickey et al., Citation1999; Cumberland et al., Citation2001) that, like felids, have been reported to kill their prey via a precise bite to the neck (Clark et al., Citation1987). Much of the caloric content of the diet in some members of this genus, such as M. americana, is composed of larger prey items (Cumberland et al., Citation2001), such as grouse (Bonasa umbellus and Canachites canadensis) and snowshoe hares (Lepus americanus). Similar hunting behaviors have also been reported in some predatory marsupials. The spotted-tailed quoll (Dasyurus maculatus), which has the least mediolaterally compressed canines of any living large-bodied dasyurid (aside from the highly specialized Tasmanian devil, Sarcophilus harrisii), has also been observed to kill prey with a precise neck bite and is known to take large prey relative to its body size (Jones, Citation1995; Jones and Stoddart, Citation1998). Based on the similarities between these carnivorous mammals and UF 27881, it seems plausible that this marsupial killed prey in a similar method to the living Dasyurus maculatus and Martes spp. Other features of UF 27881 such as its short, broad snout (see above) support this interpretation, because this type of rostral morphology is common in predatory mammals that tend to kill prey about the same size or larger than themselves (Meachen-Samuels and Van Valkenburgh, Citation2009). Therefore, the majority of the diet of UF 27881 probably consisted of similar-sized birds, mammals, and reptiles, such as the caviid Guiomys unica (Croft et al., Citation2011).
The Smallest Sparassodonts
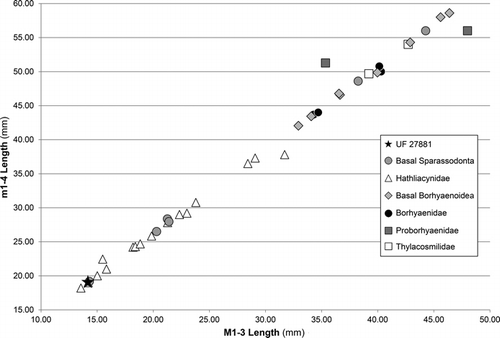
Although there has been some discussion of the size of the largest sparassodonts (Babot et al., Citation2002; Ercoli and Prevosti, Citation2011), few studies have investigated the lower size limit of this group (Marshall, Citation1981; Ercoli and Prevosti, Citation2011; Prevosti et al., Citation2012). In his review of the then-known species of small-bodied sparassodonts, Marshall (Citation1981) considered the late early Miocene (Santacrucian) hathliacynid Pseudonotictis pusillus to be the smallest known member of this group. More recently, Martín and Tejedor (Citation2007) described a second species of this genus, Pseudonotictis chubutensis, from the middle Miocene (Colloncuran SALMA) of Argentina, which appears to have been even smaller than P. pusillus. Some species, such as Patene campbelli from the Paleogene Santa Rosa local fauna of Peru (Goin and Candela, Citation2004) or Allqokirus australis from the early Paleocene of Bolivia (Marshall and Muizon, Citation1988), have also been suggested to be the smallest known sparassodonts, but these species are only known from fragmentary teeth and the identification of the latter taxon (Allqokirus) as a sparassodont is still questionable.
Based on dental measurements, UF 27881 is clearly among the smallest sparassodonts, comparable in size to Pseudonotictis pusillus, Patene simpsoni, and Notictis ortizi (). However, one specimen referred to these taxa stands out. PVL 2618 from the middle Eocene Lumbrera Formation (Goin et al., Citation1986) has been referred to Patene simpsoni, but is more than 20% smaller than the other specimens assigned to this species (Supplementary Data, Table S3). This size discrepancy and the fact that PVL 2618 is much younger than the other specimens assigned to this species (Marshall, Citation1981) suggest that it may pertain to a species distinct from P. simpsoni. If eventually substantiated, this species would represent the smallest definitive sparassodont.
The Evolution of South America's Predator Guild
UF 27881 was originally referred to the genus Sparassocynus (see Croft, Citation2007), which would have constituted the oldest known occurrence of the Sparassocynidae. However, with the reidentification of this specimen as a sparassodont, the pre-late Miocene fossil record of the Didelphoidea is almost entirely composed of small-bodied non-hypercarnivorous species, including the largely frugivorous caluromyids (Goin et al., Citation2007), the omnivorous Marmosa (Marshall, Citation1976b; Vieira and Astúa de Moraes, Citation2003), and the small, insectivorous Thylamys (Goin, Citation1997; Palma, Citation1997). The only possible occurrence of a pre-late Miocene hypercarnivorous didelphoid is an unnamed species from La Venta, Colombia, represented by three lower molars, several mandibular fragments, and possibly a partial cranium (Goin, Citation1997).
There are two possible explanations for the absence or near absence of hypercarnivorous didelphoids prior to the late Miocene (Huayquerian SALMA). One possibility is that didelphoids were unable to move into hypercarnivorous niches prior to the late Miocene because these niches were occupied by similar-sized sparassodonts. This hypothesis is supported by UF 27881, which expands the known diversity of small sparassodonts during the middle Miocene. It is also supported by the observation that many species of hypercarnivorous didelphoid taxa first appear in the fossil record only when the diversity of small sparassodonts begins to wane (Reig and Simpson, Citation1972; Goin and Pardiñas, Citation1996).
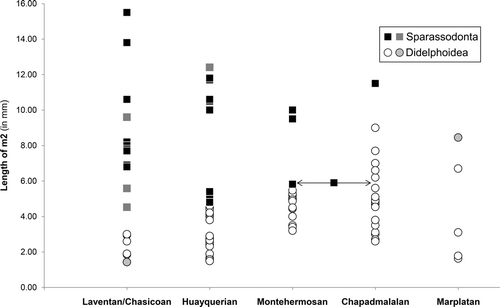
The relationship between the rise of large, predatory didelphoids and the decline of small sparassodonts can be examined by comparing the fossil record of these two groups during the late Cenozoic (). The general pattern supports the idea that these two phenomena are related. Didelphoids are limited to small forms during the Laventan and Chasicoan SALMAs, with large didelphoids first appearing during the Huayquerian SALMA. Even then, hypercarnivorous didelphoids remain smaller than sparassodonts, only increasing in size as the small hathliacynids Borhyaenidium, Notictis, and Notocynus disappear. Didelphoids reach their maximum size in the Chapadmalalan SALMA when the only sparassodont was the large-bodied Thylacosmilus atrox (jaguar to female lion-sized; Ercoli and Prevosti, Citation2011). They may have become even larger in the subsequent Marplatan SALMA after sparassodonts went extinct (Goin et al., Citation2009). The fact that didelphoids and sparassodonts coexisted for at least 11 million years prior to the emergence of hypercarnivorous didelphoids suggests that the decline in sparassodont diversity is not due to competitive exclusion (Goin et al., Citation2007; Prevosti et al., Citation2013). However, the possibility that predatory didelphoids were better adapted to changing environmental conditions of the latest Cenozoic than sparassodonts cannot be discounted. A second faunal turnover may have occurred among hypercarnivorous members of the Didelphoidea in the early/middle Pleistocene (Ensenadan SALMA), as evidenced by the extinction of the sparassocynids, Thylatheridium, and Thylophorops and the first appearance of the cold-adapted thylamyin Lestodelphys (Marshall, Citation1977b; Goin, Citation1995; Goin et al., Citation2009)
A similar case of opportunistic replacement may have also occurred between the basal, omnivorous basal sparassodont Stylocynus and the South American procyonids Cyonasua and Chapalmalania. Although Cyonasua and Stylocynus coexisted in the late Miocene (Huayquerian SALMA), the earliest species of Cyonasua are also the species with strongest adaptations towards an omnivorous/hypocarnivorous mode of life (based on RGA values; see Prevosti et al., Citation2013). Soibelzon (Citation2011) also noted that the species of Cyonasua that coexisted with Stylocynus were only about one-fourth of its size; therefore, the two animals were unlikely to be in competition. Only after the extinction of Stylocynus do larger procyonids (Chapalmalania spp.) and species of procyonids with relatively more pronounced adaptations for carnivory (Cyonasua lutaria and Chapalmalania spp.) appear in the fossil record (Soibelzon, Citation2011; Prevosti et al., Citation2013).
The other possible explanation for the rarity or absence of hypercarnivorous didelphoids prior to the late Miocene is that these animals were present in South America for much of the Cenozoic, but their remains have just not been found yet. This explanation seems plausible, because didelphoid remains in general are rare prior to the late Miocene, and no didelphoid fossils have been found in otherwise productive South American localities such as the Santa Cruz Formation (Marshall et al., Citation1983; Abello et al., Citation2012a). Furthermore, a long, unrecorded history of didelphoids is supported by molecular studies, which suggest that most didelphoid genera originated prior to the late Miocene (Steiner et al., Citation2005).
A long ghost lineage prior to the late Miocene has often been inferred for at least one group of hypercarnivorous didelphoids, the sparassocynids. These marsupials are usually considered to be a sister group to the two remaining families of didelphoids, the caluromyids and didelphids (Goin, Citation1995), suggesting that sparassocynids had diverged from other didelphoids by the earliest Miocene (the age of the earliest caluromyids and didelphids; Goin et al., Citation2007). Some authors have even suggested that sparassocynids diverged from other didelphoids by the early or middle Eocene (Marshall, Citation1987), roughly congruent with the results of molecular studies (Steiner et al., Citation2005). However, as of this writing, the geologically oldest sparassocynid remains come from the late Miocene (Huayquerian SALMA) Cerro Azul, Andalhualá, and Maimará formations of Argentina (Abello et al., Citation2002, 2012b; Forasiepi et al., Citation2009), implying a ghost lineage of at least 11 million years if sparassocynids are sister to the other didelphoids. Additionally, some features of sparassocynids, including a canine alveolus spanning the premaxillary-maxillary suture and the presence of ventrally directed posterolateral corners of the palate, suggest that sparassocynids may be more closely related to or even within the Didelphidae (Reig and Simpson, Citation1972; Voss and Jansa, Citation2003, Citation2009). Similar conclusions were independently reached by another study whose details have not yet been published (Beck and Voss, Citation2012). This is more consistent with the known fossil record of the group.
Engelman_Croft_SD.zip
Download Zip (250.5 KB)ACKNOWLEDGMENTS
We thank T. Matson and R. Muehlheim for access to CMNH zoology collections; T. Pucci, R. Wherley, and G. Svenson (CMNH) for photographic assistance with UF 27881; R. Heaney, B. Patterson, and B. Stanley (FMNH) for loans of extant didelphoid specimens; A. Kramarz (MACN) and M. Reguero (MLP) for access to and information about sparassodont specimens in their care; and J. Bloch, R. Hulbert, and B. MacFadden (UF) for allowing us to describe UF 28871 and providing information on its provenance. We are grateful to A. M. Forasiepi for helpful discussions and providing a digital version of her matrix. We thank editor G. W. Rougier and reviewers F. Prevosti and A. M. Forasiepi for helpful criticisms and comments on earlier drafts of the manuscript. This research was supported by NSF EAR 0958733 to D. Croft.
Handling editor: Guillermo Rougier
High resolution color images of UF 27881 are available online in Morphobank.
LITERATURE CITED
- Abello, A., C.I. Montalvo, and F.J. Goin. 2002. Marsupiales del Mioceno Superior de Caleufú (La Pampa, Argentina). Ameghiniana 39:433–442.
- Abello, M.A., E. Ortiz-Jaureguizar, and A.M. Candela. 2012a. Paleoecology of the Paucituberculata and Microbiotheria (Mammalia, Marsupialia) from the late Early Miocene of Patagonia; pp. 156–172 in S.F. Vizcaíno, R.F. Kay, and M.S. Bargo (eds.), Early Miocene Paleobiology in Patagonia: High-Latitude Paleocommunities of the Santa Cruz Formation. Cambridge University Press, Cambridge, U.K.
- Abello, M.A., M. de Los Reyes, D. Voglino, A.M. Candela, B. Coira, and C.I. Galli. 2012b. Primer registro de Marsupialia (Mammalia, Metatheria) para la Formación Maimará (Mioceno tardío), Quebrada de Humahuaca (Provincia de Jujuy, Argentina). Ameghiniana 49(4, Supplement
- Akersten, W.A. 1985. Canine function in Smilodon (Mammalia; Felidae; Machairodontinae). Contributions in Science, Natural History Museum, Los Angeles County 356:1–22.
- Albino, A.M. 2011. Evolution of Squamata reptiles in Patagonia based on the fossil record. Biological Journal of the Linnean Society 103:441–457.
- Alvarenga, H.M. F., and E. Höfling. 2003. Systematic revision of the Phorusrhacidae (Aves: Ralliformes). Papéis Avulsos de Zoologia 43:55–91.
- Alvarenga, H., L. Chiappe, and S. Bertelli. 2011. Phorusrhacids: the terror birds; pp. 187–208 in G. Dyke and G. Kaiser (eds.), Living Dinosaurs: The Evolutionary History of Modern Birds. John Wiley & Sons, Oxford, U.K.
- Ameghino, 1894. Enumération synoptique des espéces des mammiferes fósiles des formations éocenes de Patagonie. Boletín de la Academia Nacional de Ciencias en Córdoba 13:259–452.
- Andersson, K., D. Norman, and L. Werdelin. 2011. Sabretoothed carnivores and the killing of large prey. PLoS ONE 6:e24971. doi: 10.1371/journal.pone.0024971.
- Argot, C. 2004a. Evolution of South American mammalian predators (Borhyaenoidea): anatomical and palaeobiological implications. Zoological Journal of the Linnean Society 140:487–521.
- Argot, C. 2004b. Functional-adaptive features and palaeobiological implications of the postcranial skeleton of the late Miocene sabretooth borhyaenoid, Thylacosmilus atrox (Metatheria). Alcheringa 28:229–266.
- Argot, C., and J. Babot. 2011. Postcranial morphology, functional adaptations and palaeobiology of Callistoe vincei, a predaceous metatherian from the Eocene of Salta, north-western Argentina. Palaeontology 54:447–480.
- Astúa, D., and N.O. Leiner. 2008. Tooth eruption sequence and replacement pattern in wooly opossums, genus Caluromys (Didelphimorphia: Didelphidae). Journal of Mammalogy 89:244–251.
- Babot, M.J. 2005. Los Borhyaenoidea (Mammalia, Metatheria) del Terciario inferior del Noroeste argentino. Aspectos filogenéticos, paleobiológicos, y bioestratigrafícos. Unpublished Ph.D. dissertation, Universidad Nacional de Tucumán, Tucumán, Argentina, 454 pp.
- Babot, M.J., J.E. Powell, and C. de Muizon. 2002. Callistoe vincei, a new Proborhyaenidae (Borhyaenoidea, Metatheria, Mammalia) from the early Eocene of Argentina. Geobios 35:615–629.
- Beck, R.M. 2012. An ‘ameridelphian’ marsupial from the early Eocene of Australia supports a complex model of Southern Hemisphere marsupial biogeography. Naturwissenschaften 99:715–729.
- Beck, R.M., and R.S. Voss. 2012. A comprehensive genus-level phylogeny of living and extinct marsupials based on craniodental and molecular data. Journal of Vertebrate Paleontology, Program and Abstracts 2012:62A
- Ben-David, M., R.W. Flynn, and D.M. Schell. 1997. Annual and seasonal changes in diets of martens: evidence from stable isotope analysis. Oecologia 111:280–291.
- Christiansen, P. 2008. Evolutionary convergences of primitive sabertooth craniomandibular morphology: the clouded leopard (Neofelis nebulosa) and Paramachairodus ogygia compared. Journal of Mammalian Evolution 15:155–179.
- Cione, A.L., and E.P. Tonni. 2005. Bioestratigrafía basada en mamíferos del Cenozoico superior de la provincia de Buenos Aires, Argentina; pp. 183–200 in R.E. de Barrio, R.O. Etcheverry, M.F. Caballé, and E. Llambías (eds.), Geología y Recursos Minerales de la Provincia de Buenos Aires XVI Congresos Geológica, 20–23 September 2005, Argentina, La Plata.
- Clark, T.W., E. Anderson, C. Douglas, and M. Strickland. 1987. Martes americana. Mammalian Species 289:1–8.
- Croft, D.A. 2007. The middle Miocene (Laventan) Quebrada Honda fauna, southern Bolivia and a description of its notoungulates. Palaeontology 50:277–303.
- Croft, D.A., and F. Anaya. 2006. A new middle Miocene hegetotheriid (Notoungulata: Typotheria) and a phylogeny of the Hegetotheriidae. Journal of Vertebrate Paleontology 26:387–399.
- Croft, D.A., J.M. H. Chick, and F. Anaya. 2011. New middle Miocene caviomorph rodents from Quebrada Honda, Bolivia. Journal of Mammalian Evolution 18:245–268.
- Cumberland, R.E., J.A. Dempsey, and G.J. Forbes. 2001. Should diet be based on biomass? Importance of larger prey to the American marten. Wildlife Society Bulletin 29:1125–1130.
- Elbroch, M. 2006. Animal Skulls: A Guide to North American Species. Stackpole Books, Mechanicsburg, Pennsylvania, 727 pp.
- Ercoli, M.D., and F.J. Prevosti. 2011. Estimación de masa de las especies de Sparassodonta (Mammalia, Metatheria) de edad Santacrucense (Mioceno temprano) a partir del tamaño del centroide de los elementos apendiculares: inferencias paleoecológicas. Ameghiniana 48:462–479.
- Ercoli, M.D., F.J. Prevosti, and A. Álvarez. 2012. Form and function within a phylogenetic framework: locomotory habits of extant predators and some Miocene Sparassodonta (Metatheria). Zoological Journal of the Linnean Society. 165:224–251.
- Estes, R. 1961. Cranial anatomy of the cynodont reptile Thrinaxodon liorhinus. Bulletin of the Museum of Comparative Zoology 125:163–180.
- Figueirido, B., and C.M. Janis. 2011. The predatory behavior of the thylacine: Tasmanian tiger or marsupial wolf? Biology Letters 7:937–940.
- Figueirido, B., N. MacLeod, J. Krieger, M. De Renzi, J.A. Pérez-Claros, and P. Palmqvist. 2011. Constraint and adaptation in the evolution of carnivoran skull shape. Paleobiology 37:490–518.
- Flynn, J.J., and C.C. Swisher III. 1995. Cenozoic South American Land Mammal Ages: correlation to global geochronologies; pp. 317–333 in W.A. Berggren, D.V. Kent, M.-P. Aubry, and J. Hardenbol (eds.), Geochronology, Time Scales, and Global Stratigraphic Correlation. SEPM (Society For Sedimentary Geology), Special Publication No. 54.
- Flynn, J.J., D.A. Croft, R. Charrier, G. Hérail, and A.R. Wyss. 2002. The first Cenozoic mammal fauna from the Chilean Altiplano. Journal of Vertebrate Paleontology 22:200–206.
- Forasiepi, A.M. 2009. Osteology of Arctodictis sinclairi (Mammalia, Metatheria, Sparassodonta) and phylogeny of Cenozoic metatherian carnivores from South America. Monografías del Museo Argentino de Ciencias Naturales 6:1–174.
- Forasiepi, A.M., and A.A. Carlini. 2010. A new thylacosmilid (Mammalia, Metatheria, Sparassodonta) from the Miocene of Patagonia, Argentina. Zootaxa 2552:55–68.
- Forasiepi, A.M., F.J. Goin, and A.G. Martinelli. 2009. Contribution to the knowledge of the Sparassocynidae (Mammalia, Metatheria, Didelphoidea) with comments on the age of the Aisol Formation (Neogene), Mendoza Province, Argentina. Journal of Vertebrate Paleontology 29:1252–1263.
- Forasiepi, A.M., A.G. Martinelli, and F.J. Goin. 2007. Revisión taxonómica de Parahyaenodon argentinus Ameghino y sus implicancias en el conocimiento de los grandes mamíferos carnívoros del Mio-Plioceno de América del Sur. Ameghiniana 44:143–159.
- Forasiepi, A.M., M.R. Sánchez-Villagra, F.J. Goin, M. Takai, N. Shigehara, and R.F. Kay. 2006. A new species of Hathliacynidae (Metatheria, Sparassodonta) from the middle Miocene of Quebrada Honda, Bolivia. Journal of Vertebrate Paleontology 26:670–684.
- Fox, R.C., and B.G. Naylor. 1995. The relationships of the Stagodontidae, primitive Late Cretaceous mammals; pp. 247–250 in A. Sun and Y. Wang (eds.), VI Symposium on Mesozoic Terrestrial Ecosystems and Biota, Short Papers, China Ocean Press, Beijing.
- Gelfo, J.N., F.J. Goin, M.O. Woodburne, C. de Muizon. 2009. Biochronological relationships of the earliest South American Paleogene faunas. Palaeontology 52:251–269.
- Goin, F.J. 1995. Los marsupiales; pp. 165–179 in M.T. Alberdi, G. Leone, and E.P. Tonni (eds.), Evolución Biológica y Climática de la Región Pampeana durante los Últimos Cinco Millones de Anos. Un ensayo de Correlación con el Mediterráneo Occidental. Monografías del Museo Nacional de Ciencias Naturales 12, Madrid.
- Goin, F.J. 1997. New clues for understanding Neogene marsupial radiations; pp. 185–204 in R.F. Kay, R.H. Madden, R.L. Cifelli, and J. Flynn (eds.), A History of the Neotropical Fauna: Vertebrate Paleobiology of the Miocene in Colombia. Smithsonian Institution Press, Washington, D.C.
- Goin, F.J., and A.M. Candela. 2004. New Paleogene marsupials from the Amazon Basin of eastern Perú. Science Series, Los Angeles Museum of Natural History 40:15–60.
- Goin, F.J., and U.F. J. Pardiñas. 1996. Revisión de las especies del género Hyperdidelphys Ameghino, 1904 (Mammalia, Marsupialia, Didelphidae). Su significación filogenética, estratigráfica y adaptativa en el Neógeno del Cono Sur sudamericano. Estudios Geológicos 52:327–359.
- Goin, F.J., and R. Pascual. 1987. News on the biology and taxonomy of the marsupials Thylacosmilidae (late Tertiary of Argentina). Anales de la Academia Nacional de Ciencias Exactas, Fisicas, y Naturales de Buenos Aires 39:219–246.
- Goin, F.J., M.A. Abello, and L. Chornogubsky. 2010. Middle Tertiary marsupials from central Patagonia (early Oligocene of Gran Barranca): understanding South America's Grande Coupre; pp. 69–105 in R.H. Madden, A.A. Carlini, M.G. Vucetich, and R.F. Kay (eds.), The Paleontology of Gran Barranca: Evolution and Environmental Change through the Middle Cenozoic of Patagonia. Cambridge University Press, Cambridge, U.K.
- Goin, F.J., R.M. Palma, R. Pascual, and J.E. Powell. 1986. Persistencia de un primitivo Borhyaenidae (Mammalia, Marsupialia) en el Eoceno Temprano de Salta (Fm. Lumbrera, Argentina). Aspectos geologicos y paleoambientales relacionados. Ameghiniana 23:47–56.
- Goin, F.J., N. Zimicz, M. de Los Reyes, and L. Soibelzon. 2009. A new large didelphid of the genus Thylophorops (Mammalia:Didelphimorphia:Didelphidae), from the late Tertiary of the Pampean Region (Argentina). Zootaxa 2005:35–46.
- Goin, F.J., A. Abello, E. Bellosi, R.F. Kay, R. Madden, and A. Carlini. 2007. Los Metatheria sudamericanos de comienzos del Neógeno (Mioceno temprano, edad mamífero Colhuehuapense). Parte I: introducción, Didelphimorphia y Sparassodonta. Ameghiniana 44:29–71.
- Goloboff, P.A., J.S. Farris, and K.C. Nixon. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24:774–786
- Goswami, A., N. Milne, and S. Wroe. 2011. Biting through constraints: cranial morphology, disparity, and convergence across living and fossil carnivorous mammals. Proceedings of the Royal Society B. 278:1831–1839.
- Head, J.J., J.I. Bloch, A.K. Hastings, J.R. Bourque, E.A. Cadena, F.A. Herrera, P.D. Polly, and C.A. Jaramillo. 2009. Giant boid snake from the Palaeocene Neotropics reveals hotter past equatorial temperatures. Nature 457:715–717.
- Hickey, J.R., R.W. Flynn, S.W. Buskirk, K.G. Gerow, and M.F. Wilson. 1999. An evaluation of a mammalian predator, Martes americana, as a disperser of seeds. Oikos 87:499–508.
- Hoffstetter, R. 1977. Un gisement de mammifères miocènes à Quebrada Honda (Sud Bolivien). Comptes Rendus de la Académie des Sciences, Paris, Série D 284:1517–1520.
- Horovitz, I., and M.R. Sánchez-Villagra. 2003. A morphological analysis of marsupial mammal higher-level phylogenetic relationships. Cladistics 19:181–212.
- Horovitz, I., T. Martin, J. Bloch, S. Ladevèze, C. Kurz, M.R. Sánchez-Villagra. 2009. Cranial anatomy of the earliest marsupials and the origin of opossums. PLoS ONE 4:e8278. doi: 10.1371/journal.pone.0008278.
- Huttenlocker, A.K., C.A. Sidor, and R.M. H. Smith. 2011. A new specimen of Promoschorhynchus (Therapsida: Therocephalia: Akidnognathidae) from the Lower Triassic of South Africa and its implications for theriodont survivorship across the Permo-Triassic boundary. Journal of Vertebrate Paleontology 31:405–421.
- Huxley, 1894.
- Jones, M.E. 1995. Guild structure of the large marsupial carnivores in Tasmania. Ph.D. dissertation, University of Tasmania, Hobart, Australia, 143 pp.
- Jones, M.E., and D.M. Stoddart. 1998. Reconstruction of the predatory behavior of the extinct marsupial thylacine (Thylacinus cynocephalus). Journal of the Zoological Society of London 246:239–246.
- Leyhausen, P. 1979. Cat Behavior: The Predatory and Social Behavior of Domestic and Wild Cats. Garland Publishing Incorporated, New York, 340 pp.
- Linnaeus, 1754.
- MacFadden, B.J., and R.G. Wolff. 1981. Geological investigations of late Cenozoic vertebrate-bearing deposits in southern Bolivia. Anales del II Congreso Latinoamericano de Paleontología 2:765–788.
- MacFadden, B.J., F. Anaya, H. Perez, C.W. Naeser, P.K. Zeitler, and K.E. Campbell Jr. 1990. Late Cenozoic paleomagnetism and chronology of Andean basins of Bolivia: evidence for possible oroclinal bending. Journal of Geology 98:541–555.
- Maddison, W.P., and D.R. Maddison. 2011. Mesquite: A Modular System for Evolutionary Analysis, version 2.75. Available at http://mesquiteproject.org. Accessed February 7, 2012.
- Marshall, L.G. 1976a. Evolution of the Thylacosmilidae, extinct saber-tooth marsupials of South America. PaleoBios 23:1–30.
- Marshall, L.G. 1976b. New didelphine marsupials from the La Venta fauna (Miocene) of Colombia, South America. Journal of Paleontology 50:402–418.
- Marshall, L.G. 1976c. Notes on the deciduous dentition of the Borhyaenidae (Marsupialia: Borhyaenoidea). Journal of Mammalogy 57:751–754.
- Marshall, L.G. 1977a. A new species of Lycopsis (Borhyaenidae, Marsupialia) from the La Venta fauna (late Miocene) of Columbia, South America. Journal of Paleontology 51:633–642.
- Marshall, L.G. 1977b. Lestodelphys halli. Mammalian Species 81:1–3.
- Marshall, L.G. 1978. Evolution of the Borhyaenidae, extinct South American predaceous marsupials. University of California Publications in Geological Sciences 117:1–89.
- Marshall, L.G. 1979. Review of the Prothylacyininae, an extinct subfamily of South America “dog-like” marsupials. Fieldiana: Geology, New Series 3:1–50.
- Marshall, L.G. 1981. Review of the Hathlylacyninae, an extinct subfamily of South American “dog-like” marsupials. Fieldiana: Geology, New Series 7:1–120.
- Marshall, L.G. 1987. Systematics of Itaboraian (middle Paleocene) age opossum-like marsupials from the limestone quarry at São José de Itaboraí, Brazil; pp. 91–160 in M. Archer (ed.), Possums and Opossums: Studies in Evolution. Surrey Beatty and Sons and the Royal Zoological Society of New South Wales, Sydney, New South Wales, Australia.
- Marshall, L.G., and C. de Muizon. 1988. The dawn of the age of mammals in South America. National Geographic Research 4:23–55.
- Marshall, L.G., J.A. Case, and M.O. Woodburne. 1990. Phylogenetic relationships of the families of marsupials. Current Mammalogy 2:433–502.
- Marshall, L.G., R. Hoffstetter, and R. Pascual. 1983. Mammals and stratigraphy: geochronology of the continental mammal-bearing Tertiary of South America. Palaeovertebrata, Mémoire Extraordinaire 1983:1–93.
- Marshall, C.D., H. Amin, K.M. Kovacs, and C. Lydersen. 2006. Microstructure and innervation of the mystacial vibrissal follicle-sinus complex in bearded seals, Erignathus barbatus (Pinnipedia: Phocidae). The Anatomical Record Part A 288:13–25.
- Martín, G.M., and M.F. Tejedor. 2007. Nueva especie de Pseudonotictis Ameghino (Metatheria, Sparassodonta, Hathliacynidae) del Mioceno medio de Chubut noroccidental, Argentina. Ameghiniana 44:747–750.
- Meachen-Samuels, J.A. 2012. Morphological convergence of the prey-killing arsenal of sabertooth predators. Paleobiology 1:1–14.
- Meachen-Samuels, J., and B. Van Valkenburgh. 2009. Craniodental indicators of prey size preference in the Felidae. Biological Journal of the Linnaean Society 96:784–799.
- Muchlinski, M.N. 2010. A comparative analysis of vibrissa count and infraorbital foramen area in primates and other mammals. Journal of Human Evolution 58:447–473.
- Muizon, C.de. 1994. A new carnivorous marsupial from the Palaeocene of Bolivia and the problem of marsupial monophyly. Nature 370:208–211.
- Muizon, C.de. 1998. Mayulestes ferox, a borhyaenoid (Metatheria, Mammalia) from the early Palaeocene of Bolivia. Phylogenetic and palaeobiologic implications. Geodiversitas 20:19–142.
- Noriega, J.I., S.F. Vizcaíno, and M.S. Bargo. 2009. First record and a new species of seriema (Aves: Ralliformes: Cariamidae) from Santacrucian (early-middle Miocene) beds of Patagonia. Journal of Vertebrate Paleontology 29:620–626.
- Palma, R.E. 1997. Thylamys elegans. Mammalian Species 572:1–4.
- Petter G., and R. Hoffstetter. 1983. Les marsupiaux du déséadién (Oligocene inférieur) de Salla (Bolivie). Annales de Paléontologie 69:175–234.
- Pol, D., J.M. Leardi, A. Lecuona, and M. Krause. 2012. Postcranial anatomy of Sebecus icaeorhinus (Crocodyliformes, Sebecidae) from the Eocene of Patagonia. Journal of Vertebrate Paleontology 32:328–354.
- Prevosti, F.J., A.M. Forasiepi, and N. Zimicz. 2013. The evolution of the Cenozoic terrestrial mammal guild in South America: competition or replacement? Journal of Mammalian Evolution 20:3–21.
- Prevosti, F.J., A.M. Forasiepi, M.D. Ercoli, and G.F. Turazzini. 2012. Paleoecology of the mammalian carnivores (Metatheria, Sparassodonta) of the Santa Cruz Formation (late Early Miocene); pp. 173–193 in S.F. Vizcaíno, R.F. Kay, and M.S. Bargo (eds.), Early Miocene Paleobiology in Patagonia: High-Latitude Paleocommunities of the Santa Cruz Formation. Cambridge University Press, Cambridge, U.K.
- Pujos, F., G. De Iuliis, and B.M. Quispe. 2011. Hiskatherium saintandrei, gen. et sp. nov.: an unusual sloth from the Santacrucian of Quebrada Honda (Bolivia) and an overview of middle Miocene, small megatherioids. Journal of Vertebrate Paleontology 31:1131–1149.
- Reguero, M.A., and A.M. Candela. 2011. Late Cenozoic mammals from the northwest of Argentina; pp. 411–426 in J.A. Salfity and R.A. Marquillas (eds.), Cenozoic Geology of the Central Andes of Argentina, SCS Publisher, Salta, Argentina.
- Reig, O.A., and G.G. Simpson. 1972. Sparassocynus (Marsupialia, Didelphidae), a peculiar mammal from the late Cenozoic of Argentina. Journal of Zoology 167:511–539.
- Riviere, H.L., and H.T. Wheeler. 2005. Cementum on Smilodon sabers. The Anatomical Record Part A 285:634–642.
- Rougier, G.W., J.R. Wible, and M.J. Novacek. 1998. Implications of Deltatheridium specimens for early marsupial history. Nature 396:459–463.
- Salesa, M.J. M. Antón, A. Turner, and J. Morales. 2005. Aspects of the functional morphology in the cranial and cervical skeleton of the sabre-tooth cat Paramachairodus ogygia (Kaup, 1832) (Felidae, Machairodontinae) from the Late Miocene of Spain: implications for the origins of the machairodont killing bite. Zoological Journal of the Linnean Society 144:363–377.
- Sánchez-Villagra, M.R., S. Ladevèze, I. Horovitz, C. Argot, J.J. Hooker, T.E. Macrini, T. Martin, S. Moore-Fay, C. de Muizon, T. Schmelzle, and R.J. Asher. 2007. Exceptionally preserved North American Paleogene metatherians: adaptations and discovery of a major gap in the opossum fossil record. Biology Letters 3:318–322.
- Sinclair, W.J. 1906. Mammalia of the Santa Cruz beds: Marsupialia. Reports of the Princeton University Expeditions to Patagonia 4:333–460.
- Slater, G.J., and B. Van Valkenburgh. 2009. Allometry and performance: the evolution of skull form and function in felids. Journal of Evolutionary Biology 22:2278–2287.
- Soibelzon, L.H. 2011. First description of milk teeth of fossil South American procyonid from the lower Chapadmalalan (late Miocene-early Pliocene) of “Farola Monte Hermoso,” Argentina: paleoecological considerations. Paläontologische Zeitschrift 85:83–89.
- Steiner, C., M. Tilak, E.J. P. Douzery, and F.M. Catzeflis. 2005. New DNA data from a transthyretin nuclear intron suggest an Oligocene to Miocene diversification of living South American opossums (Marsupialia:Didelphidae). Molecular Phylogenetics and Evolution 35:363–379.
- Swofford, D.L. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (*And Other Methods), version 4.0b10. Sinauer Associates, Sunderland, Massachusetts.
- Tribe, C.J. 1990. Dental age classes in Marmosa incana and other didelphoids. Journal of Mammalogy 71:566–569.
- Turner, A., and M. Antón. 1997. The Big Cats and Their Fossil Relatives. Columbia University Press, New York, 234 pp.
- Ungar, P.S. 2010. Mammal Teeth. John Hopkins University Press, Baltimore, Maryland, 304 pp.
- Van Valkenburgh, B. 2007. Déjà vu: the evolution of feeding morphologies in the Carnivora. Integrative and Comparative Biology 47:147–163.
- Van Valkenburgh, B., and C.B. Ruff. 1987. Canine tooth strength and killing behavior in large carnivores. Journal of the Zoological Society of London 212:379–397.
- Vieira, E.M., and D. Astúa de Moraes. 2003. Carnivory and insectivory in Neotropical marsupials; pp. 271–284 in M. Jones, C.R. Dickman, and M. Archer (eds.), Predators With Pouches: The Biology of Carnivorous Marsupials. CSIRO Publishing, Collingwood, Victoria, Australia.
- Villarroel, C., and L.G. Marshall. 1983. Two new late Tertiary marsupials (Hathylacininae and Sparassocyninae) from the Bolivian Altiplano. Journal of Paleontology 57:1061–1066.
- Voss, R.S., and S.A. Jansa. 2003. Phylogenetic studies on didelphid marsupials II. Nonmolecular data and new IRBP sequences: separate and combined analyses of didelphine relationships with denser taxon sampling. Bulletin of the American Museum of Natural History 276:1–82.
- Voss, R.S., and S.A. Jansa. 2009. Phylogenetic relationships and classification of didelphid marsupials, an extant radiation of new world metatherian mammals. Bulletin of the American Museum of Natural History 322:1–177.
- Wheeler, H.T. 2011. Experimental paleontology of the scimitar-tooth and dirk-tooth killing bites; pp. 19–33 in V.L. Naples, L.D. Martin, and J.P. Babiarz (eds.), The Other Saber-Tooths: Scimitar-Tooth Cats of the Western Hemisphere. John Hopkins University Press, Baltimore, Maryland.
- Wroe, S., and N. Milne. 2007. Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution 61:1251–1260.
