ABSTRACT
The skull of Erlikosaurus andrewsi from the Upper Cretaceous Baishin Tsav locality of Mongolia represents the only known three-dimensionally preserved and nearly complete skull of a therizinosaurian. Computed tomographic (CT) scanning of the original specimen and three-dimensional visualization techniques allow the cranial skeleton to be digitally prepared, disarticulated, and restored. Here, we present a detailed description of the restored skull morphology and the individual cranial elements, including visualization of the internal neurovascular and pneumatic structures. Information gained from this study is used in a revised and emended diagnosis for E. andrewsi. A reappraisal of the evolutionary and functional changes in the cranial skeleton as provided by this study supports prior proposals that a keratinous sheath or rhamphotheca was developed early in the evolution of Therizinosauria. Paralleled by the reduction of functional and replacement teeth, this development indicates a shift in the manner of food processing/procurement at the tip of the snout. Extensive pneumatization of the braincase, most evidently developed in E. andrewsi in comparison with other known therizinosaurians, appears to have led to a reduction of the adductor musculature and thus the potential bite force in derived therizinosaurians. In addition, the application of digital data, as presented in this study, introduces a novel way to document fossil data that will allow for morphological and anatomical data to be made widely accessible.
SUPPLEMENTAL DATA—Supplemental materials are available for this article for free at www.tandfonline.com/UJVP
INTRODUCTION
Therizinosauria is an enigmatic clade of derived, specialized theropod dinosaurs found in the Cretaceous deposits of North America and Asia (Clark et al., Citation2004; Kirkland et al., Citation2005b). They are characterized by unusual cranial and postcranial anatomy, including blunt, leaf-shaped teeth, an edentulous premaxilla (except in the basal-most taxon), a ventrally deflected dentary, an elongate neck, wide pelvis, long arms lined with primitive feathers (Xu and Wang, Citation1999), and hands tipped with large, sickle-shaped claws. Due to this unique morphology, the phylogenetic relationships of Therizinosauria have been in flux for many years. Originally identified as the remains of a giant turtle (Maleev, Citation1954), they have since been allied to prosauropods, theropods, or intermediates between the two saurischian clades, Theropoda and Sauropodomorpha (Barsbold and Perle, Citation1980; Paul, Citation1984; Gauthier, Citation1986). The discovery of more complete skeletons from China and Mongolia (Perle, Citation1981; Russell and Dong, Citation1993) later revealed their derived theropod affinities. Since then, numerous discoveries within the last 20 years have added further to our knowledge of therizinosaurian anatomy and provided more information on their paleobiology (e.g., Xu et al., Citation2002a; Li et al., Citation2007; Zanno et al., Citation2009; Lautenschlager et al., Citation2012). Challenging the long-held, classical assumption that all theropod dinosaurs were nearly exclusively carnivorous, therizinosaurians appear to have evolved specialized dietary preferences. Their leaf-shaped, tightly packed teeth and edentulous premaxilla have been suggested to represent adaptations to an herbivorous diet (Paul, Citation1984; Zanno and Makovicky, Citation2011).
Despite the large number of recent discoveries, the fossil record for Therizinosauria is still poor. Most taxa are represented by single, often fragmentary, specimens. With few exceptions, cranial remains are especially rare and descriptions are restricted to a handful of taxa or consist of isolated fragments (Zanno, Citation2010a; Pu et al., Citation2013). Although other, partially articulated cranial remains have been discovered, these have not been thoroughly described (Xu et al., Citation2009a; see also Zanno, Citation2010b). Consequently, the skull of Erlikosaurus andrewsi, from the Upper Cretaceous Baishin Tsav locality of Mongolia, represents the only three-dimensionally preserved, nearly complete skull of a therizinosaurian and is of fundamental phylogenetic, functional, and biomechanical interest.
The cranial osteology of E. andrewsi was described briefly by Barsbold and Perle (Citation1980) and in more detail by Perle (Citation1981), Barsbold (Citation1983), and Clark et al. (Citation1994). However, because the majority of therizinosaurian discoveries have been made since the latter date, a reconsideration of E. andrewsi is necessary. Furthermore, detailed computed tomographic (CT) scans of the skull and mandible provide an opportunity for a detailed description of its external and, for the first time, internal cranial structures. Employing three-dimensional (3D) visualization techniques, CT scan data allow for an accurate, digital restoration and reconstruction of the cranial anatomy. Here, we present a detailed description of the digitally restored cranial osteology, including a revised and emended diagnosis for E. andrewsi. Instead of describing the external anatomy of the articulated specimen only, which has been covered in previous publications, we focus on the individual, digitally disarticulated and restored cranial elements. This description is supplemented by new information on the internal anatomy of the therizinosaurians Falcarius utahensis and Nothronychus mckinleyi based on CT scans of these specimens. The description is further accompanied by extensive 3D documentation of the material (see Supplementary Data), which could serve as a general method to document rare or difficult-to-access fossil specimens and make them accessible in a manner similar to, for example, cladistic data sets.
MATERIALS AND METHODS
The skull of E. andrewsi (IGM 100/111) was CT scanned at X-Tek Systems Ltd. (now Nikon Metrology), Tring, Hertfordshire, U.K., using a XT-H-225ST CT scanner. Scan parameters were set at 180 kV and 145 μA for the complete skull. Additional scans were performed for the braincase region at 180 kV and 135 μA. The resulting rotational projections were processed with custom-built software provided by X-Tek Systems Ltd. creating a VGI and a VOL file, containing 1998 slices with a slice thickness of 145 μm for the complete skull and 1000 slices with a slice thickness of 108 μm for the braincase region. CT scans of the cranial elements of Falcarius utahensis and Nothronychus mckinleyi were performed at Ohio University using a General Electric eXplore Locus in vivo Small Animal MicroCT Scanner at 80 kV and 498 mA. The final data files were subsequently imported into Avizo (versions 6.3.1. and 7.0.0 VSG; Visualization Science Group) for segmentation, visualization, and reconstruction processes.
For the reconstruction process, a stepwise approach was applied: (1) Each individual skull element was highlighted and separately labeled using Avizo's segmentation editor. Minor breaks, cracks, or holes in the bone structure visible in the CT scans were removed by interpolating over the incomplete surface. Subsequently, 3D surface models and volumes of each element were created. (2) In order to restore incomplete or fragmentary elements, respective surface models of opposing sides were compared. The more complete one was chosen as a base model. The counterpart was then reflected and superimposed on to the base model to create a composite outline. Segmented label fields of the latter were then combined into a single label and surface, respectively. To superimpose the individual counterparts, prominent surface structures were landmarked as reference points. To facilitate the mirroring and landmark alignment, two tcl (tool command language) scripts were created for Avizo. (3) In a final step, the individual elements were articulated, following the information provided by undeformed regions of the skull or as indicated by sutures and articulation facets on each element.
Surfaces of the individual elements and the articulated skull and mandible were created to illustrate this article with traditional figures. Additionally, the final restored model and its individual components were embedded into an interactive 3D PDF document (provided in Supplementary Data) using Adobe Acrobat 9 Pro Extended and Adobe 3D Reviewer (CitationLautenschlager, 2014).
Institutional Abbreviations
AZMNH, Arizona Museum of Natural History, Mesa, Arizona, U.S.A.; IGM, Geological Institute of the Mongolian Academy of Sciences, Ulaan Baatar, Mongolia; UMNH, Natural History Museum of Utah, Salt Lake City, Utah, U.S.A.
SYSTEMATIC PALEONTOLOGY
DINOSAURIA Owen, Citation1842
SAURISCHIA Seeley, Citation1887
THEROPODA Marsh, Citation1881
COELUROSAURIA von Huene, Citation1914
THERIZINOSAURIA Russell, Citation1997 (sensu Zanno, Citation2010b)
THERIZINOSAURIDAE Maleev, Citation1954 (sensu Zanno, Citation2010b)
ERLIKOSAURUS ANDREWSI Barsbold and Perle, Citation1980
()
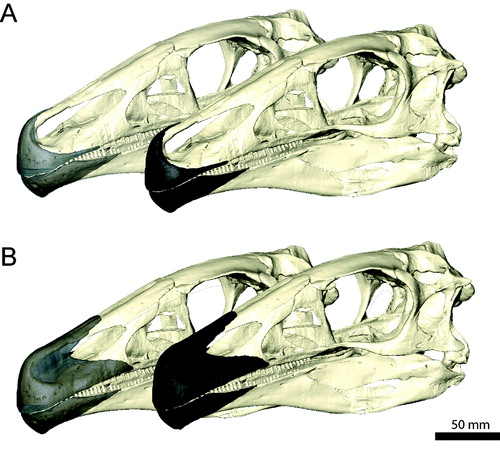
FIGURE 1. Digital representation of the skull of Erlikosaurus andrewsi (IGM 100/111). A, left lateral; B, dorsal; and C, ventral views. Abbreviations: bsp, basisphenoid; exoc, exoccipital; f, frontal; j, jugal; la, lacrimal; mx, maxilla; n, nasal; oc, occipital condyle; pa, parietal; pal, palatine; pmx, premaxilla; po, postorbital; prf, prefrontal; pty, pterygoid; q, quadrate; qj, quadratojugal; soc, supraoccipital; sq, squamosal; vo, vomer.
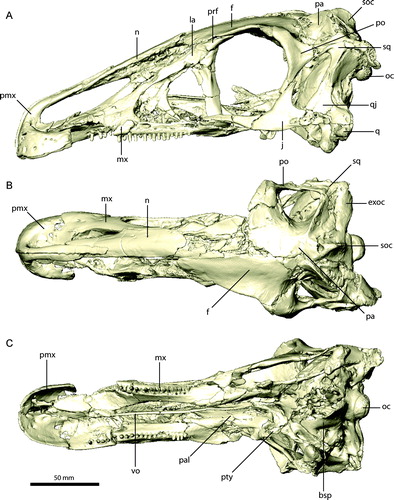
FIGURE 2. Restored skull of Erlikosaurus andrewsi (IGM 100/111). A, left lateral; B, dorsal; and C, ventral views. Abbreviations: bsp, basisphenoid; exoc, exoccipital; f, frontal; j, jugal; la, lacrimal; mx, maxilla; n, nasal; oc, occipital condyle; pa, parietal; pal, palatine; pmx, premaxilla; po, postorbital; prf, prefrontal; pty, pterygoid; q, quadrate; qj, quadratojugal; scr, sclerotic ring; soc, supraoccipital; sq, squamosal; vo, vomer.
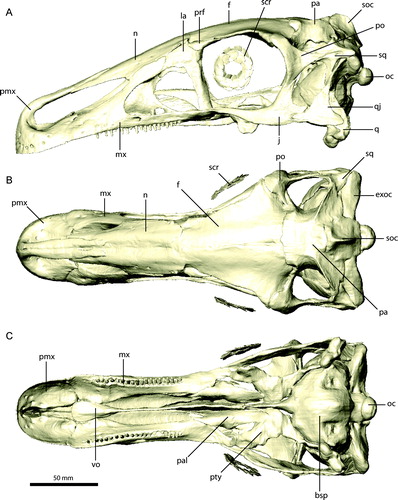
FIGURE 3. Right premaxilla of Erlikosaurus andrewsi (IGM 100/111). A, B, lateral; C, medial; D, dorsal; and E, ventral views. Bone in B rendered transparent to visualize internal neurovascular structures (in yellow). Abbreviations: a.n, nasal articulation; a.pmx, premaxilla articulation; af, ancillary foramina; f1–18, neurovascular foramina; fi, incisive foramen; ml, medial lamina; mp, maxillary process; nc, narial chamber; np, narial process.
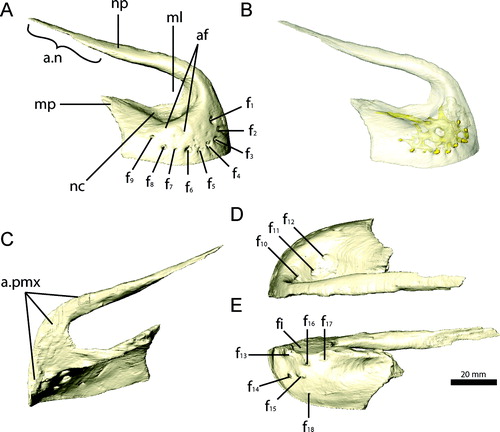
FIGURE 4. Right maxilla of Erlikosaurus andrewsi (IGM 100/111). A, G, lateral; B, F, medial; and C, caudolateral views. 11th maxillary tooth in D, lateral and E, rostral views. Right maxilla fragment of Falcarius utahensis (UMNH VP 14565) in H, medial and I, lateral views. Bone in F–I rendered transparent to visualize teeth (in blue), replacement teeth (in red), and neuropalatine nerve canal (in yellow). Abbreviations: a.pmx, premaxilla articulation; aofo, antorbital fossa; ap, ascending process; cp, caudal process; fo, neurovascular foramina; lnfo, lateral neurovascular foramen; mf, maxillary fenestra; mnfo, medial neurovascular foramen; pnp, pneumatic pockets; ps, palatal shelf.
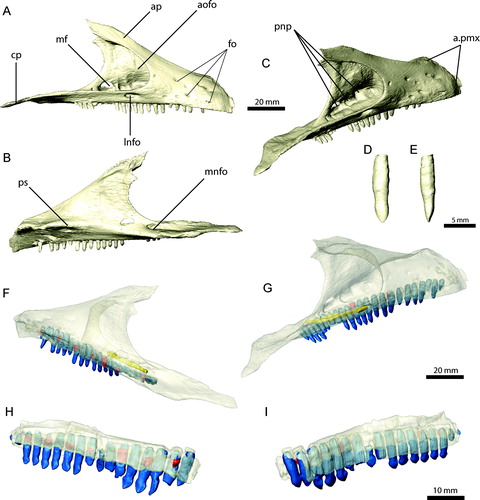
FIGURE 5. Right nasal and left lacrimal of Erlikosaurus andrewsi (IGM 100/111). Right nasal in A, dorsal and B, ventral views. Left lacrimal in C, D, lateral, E, medial, F, rostral, and G, caudal views. Bone in D rendered transparent to visualize internal neurovascular/pneumatic structures (in yellow). Abbreviations: a.f, frontal articulation; a.la, lacrimal articulation; a.mx, maxilla articulation; a.pmx, premaxilla articulation; aofo, antorbital fossa; cp, caudal process; fo, neurovascular foramina; lfo, lacrimal foramen; ra, rostral aperture; rp, rostral process; vp, ventral process.
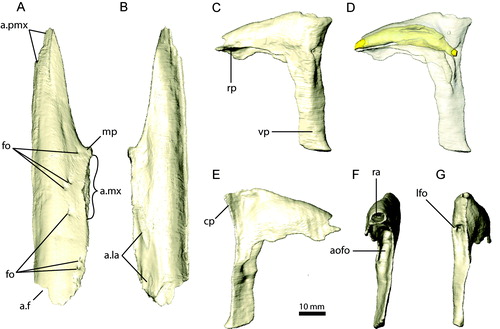
FIGURE 6. Right prefrontal and left jugal of Erlikosaurus andrewsi (IGM 100/111). Right prefrontal in A, lateral, B, dorsal, and C, ventral views. Left jugal in D, lateral and E, medial views. Abbreviations: a.f, frontal articulation; a.la, lacrimal articulation; a.mx, maxilla articulation; a.n, nasal articulation; a.po, postorbital articulation; a.qj, quadratojugal articulation; cp, caudal process; dp, dorsal (postorbital) process; rp, rostral (maxillary) process; vr, ventral ridge.
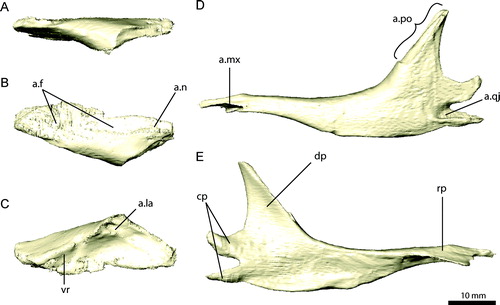
FIGURE 7. Left frontal and parietal of Erlikosaurus andrewsi (IGM 100/111). Left frontal in A, dorsal, B, ventral, C, lateral, and D, medial views. Parietal in E, rostral, F, dorsal, and G, caudal views. Abbreviations: a.f, frontal articulation; a.ls, laterosphenoid articulation; a.n, nasal articulation; a.pa, parietal articulation; a.po, postorbital articulation; a.prf, prefrontal articulation; a.soc, supraoccipital articulation; cc, crista cranii; cer, impression of cerebral hemispheres; lac, lambdoidal crest; ob, impression of olfactory bulbs; orb, orbital cavity; orbm, orbital margin; pop, postorbital process; sqp, squamosal process; stf, supratemporal fenestra; vr, ventral ridge.
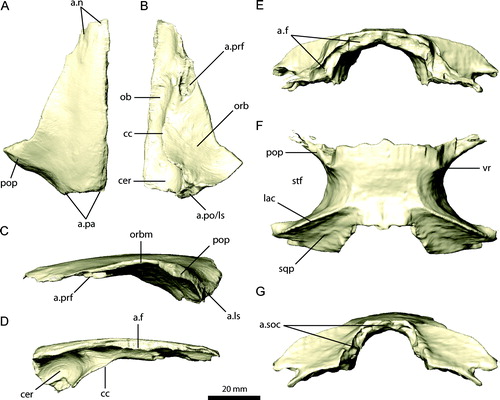
FIGURE 8. Left postorbital, squamosal, and quadratojugal of Erlikosaurus andrewsi (IGM 100/111). Left postorbital in A, lateral, B, medial, and C, caudal views. Left squamosal in D, lateral, E, medial, and F, dorsal views. Left quadratojugal in G, lateral and H, medial views. Abbreviations: a.exoc, exoccipital articulation; a.f, frontal articulation; a.j, jugal articulation; a.ls, laterosphenoid articulation with; a.pa, parietal articulation; a.po, postorbital articulation; a.q, quadrate articulation; a.sq, squamosal articulation; afp, accessory frontal process; dp, dorsal process; fp, frontal process; jp, jugal process; ob, orbit; qp, quadrate process; sqp, squamosal process; stf, supratemporal fenestra.
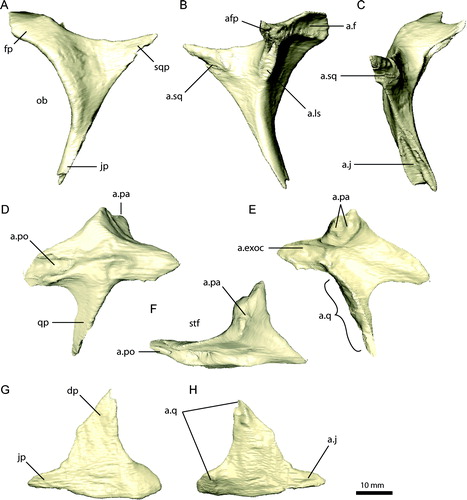
FIGURE 9. Left quadrate of Erlikosaurus andrewsi (IGM 100/111). A, lateral; B, medial; and C, caudal views. Abbreviations: a.bsp, basisphenoid articulation; a.exoc, exoccipital articulation; a.pty, pterygoid articulation; a.qj, quadratojugal articulation; a.sq, squamosal articulation; ac, accessory condyle; lc, lateral condyle; mc, medial condyle; pc, external pneumatic chamber; pqf, paraquadrate foramen; ptf, pterygoid flange; sc, squamosal capitulum.
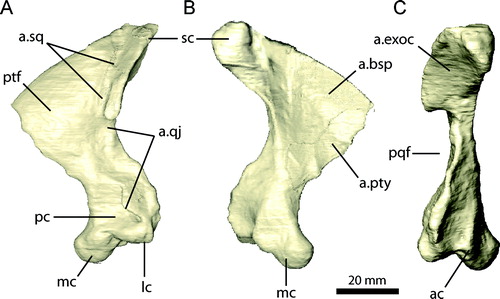
← FIGURE 10. Braincase of Erlikosaurus andrewsi (IGM 100/111) in A, C, and E, natural articulation and B, D, and F, separated into elements in A, B, caudal, C, D, left lateral, and E, F, rostral views. Abbreviations: boc, basioccipital; bsp, basisphenoid; bsr, basisphenoid recess; ls, laterosphenoid; osp, orbitosphenoid; parp, paroccipital process; pro, prootic; soc, supraoccipital; V1, foramen for ophthalmic branch of the trigeminal nerve; V2, foramen for maxillary branch of the trigeminal nerve; V3, foramen for mandibular branch of the trigeminal nerve; VI, foramen for the abducens nerve; IX–XI, foramina for the glossopharyngal, vagus, and spinal accessory nerves; XII, foramen for the hypoglossal nerve.
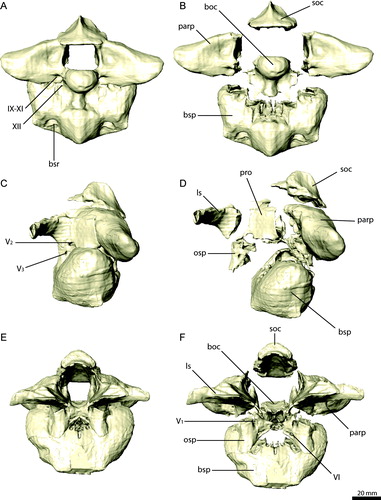
FIGURE 11. Braincase elements of Erlikosaurus andrewsi (IGM 100/111). Supraoccipital in A, dorsal, B, ventral, and C, left lateral views. Right exoccipital/opisthotic in D, rostral, E, G, caudal, and F, dorsal views. Basioccipital in H, caudal, I, dorsal, and J, left lateral views. Bone in G rendered transparent to visualize internal pneumatic structures (in yellow). Abbreviations: a.boc, basioccipital articulation; a.bsp, basisphenoid articulation; a.exoc, exoccipital articulation; a.osp, orbitosphenoid articulation; a.pa, parietal articulation; a.pro, prootic articulation; a.q, quadrate articulation; a.soc, supraoccipital articulation; cp, cerebellar prominence; d, depression; ecf, endocranial floor; nc, nuchal crest; oc, occipital condyle; vr, vertical ridge; stg, stapedial groove; IX–XI, foramina for the glossopharyngal, vagus, and spinal accessory nerves; XII, foramen for the hypoglossal nerve.
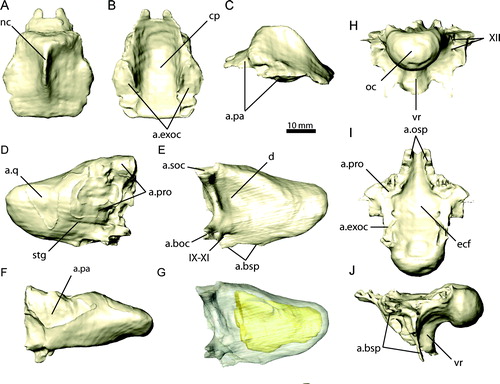
FIGURE 12. Basisphenoid and cultriform process of the parasphenoid of Erlikosaurus andrewsi (IGM 100/111) in A, left lateral, B, ventral, and C, caudal views. Sagittal cross-sections through the braincase of D, Erlikosaurus andrewsi (IGM 100/111) and E, Nothronychus mckinleyi (AZMNH-2117) in left lateral view, revealing pneumatic cavities within the basisphenoid. Abbreviations: bsp, basisphenoid; bsr, basisphenoid recess; cup, cultriform process.
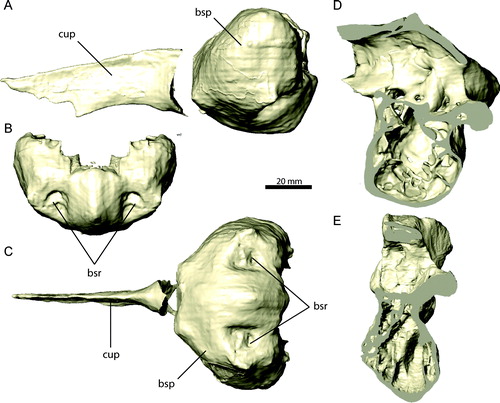
FIGURE 13. Individual braincase elements of Erlikosaurus andrewsi (IGM 100/111). Right prootic (in articulation with exoccipital) in A, lateral and B, medial views. Right laterosphenoid in C, rostral and D, lateral views. Right orbitosphenoid in E, rostral and F, lateral views. Abbreviations: a.bsp, basisphenoid articulation; a.ls, laterosphenoid articulation; a.osp, orbitosphenoid articulation; a.pa, parietal articulation; a.po, postorbital articulation; exoc, exoccipital; fc, fenestra cochleae; ff, floccular fossa; fv, fenestra vestibuli; pop, postorbital process; pro, prootic; rr, rostral horizontal ridge; V1, foramen for ophthalmic branch of the trigeminal nerve; V2, foramen for maxillary branch of the trigeminal nerve; V3, foramen for mandibular branch of the trigeminal nerve; VI, foramen for the abducens nerve; VII, foramen for the facial nerve; VIII, foramen for the vestibulocochlear nerve.
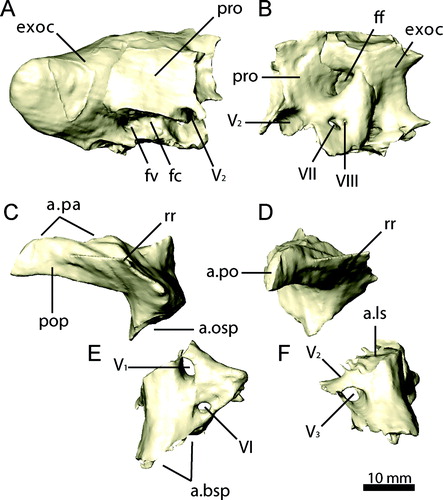
FIGURE 14. Palatal complex of Erlikosaurus andrewsi (IGM 100/111) in A, dorsal, B, left lateral, and C, ventral views. Abbreviations: cp, cultriform process; ect, ectopterygoid; pal, palatine; pty, pterygoid; spf, subsidiary palatal fenestra; vo, vomer.
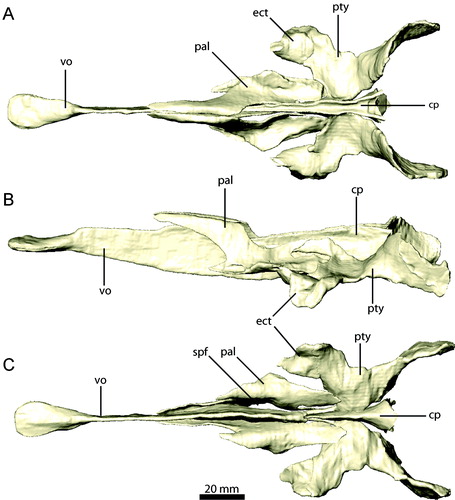
FIGURE 15. Palatal elements of Erlikosaurus andrewsi (IGM 100/111). Vomer in A, left lateral and B, dorsal views. Right palatine in C, dorsal and D, lateral views. Right pterygoid and ectopterygoid in E, dorsal and F, lateral views. Abbreviations: a.bsp, basisphenoid articulation; a.cp/vpar, cultriform process and vomeropalatine ramus of the pterygoid articulation; a.ect, articulation with ectopterygoid; a.j, jugal articulation; a.max, maxilla articulation; a.pmx/mx, premaxilla/maxilla articulation; a.pty, pterygoid articulation; a.q, quadrate articulation; a.vpal, vomeropterygoid ramus of the palatine articulation; ect, ectopterygoid; ectr, ectropterygoid ramus; papr, palatine pneumatic recess; ppp, pterygoid process of palatine; qr, quadrate ramus; spf, subsidiary palatal fenestra; tf, transverse flange; vpal, vomeropterygoid ramus; vpar, vomeropalatine ramus.
FIGURE 16. Sclerotic elements of Erlikosaurus andrewsi (IGM 100/111). A, articulated sclerotic plates as preserved (six of seven preserved elements shown). Isolated, single sclerotic plate in B, lateral and C, medial views. D, fully reconstructed sclerotic ring.
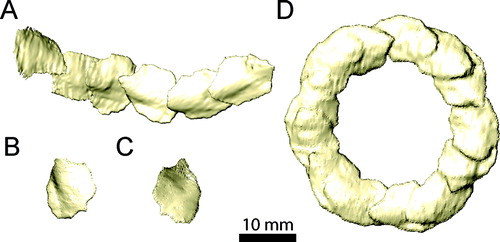
FIGURE 17. Left mandible of Erlikosaurus andrewsi (IGM 100/111) in A, lateral, B, medial, and C, dorsal views. Abbreviations: ang, angular; ar, articular; d, dentary; emf, external mandibular fenestra; maf, mandibular fossa; pra, prearticular; sp, splenial; sur, surangular.
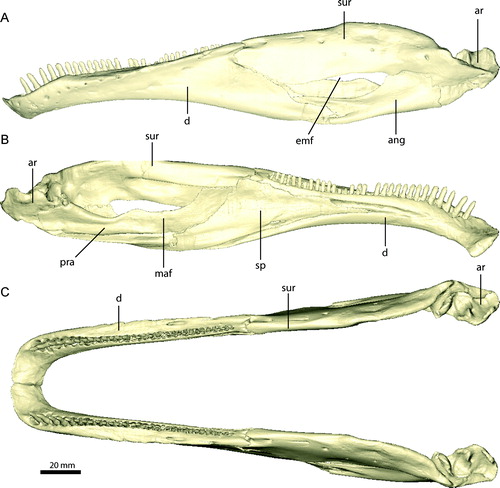
FIGURE 18. Left dentary of Erlikosaurus andrewsi (IGM 100/111) in A, C, lateral, and B, D, medial views. Right dentaries of Falcarius utahensis in medial view: E, UMNH VP 14527, F, UMNH VP 14529, G, UMNH VP 14528. Bone in C–G rendered transparent to visualise teeth (in blue), replacement teeth (in red), and neurovascular structures (in yellow). Abbreviations: a.pra, prearticular articulation; a.sur, surangular articulation; fo, neurovascular foramina; lsh, lateral shelf; mf, Meckelian fossa; sf, symphyseal facet.
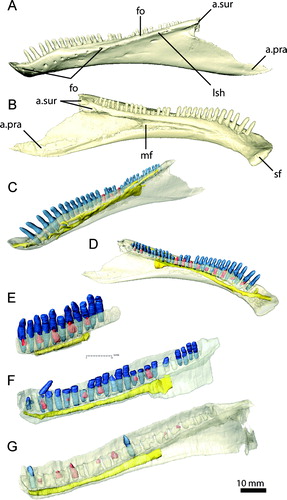
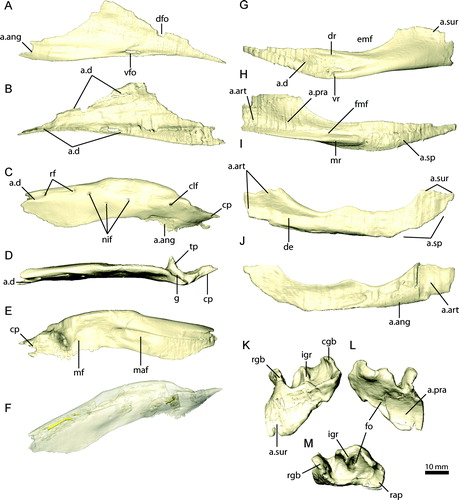
FIGURE 19. Mandibular elements of Erlikosaurus andrewsi (IGM 100/111). Left splenial in A, medial and B, lateral views. Left surangular in C, D, lateral, E, dorsal, and F, medial views. Left angular in G, lateral and H, medial views. Left prearticular in I, medial and J, lateral views. Left articular in K, lateral, L, medial, and M, dorsal views. Bone in F rendered transparent to visualize internal neurovascular structures (in yellow). Abbreviations: a.ang, angular articulation; a.art, articular articulation; a.d, dentary articulation; a.pra, prearticular articulation; a.sp, splenial articulation; a.sur, surangular articulation; cgb, caudal glenoid buttress; clf, caudolateral foramen; cp, caudal process; de, elongated depression; dfo, dorsal foramen; dr, dorsal ridge; emf, external mandibular fenestra; fmf, floor of mandibular fossa; fo, foramen for the chorda tympani; g, groove; igr, interglenoid ridge; maf, mandibular adductor fossa; mf, medial foramen; mr, medial ridge; nif, noninvasive foramina; rap, retroarticular process; rf, rostral foramina; rgb, rostral glenoid buttress tp, transverse process; vfo, ventral foramen; vr, ventral ridge.
Holotype
IGM 100/111, skull and mandible, several disarticulated and fragmentary cervical vertebrae, left humerus, and articulated right pes missing the proximal ends of two metatarsals (Clark et al., Citation1994).
Type Locality and Horizon
Baishin Tsav locality (= Baynshin, Bayshin, or Bainshin, Tsav; Clark et al., Citation1994), Southeast Mongolia, Bayanshiree Svita (Barsbold and Perle, Citation1980; Perle, Citation1981), Upper Cretaceous, Cenomanian–Turonian (Shuvalov, Citation2000).
Emended Diagnosis
Erlikosaurus andrewsi differs from other therizinosaurians by the following autapomorphies: antorbital fossa lateromedially expanded, containing three pneumatic pockets rostroventral to the maxillary fenestra; antorbital fossa with well-developed overhanging lip and a caudally located and reduced maxillary fenestra; large mediolaterally expanded caudal process of the maxilla; transversely inflated and pneumatized main body of lacrimal; supraoccipital with prominent nuchal crest; quadrate with accessory third condyle and oblique orientation of mandibular capitulum; 31 dentary teeth.
Remarks
As noted by Clark et al. (Citation1994) and Zanno (Citation2010b), the lack of comparative cranial material makes a clear differentiation and definition between autapomorphic, synapomorphic, and plesiomorphic features difficult. The following characters, noted as possible autapomorphies in the original diagnosis by Clark et al. (Citation1994), were removed by Zanno (Citation2010b), because they are now known to have a wider distribution amongst Maniraptoriformes and/or Therizinosauria: edentulous premaxilla with sharp vertical ventrolateral edge; vomer extremely elongate and extending caudally to meet cultriform process; maxilla with medially inset dentition and few nutrient foramina on caudal part of facial process; homodont maxillary dentition of numerous small, lanceolate, coarsely serrated, unrecurved, mediolaterally flattened teeth constricted at the base; extremely elongated nares reaching the level of the antorbital fossa caudally; external auditory meatus restricted ventrally by lateral expansion of braincase; trigeminal opening divides into three branches within side wall of braincase; symphyseal region of dentary ventrally deflected; lateral shelf on dentary. The remaining potential autapomorphy (passage for internal carotid artery enclosed on occiput) noted by Clark et al. (Citation1994) could not be confirmed as an autapomorphy in the reevaluation of the material and is excluded here.
DESCRIPTION
Premaxilla
The premaxilla () consists of an edentulous main body and an elongate narial process, whereas the maxillary process is short and strongly reduced. The main body is laterally expanded and convex. It can be divided into a horizontally oriented dorsomedial part, forming the floor of the narial chamber, and a vertically oriented part, forming the lateral and rostral surface of the main body. The latter part is rostrally flattened, giving the snout a blunt shape. The bone is transversely thin and tapers to a sharp ridge at its ventral margin. The dorsomedial part of the main body extends medially and partly dorsally, but does not meet the opposite premaxilla. It encloses a large incisive foramen between both elements, which perforates the ventral and dorsal surfaces. The medial margin of the premaxilla is connected rostrally to the base of the narial process by a thin, vertical lamina of bone (), supporting a cartilaginous internasal septum in life (Sampson and Witmer, Citation2007). The floor of the narial chamber is deeply excavated and forms a prominent fossa, which is clearly separated from the incisive foramen. The presence of similarly developed fossae in a therizinosauroid embryo referred to Neimongosaurus or Erliansaurus (Kundrat et al., Citation2008), and the basal taxon Jianchangosaurus yixianensis (Pu et al., Citation2013) suggests an ontogenetically early development and more widespread distribution of this feature amongst Therizinosauria.
On the rostrolateral surface, the main body bears nine prominent foramina, arranged in a semicircle and following the lateroventral outline of the premaxilla. They are of equal size (approximately 2 mm diameter). The rostral five foramina open into an elongated depression on the surface, projecting towards the ventral and medial margins. Three smaller, ancillary foramina are positioned dorsal to the main ones. The number and position of the individual foramina is similar to the condition found in a therizinosauroid embryo (Kundrat et al., Citation2008) and Jianchangosaurus (Pu et al., Citation2013). Three large foramina are present on the dorsomedial surface of the main portion of the premaxilla (). The ventral surface of the premaxilla bears five large foramina clustered around the incisive foramen (). They all face medially, with the exception of the caudal-most, which faces more ventrally. A smaller, additional foramen is located on the medioventral surface, closer to the ventral margin. The individual foramina on the lateral and medial sides are connected by an extensive meshwork of vascular canals, which pervades the whole main body of the premaxilla () and is most likely associated with the sensory branches of the ophthalmic nerve and neurovasculature supplying the rhamphotheca. Only the rostral-most foramen on the ventral surface ends blind and does not connect to the other foramina. The vascular network appears to exit the premaxilla caudally into the space of the incisive foramen between the premaxilla, maxilla, and vomer. An elongate canal is visible in the CT images, although a possible foramen is obscured by poor preservation in that region.
The premaxilla is not fused to its counterpart, but both elements are firmly joined along a thin, flattened articular surface on the rostral-most part of the main body and along the entire length of the narial process up to the premaxilla/nasal contact (). The narial process is nearly twice as long as the premaxillary main body. It extends subvertically from the main body before it slopes caudally to become subhorizontal. The narial process is semicircular in cross-section rostrally, where it attaches to the opposing premaxilla, and gradually becomes more circular caudally. At the contact with the nasal, the narial process tapers to a flat sheet of bone overlying the nasal dorsomedially, forming a flat internarial bar as in most troodontids and ornithomimids (Bever and Norell, Citation2009).
The maxillary process of the premaxilla is considerably reduced in size compared with the narial process. It projects only a little beyond the premaxilla's main body and onto the maxilla. CT scans confirm that the maxillary process does not contact the nasal, as also described by Clark et al. (Citation1994). Thus, the maxilla is not excluded in the formation of the external naris as in ornithomimids (e.g., Kobayashi and Lü, Citation2003; Kobayashi and Barsbold, Citation2005a). The premaxilla/maxilla suture curves caudodorsally, and the premaxilla only marginally overlaps the maxilla, which results in a comparably weak articulation between both elements. Clark et al. (Citation1994) discussed the possible presence of a small subnarial foramen between both elements, indicated by a matrix-filled opening on the left side, but reached no firm conclusion. The CT scans do not show clear evidence for the presence or absence of such a foramen, but the superimposition of the left and right premaxillae produces a solid surface. Subnarial foramina have not been described or illustrated in other therizinosaurians (Kundrat et al., Citation2008; Pu et al., Citation2013), although a small recess in the caudal margin of the disarticulated premaxilla in Jianchangosaurus (Pu et al., Citation2013) might indicate such a foramen.
Maxilla
The maxilla (, , ) has an elongate, triangular outline in lateral view and forms most of the lateral side of the snout, contacting the premaxilla rostrally, the nasal dorsally, and the lacrimal, jugal, and palatine caudally. The rostrodorsal margin, which forms the caudoventral border of the external naris, tapers to a sharp edge, separating the convex lateral surface and the more concave medial/palatal surface of the maxilla. The contribution of the maxilla to the external naris deviates from the plesiomorphic condition for theropods. As a result, the external naris is considerably elongated, reaching the level of the antorbital fossa caudally, and is dorsoventrally narrow. The lateral surface of the maxilla is rounded and bears a series of 10 foramina that are clustered around the rostrolateral portion of the maxilla. They are smaller (approximately 1 mm diameter) than those on the premaxilla, and CT scans show that they do not form an extensive network, but connect to the alveolar cavities or merge into the bone. An additional, larger (approximately 5 mm diameter) foramen is located ventral to the antorbital fossa and dorsal to the 13th tooth position (). It was described as an external maxillary fenestra by Clark et al. (Citation1994). However, CT scans show that the foramen is connected internally to the palatal region and was therefore likely to have transmitted the nasopalatine nerve (which corresponds to parts of the maxillary branch of the trigeminal nerve). Rostrally, the ventral edge of the maxilla is transversely thin and continuous with the ventral edge of the premaxilla. Approximately 15 mm caudal to the premaxilla/maxilla suture, the ventral margin becomes wider to accommodate the first alveolus. Altogether, there are 24 alveoli in the maxilla. From the 16th tooth position caudally, the orientation of the alveoli gradually changes from vertical to laterally inclined. In the caudal-most teeth, this inclination is at 45° to the vertical. This pattern is found on both sides of the original specimen, giving the caudal teeth a splayed appearance. Clark et al. (Citation1994) suggested that this may be due to distortion, because the occluding/opposing teeth on the dentary do not show a similar orientation. However, no signs of deformation (such as small cracks or gaps) are either visible on the bone surface (Clark et al., Citation1994) or in the CT scans. The lateral and medial margins of the maxilla are continuous in this region, which would not be expected had the element been twisted to result in the ‘splayed’ orientation. It is not clear if these teeth were still functional and actively used in food processing or whether they represent a pathological condition or a vestigial state due to a change of dietary habits among therizinosaurians. Given the lack of contradicting information, the maxilla was restored with the ‘splayed’ tooth orientation.
The ventromedial surface of the maxilla is rostrally concave and separated from the dorsal portion and the ascending process by a short palatal process. The latter extends only a little medially to meet the expanded tip of the vomer. Combined, the palatal processes of the opposing maxillae form the rostral margins of the choanae. Caudally, the medial surface of the maxilla becomes less convex and forms a flat wall, which stands at an angle of approximately 45° to the alveolar margin. At the last alveolus, it merges into the horizontally inclined caudal process of the maxilla.
The antorbital fossa is large and deeply excavates the main body of the maxilla caudally and the ascending process ventrally. A thin osseous wall forms the medial surface of the antorbital fossa. The caudal margin of this wall is gently curved and connects the ascending process of the maxilla with the dorsal surface of the caudal process. Medial to its junction with the caudal process of the maxilla, the wall of the antorbital fossa is pierced by an elongate foramen (). The CT images show that this foramen is connected internally to the large nasopalatine foramen on the lateral surface above the 13th tooth position. The floor of the antorbital fossa is subdivided into three depressions or pneumatic spaces, as noted by Clark et al. (Citation1994) (). The caudal-most of these is located directly ventral to the small maxillary fenestra and is partly exposed on the left maxilla, but covered by matrix on the right side and is only visible in the CT scans. The superposition of the respective elements reveals its full outline. The fenestra is oval and approximately 7 mm wide and 3 mm high. The medial wall of the antorbital fossa is otherwise unperforated. In comparison with other maniraptoriformes, the maxillary fenestra is small and located more caudally. A promaxillary fenestra, as found in troodontids (Xu et al., Citation2002b), dromaeosaurids (Norell et al., Citation2006), and other theropods (Witmer, Citation1997), is absent in E. andrewsi. A small promaxillary fenestra has been described for a therizinosauroid embryo (Kundrat et al., Citation2008), but is absent in the basal therizinosaurian Jianchangosaurus (Pu et al., Citation2013). In contrast to E. andrewsi, the latter taxon was reconstructed with a large maxillary fenestra, approximately half the size of the antorbital fenestra. However, the structure forming the caudal border of the maxillary fenestra described by Pu et al. (Citation2013) as being part of the maxilla appears actually to be the right lacrimal, as indicated by a suture connecting both elements (J. Clark, pers. comm., 2013). A similarly deep excavation of the antorbital fossa as in E. andrewsi is indicated in Jianchangosaurus, but its full extent cannot be quantified due to the compaction of the specimen.
The ascending process projects at an angle of approximately 35° from the main portion of the maxilla, from which it is only slightly offset. The dorsal margin tapers to a thin, vertical edge, which contacts the descending process of the nasal dorsally. Medial to the maxilla/nasal suture, the ascending process of the maxilla has a convex, dorsal surface, which is overlain by the concave ventral surface of the lacrimal. Ventrally, the ascending process is deeply excavated, forming the roof of the antorbital fossa. As in ornithomimids (Makovicky et al., Citation2004) and troodontids (Makovicky and Norell, Citation2004), the nasal is excluded from the antorbital fenestra and fossa by the maxilla/lacrimal contact. In a reconstruction of the basal therizinosaurian Jianchangosaurus, the nasal contributes to the dorsal border of the antorbital fenestra (Pu et al., Citation2013). However, Pu et al. (Citation2013) apparently attributed parts of the right frontal to the nasal, which results in an extensive ventral process of the nasal in their reconstruction (J. Clark, pers. comm., 2013). Hence, the contribution of the nasal to the antorbital fenestra could be less extensive than suggested by these authors.
The caudal process of the maxilla forms a broad and dorsoventrally flat shelf, which tapers caudolaterally. Its dorsal surface is continuous with the floor of the antorbital fossa. The caudal-most part is overlain by the rostral or maxillary process of the jugal in a simple lap joint suture. Directly rostral to the maxilla/jugal joint, the ventral process of the lacrimal contacts the dorsal surface of the maxilla. It appears that both the jugal and the lacrimal are attached only weakly to the maxilla at this point. Along the caudomedial margin, the palatine attaches to the caudal process.
The maxillary teeth are homodont throughout the tooth row, with a lanceolate outline in lateral aspect and a slight constriction dorsal to the crown (Clark et al., Citation1994). The roots are elongate and circular in cross-section. The length and crown expansion is equal throughout the tooth row, with insignificant variation between teeth. The labial and lingual crown surfaces are weakly convex and do not show the pronounced concavity and crown asymmetry illustrated for Jianchangosaurus (Pu et al., Citation2013). About one-third of the teeth are exposed for half of their length, whereas the rest appear to be partially erupted. This results in an uneven outline for the tooth row. CT scans show only a few replacement teeth (), with five on the left and eight on the more complete right side, which accounts for one replacement tooth for every third erupted tooth on average. As noted by Clark et al. (Citation1994), the teeth gradually change their orientation from vertical rostrally to laterally inclined from the 16th tooth position caudally. Wear facets are not visible on the specimen (Clark et al., Citation1994) or in CT scans. The scans, however, may not provide high enough resolution to identify microwear patterns. Wear surfaces are generally absent from therizinosaurian teeth (Russell and Dong, Citation1993; Clark et al., Citation1994; Zanno, Citation2010a), with the exception of Segnosaurus galbinensis, for which weakly developed wear facets have been reported on the distal edges of some dentary teeth (Clark et al., Citation2004).
CT scans of a fragmentary maxilla of Falcarius utahensis (UMNH VP 14565) (Zanno, Citation2010a) show that the pattern of tooth eruption is more consistent than in E. andrewsi, not including several broken teeth. Seven replacement teeth () are present in the maxilla fragment, with one replacement tooth for every other erupted tooth on average. Except for pronounced difference in size (Zanno, Citation2010a), the maxillary teeth of Falcarius do not show considerable morphology heterodonty.
Nasal
The nasal (, , ) is elongate, transversely vaulted, and forms the long rostrum of E. andrewsi. It abuts its counterpart medially along most of its length, with the exception of the caudal-most part, where both nasals diverge from each other to contact the frontals. The frontal/nasal contact is located at the level of the preorbital bar. The nasal tapers rostrally and bears an elongated depression dorsomedially, which accommodates the nasal process of the premaxilla. The maxillary process is strongly reduced and projects only a little ventrally from the main portion of the nasal. It is lateroventrally notched to receive the ascending process of the maxilla. The lateroventral margin rostral to the maxillary process forms the dorsal border of the external naris.
Caudal to the nasal/maxilla suture, the nasal overlies the dorsal surface of the lacrimal. Dorsolaterally, a series of six small (1 mm) foramina pit the surface of the nasal (), similar to most ornithomimids (Russell, Citation1972; Makovicky et al., Citation2004; Kobayashi and Barsbold, Citation2005a) and troodontids (Makovicky et al., Citation2003; Bever and Norell, Citation2009), but absent in Falcarius utahensis (UMNH VP 16022, Zanno, Citation2010a), whereas Jianchangosaurus yixianensis (Pu et al., Citation2013) shows only one small foramen near the nasal/maxilla contact.
Ventrally, the nasal is concave, roofing the nasal passage. When articulated, both nasals form a pronounced, ventral ridge along the articular contact, which served as an attachment site for the internasal septum. A corresponding structure is present on the medial surface of the premaxilla, which indicates that the nasal passage was separated by the internasal septum for most of its length.
Pu et al. (Citation2013) reconstructed a large nasal due to a probably erroneous identification of the right frontal, which is still semiarticulated and partly thrust onto the nasal resulting from the shearing of the right side of the specimen. Consequently, the nasal of Jianchangosaurus is very similar to that of Erlikosaurus, with a reduced maxillary process.
Lacrimal
The lacrimal (, , ) has an inverted ‘L’-shaped outline as in ornithomimosaurs (Makovicky et al., Citation2004) and tyrannosaurs, which is unlike the ‘T’-shaped morphology found in paravians. The rostral and ventral processes are elongate, whereas the caudal process is reduced to a short and mediolaterally thin lamina of bone. The main body and the rostral process are transversely expanded, prominently inflated, and hollow. The dorsal surface is convex and gently rounded. The ventral and caudoventral surfaces, forming the dorsal and caudal walls of the antorbital fossa/fenestra, are deeply excavated and concave (). The rostral process overlies the convex dorsomedial surface of the ascending process of the maxilla dorsally and medially. The lateral and medial margins of the rostral process are ventrally extended and continuous with the ascending process of the maxilla and the antorbital wall, respectively. The lateral rim curves caudally to become vertical as part of the ventral process.
The ventral process (forming the preorbital bar) is nearly vertical and transversely flattened. The lateral surface is marked by a vertically elongated, bulging ridge, giving the ventral process an approximately triradiate cross-section. The ridge starts dorsally at the base of the main portion of the lacrimal and gradually merges with the ventral process distally. Unlike in other maniraptorans (Makovicky and Norell, Citation2004; Makovicky et al., Citation2004), the lacrimal does not contact the jugal, as indicated by the CT scans. Medially, the ventral process is slightly concave and separated from the caudal process and the main body by a thin lamina of bone. The latter forms part of the caudoventral margin of the antorbital wall medially.
The small and abbreviated caudal process does not extend beyond the level of the ventral process caudally and projects only slightly medially. Dorsally, it inserts into a pit on the ventral surface of the prefrontal. As noted by Clark et al. (Citation1994), a lacrimal foramen (2–3 mm in diameter) opens into the orbital region caudally (). It is located at the base of the caudal process and bounded by the vertical ridge on the ventral process laterally. The foramen connects to the large cavity within the main portion of the lacrimal () and opens into the nasopharyngeal cavity via a large circular aperture in the rostral process. Unlike in other theropods (Witmer, Citation1997; Sampson and Witmer, Citation2007), the internal space is undivided in E. andrewsi, without clear differentiation between the pneumatic recess and the nasolacrimal canal.
A left lacrimal preserved with a therizinosauroid embryo (Kundrat et al., Citation2008) shows a similar, though slightly more ‘T’-shaped, morphology to E. andrewsi. The rostral and ventral processes are elongated and pronounced, but unlike in E. andrewsi, the embryonic lacrimal appears to lack the pneumatic inflation of the main body and the rostral excavation contributing to the antorbital fossa. In Jianchangosaurus yixianensis, the lacrimal lacks the inflated main body, which is apparent in the right lacrimal, erroneously identified as the caudal border of the maxillary fenestra. A suture between the lacrimal and the maxilla is visible between both elements. The element identified as the right lacrimal by Pu et al. (Citation2013) appears to be the right postorbital. Therefore, in comparison with E. andrewsi, the lacrimal of Jianchangosaurus yixianensis has a more hourglass-shaped morphology resulting from a short rostral process and a rostrocaudally inflated ventral process (Pu et al., Citation2013). This morphology, however, might have been exaggerated by compaction of the specimen.
Prefrontal
The prefrontal (, , ) forms a distinct element in E. andrewsi, which can be clearly distinguished from the frontal and lacrimal. It is only loosely attached to the latter and not coossified as in troodontids (Makovicky and Norell, Citation2004) or some dromaeosaurids (Norell et al., Citation2006). The wedge-shaped prefrontal of E. andrewsi is dorsoventrally flat and bears no ventral process, in contrast to the condition found in ornithomimosaurs (Makovicky et al., Citation2003). The rostral and caudal processes are reduced. The rostral process contacts the nasal for a short distance dorsally. The dorsomedial surface of the prefrontal bears a large facet for the reception of the frontal along most of its length. The dorsolateral surface is smooth and tapers to a distinct spur laterally. The ventral surface is divided into a caudolateral and a rostromedial part by a distinct, ventral ridge (). Rostral to this ridge, the prefrontal bears an elongated pit, which serves as an attachment site for the caudal process of the lacrimal. The caudolateral surface is rounded and forms part of the rostroventral margin of the orbit.
A left prefrontal identified by Pu et al. (Citation2013) in Jianchangosaurus yixianensis might be the left postorbital, because it would be unusually large for a prefrontal. It is more similar to the right postorbital, which was identified as right lacrimal by Pu et al. (Citation2013).
Jugal
The jugal (, , ) is a triradiate element consisting of an elongate rostral maxillary process, a caudodorsally projecting postorbital process, and a short caudal quadratojugal process. The main portion of the jugal is transversely thin and flattened. Surface structures, such as ridges or depressions indicative of a jugal diverticulum, are absent. No foramina indicative of internal pneumatization are present, in contrast to other derived theropods (Witmer, Citation1997; Norell et al., Citation2006, Citation2009). The ventral margin is slightly curved and bears a flat depression ventromedially, possibly for the origin of m. pterygoideus dorsalis (m. PTd) (Lautenschlager, Citation2013). Clark et al. (Citation1994) considered an attachment for m. adductor mandibulae externus dorsal to it on the medial surface. However, based on the muscle topology, such an arrangement would be in conflict with the other adductor muscles of E. andrewsi (Lautenschlager, Citation2013) and unknown in theropod dinosaurs in general (Holliday, Citation2009).
The rostral process projects rostrally from the main body of the jugal with a slight dorsal curvature. It is equal in length to the main body, unlike in Jianchangosaurus yixianensis, in which the rostral process is nearly twice the length of the main body (Pu et al., Citation2013). The dorsal surface of the rostral process of E. andrewsi is rounded and forms the major part of the ventral border of the orbit. Distally, the rostral process rotates into a horizontally expanded and dorsoventrally flattened shelf overlying the caudal process of the maxilla. Its rostral-most part tapers laterally to accommodate the ventral process of the lacrimal medially. In their description, Clark et al. (Citation1994:11–12) made contradictory statements regarding a medial extension of the rostral process contacting the palatine. Such a contact cannot be confirmed on the specimen or in the CT scans.
The dorsal process has a triangular outline in lateral aspect and projects at an angle of approximately 45° dorsally and caudally, terminating at orbital mid-height. Medially, it has a shallow depression bounded by the sharp medial and caudal margins of the process. Rostrally, the dorsal process bears an extensive facet for the reception for the postorbital along most of its length. The facet is marked by a prominent, elongated ridge that fits into a groove on the ventral process of the postorbital, establishing a firm contact between both elements.
The caudal process is dorsoventrally expanded and bifurcated. The ventral portion bears an elongate, trough-like groove for the reception of the quadratojugal, whereas the dorsal portion of the process is curved, marking the ventral margin of the infratemporal fenestra. It approaches the quadratojugal to form a continuous, transversely thin lamina.
Frontal
The frontal of E. andrewsi (, , ) is triangular to wedge-shaped. Rostrally, the frontal is narrow and widens considerably caudally towards the postorbital process. The frontals are firmly attached to each other along the medial margin by a tongue and groove suture. The individual counterparts are vaulted dorsomedially, which results in a single, domed dorsal surface, unlike the condition in Falcarius utahensis, which shows a flattened skull roof between the dorsally raised orbital margins (UMNH VP 14524, 12525; Zanno, Citation2010a). The pronounced midline depression seen in some ornithomimids (Osmólska et al., Citation1972; Kobayashi and Lü, Citation2003) is absent in E. andrewsi. Lateral to the vaulted medial portion, the frontal bears a shallow and rostrocaudally elongated depression. The dorsal surface is smooth. The lateral margin forms most of the dorsal and dorsocaudal borders of the orbit and is continuous with the respective margins on the prefrontal rostrally and the postorbital caudally. In lateral aspect, it is dorsally convex, outlining the large circular orbits. In a right frontal of a therizinosauroid embryo (Kundrat et al., Citation2008), the lateroventral surface of the orbital margin bears a line of neurovascular foramina, which are absent in E. andrewsi, Falcarius utahensis (Zanno, Citation2010a), and Jianchangosaurus yixianensis (Pu et al., Citation2013).
Rostrally, the frontals taper to contact the nasals. The nasals underlie the latter along the medial margins for a short distance. Caudally, the frontals abut the parietal in an extensive suture, which extends along the entire width of the caudal margin of the frontals up to the postorbital process. The parietal is only insignificantly raised above the frontals, forming a confluent dorsal surface of the skull table. The rostral wall of the supratemporal fenestra formed by the caudolateral portion of the frontal is steeply inclined, with a poorly developed ridge. A lateral process projecting from the parietal and the postorbital contacts the frontal along the lateral portion of the caudal margin and along the postorbital process. A small portion of the frontal also contacts the laterosphenoid below the postorbital/parietal suture.
The ventral surface of the frontals is dominated by the crista cranii, which separate the orbital and the endocranial cavities (). In comparison with Falcarius utahensis (Zanno, Citation2010a), the cristae cranii are oriented more horizontally in E. andrewsi. They gradually merge with the ventral surface of the frontal rostrally, which probably prompted Clark et al. (Citation1994) to describe the crista cranii as poorly developed. The orbital cavity is a large concave surface, which extends over the major part of the frontal lateral to the crista cranii. Rostrally, the frontal bears a large, deeply excavated facet for the attachment of the prefrontal, indicating a firm contact between both elements. The crista cranii envelop the rostral part of the endocranial cavity medially. However, the dimensions of the endocranial space bordered by the crista cranii are nearly equal in both taxa. This indicates that the (lateral) extension of the cerebral hemispheres and the olfactory apparatus is similar in both taxa despite the apparently narrower skull of Falcarius utahensis. As a result, the extended endocranial space is compensated by a transversely compressed orbital cavity in the latter taxon.
The impressions of the cerebral hemispheres, olfactory tracts, and olfactory bulbs are clearly demarcated on the ventromedial surface of both frontals of E. andrewsi (). The impressions of the olfactory bulbs are separated from the nasal passage rostrally by a raised, transversely oriented swelling.
Parietal
The parietal (, , ) consists of a single, fused element. CT scans indicate the absence of a suture, but a series of breaks pervade the bone obscuring finer details. Parietal fusion occurs in various basal theropods (Coria and Currie, Citation2002; Brusatte and Sereno, Citation2007), oviraptorosaurs (Clark et al., Citation2002; Balanoff et al., Citation2009), and troodontids (Makovicky and Norell, Citation2004). The main body of the parietal, forming a large part of the caudal skull roof, is approximately horizontal and dorsoventrally flat. Rostrally, it is slightly raised to meet the frontals. The rostral margin of the parietal has an overlapping suture with the frontal. The ventral surface of the parietal is prominently vaulted and concave, forming the rostral extent of the cerebellar prominence.
Two processes project laterally from each side of the parietal, a rostral postorbital and a caudal squamosal process. The postorbital process is inclined ventrolaterally and partly rostrally. It overlies the laterosphenoid, and together both elements contact the postorbital distally. The squamosal process of the parietal is rostrocaudally enlarged and contacts the exoccipital and paroccipital process ventrally. It bifurcates distally to meet the parietal process of the squamosal. A sharp lambdoidal crest runs along the dorsal surface of the squamosal process (). It is confluent with a crest on the squamosal and delimits the caudal border of the adductor chamber. The lambdoidal crest merges with the main body of the parietal, where it splits up into two inconspicuous ridges directed medially and rostrally. The latter marks the lateral extent of the dorsal surface of the parietal. Lateral to it, the parietal is inclined nearly vertically, forming the medial wall of the adductor chamber and the attachment area for the lateral jaw adductor musculature. A vertical ridge on the base of the postorbital process distinctly separates the muscle origin sites of m. adductor mandibulae externus profundus (m. AMEP) and m. pseudotemporalis superficialis (m. PSTs) () (Lautenschlager, Citation2013). Caudally, the squamosal processes of the parietal extend beyond the main portion and envelop the supraoccipital.
Postorbital
The postorbital (, , ) is a triradiate bone, with a rostral frontal process, a caudal squamosal process, and a ventral jugal process. The frontal process is transversely expanded and curves dorsally from the main body of the postorbital as in other maniraptorans (Currie, Citation1985; Norell et al., Citation2006, Citation2009). Medially, it splits into a main process and smaller accessory medial projection. The main process bears a large, concave facet rostrally that contacts the frontal in an overlapping suture. The medially located accessory process tapers to a point and fits into a groove on the postorbital process of the parietal, forming part of the rostral wall of the supratemporal fenestra. The squamosal process is transversely thin and spike-like with a triangular outline in lateral view, with slightly concave lateral and medial surfaces. It is connected to the frontal process by a thin lamina, which forms part of the ridge-like lateral wall of the supratemporal fenestra. The dorsal and ventral margins of the squamosal process are continuous with the postorbital process of the squamosal. It attaches to the latter by inserting into a longitudinal groove on the lateral surface of the squamosal. The caudoventral margin of the squamosal process tapers to a sharp ridge, which is confluent with a similar structure on the postorbital process of the jugal.
The jugal process of the postorbital is rostrally inclined and meets the postorbital process of the jugal in an extensive rostroventral-caudodorsal suture. Dorsally, the rostral surface of the postorbital expands in width and becomes more concave as part of the dorsocaudal corner of the orbital cavity. A medial ridge continues from the suture with the jugal and gradually merges into the frontal process of the postorbital. It bears an ovoid facet for the reception of the laterally projecting process of the laterosphenoid (), similar to the condition found in a fragmentary postorbital of Falcarius utahensis (UMNH VP 14567; Zanno, Citation2010a). As noted by Kundrat et al. (Citation2008), the postorbital of E. andrewsi is nearly identical to that of a therizinosauroid embryo referred to either Neimongosaurus or Erliansaurus. It is also similar to the postorbitals of Jianchangosaurus yixianensis, which were identified originally as the right lacrimal and the left prefrontal by Pu et al. (Citation2013).
Squamosal
The squamosal (, , ) consists of four processes, a rostrally directed postorbital process, a caudal process, a ventrally projecting quadrate process, and a medial parietal process. The postorbital process is transversely flattened and medially convex. On its lateral surface, it bears a rostrocaudally elongated, deep socket for the reception of the squamosal process of the postorbital. The thin and sharp dorsal edge of the latter continues onto the postorbital process of the squamosal, forming the dorsal margin of the supratemporal bar. Ventral to the postorbital/squamosal contact, a horizontal ridge extends for the full length of the lateral surface of the postorbital process. It continues onto the main portion and the caudal process, gradually merging with the latter caudally. The ridge separates the postorbital process from the quadrate process. The latter is rostroventrally inclined and tapers to a point distally. It extends down to mid-height of the infratemporal fenestra ventrally, but does not contact the quadratojugal. Its caudal surface is concave, whereas the rostral surface bears an elongated, median ridge or lamina, giving the process a triangular to triradiate cross-section. The ridge curves prominently rostrodorsally and joins the postorbital process, forming the caudal and partly the dorsal border of the infratemporal fenestra. At the caudodorsal corner of the infratemporal fenestra, the squamosal is markedly concave at the junction between the postorbital and quadrate process. Along the caudal surface, the quadrate process overlies the rostral surface of the quadrate for its entire length, forming a simple lap joint. The pronounced quadrate cotyle typically found in other theropods is absent in E. andrewsi. The medial margin of the process is curved caudally and dorsally and joins with the base of the caudal process.
The caudal process of the squamosal is laterally convex and medially concave, which results in a lunate or ‘L’-shaped cross-section. It overlies the head of the quadrate and the lateral-most extent of the paroccipital process. The caudal process extends medially and merges with the parietal process. As a result, the medial surfaces of the caudal process and the main portion of the squamosal are markedly concave. This concavity is constricted dorsoventrally by the parietal and quadrate process.
The parietal process is dorsally raised and, compared with the other processes of the squamosal, prominently inflated and stout. It tapers medially and bifurcates, contacting the squamosal process of the parietal in an interdigitate suture. A distinct dorsal ridge, connecting the postorbital process of the squamosal rostrally, is present on the dorsal surface. It is confluent with the lambdoidal crest of the parietal, separating the caudal surface of the supratemporal fenestra from the occipital region.
Quadratojugal
The quadratojugal (, , ) is triangular in lateral view, with three processes: a rostral jugal process, a short and blunt caudal process, and an ascending dorsal process. Transversely, the quadratojugal is thin and flattened. The lateral and medial surfaces are smooth. The jugal process is spike-like and inserts into a trough-like facet on the jugal. It is enclosed by the bifurcating process of the latter ventrally, but not entirely dorsally. The dorsal portion of the quadratojugal process of the jugal tapers to a thin edge and approximates the rostral margin of the quadratojugal. The latter is nearly continuous with the jugal, forming the caudoventral border of the infratemporal fenestra.
The dorsal process is caudally inclined and contacts the lateral flange of the quadrate. The caudal process is rounded and slightly inclined medially. It overlies the condyle of the quadrate laterally. The caudal edge, contacting the dorsal process, is nearly straight in caudal and lateral aspects. It forms the medial wall of the large, oval paraquadrate foramen.
Quadrate
The quadrate (, , ) consists of a main body, a large pterygoid flange, a dorsal squamosal capitulum, and a ventral mandibular capitulum. The main body can be further divided into a caudodorsally arched dorsal portion and a vertical, transversely more flattened ventral portion. The caudal surface is flat and transversely expanded. It contacts the paroccipital process just below the single-headed squamosal capitulum caudally. Rostrally, the dorsal portion of the main body bears an elongated groove at the junction with the pterygoid flange for the reception of the ventral process of the squamosal. A spike-like projection extends lateral and ventral to this contact from the main body. It serves as the dorsal attachment site for the quadratojugal and forms the dorsal margin of the paraquadrate foramen ().
The ventral portion of the quadrate is constricted near the dorsal quadratojugal contact and widens distally. A bulging ridge extends along the caudal margin and connects the main body with the lateral mandibular condyle. A slight concavity lateral to this ridge marks the medial extent of the paraquadrate foramen. A thin, rostrally directed lamina extends from the lateral surface of the main body, forming the ventral contact point of the quadratojugal. The region medial to the quadratojugal lamina is excavated and could represent an equivalent to a quadrate diverticulum. Except for this, the quadrate bears no foramina. Clark et al. (Citation1994) remarked on the absence of a siphonial opening. CT scans confirm that the quadrate has no internal pneumatic spaces. However, an external (pneumatic) fossa is present dorsal to the mandibular capitulum. In contrast, a large pneumatic foramen opening medially is located dorsal to the mandibular capitulum in Falcarius utahensis (Zanno, Citation2010a), with a large pneumatic pocket extending internally into the medial and lateral condyles.
The quadrate head is blunt and rounded and only moderately demarcated from the main body in E. andrewsi. It has a subquadrangular outline in lateral view and a triangular outline in caudal view. This condition differs distinctly from the morphology found in Falcarius utahensis (UMNH VP 14558, 14559). In the latter, the quadrate head is well defined and clearly offset from the main body (Zanno, Citation2010a), suggesting a ball-and-socket joint between the quadrate and squamosal. In Jianchangosaurus yixianensis, the quadrate appears to be similar to that of E. andrewsi in having a stouter, quadrangular head (Pu et al., Citation2013). However, the compaction and poor preservation of the specimen in this region confounds clear characterization of this element.
The pterygoid flange is large, rostrocaudally expanded, and transversely thin. It tapers rostrally, giving the whole structure a triangular outline. It extends from the main body and is confluent with the ventral portion, but perpendicular to the dorsal part. The lateral surface of the pterygoid flange is laterally vaulted and convex, whereas the medial surface is considerably excavated and bowl-shaped (). It closely approximates the outline of the lateral surface of the inflated basisphenoid and overlies the latter firmly, with the quadrate process of the pterygoid wedged between both elements rostrally. The pterygoid flange covers nearly completely the ventrolateral part of the basisphenoid and parts of the braincase ventral and caudal to the trigeminal foramen, as in many oviraptorosaurs (Balanoff et al., Citation2009).
The mandibular articulation consists of two large condyles located laterally and medially, and an accessory, smaller condyle located dorsomedial to them (). The main condyles are not equally preserved on both sides, but superposition of the left and right quadrates shows that the condyles are more similar in size than described by Clark et al. (Citation1994). They are clearly separated and are oriented in a more rostrocaudally inclined direction than in other maniraptorans. The medial condyle is rounded, ball-shaped, and directed rostrally, whereas the lateral condyle is dorsoventrally flattened and blunt. The accessory condyle is positioned between the main condyles. A tricondylar condition has also been described for Sinornithomimus (Kobayashi and Lü, Citation2003), but is absent in other theropods, including Falcarius utahensis (Zanno, Citation2010a). Jianchangosaurus yixianensis has a possible additional condyle on the right quadrate (Pu et al., Citation2013:), but due to compaction it is not clear whether this is a preservational artifact or not.
Braincase
The braincase of E. andrewsi is well preserved and largely complete (). Only the sphenethmoid-mesethmoid complex is missing, whereas the orbitosphenoids and laterosphenoids are incompletely preserved medially. The braincase bones are firmly coossified and, as noted by Clark et al. (Citation1994), the sutures between individual elements are not visible superficially, except for a few minor variations in the bone surface. However, internal sutures could be traced in the CT scans and the individual braincase elements could be largely differentiated.
Supraoccipital
The supraoccipital (, ) of E. andrewsi has a quadrangular to slightly circular outline in dorsal aspect. The region caudal to the parietal contact is inclined at an angle of 30–35°, whereas the rostral part underlying the parietal is nearly horizontal. The dorsal surface of the supraoccipital is dominated by a pronounced midline nuchal crest (). The nuchal crest rises vertically just caudal to the contact with the parietal. It forms a dorsally curved projection, which merges caudally with the flat surface of the supraoccipital. The nuchal crest is excluded from the parietal suture and does not continue onto the latter element. A large dorsal opening rostral to the nuchal crest in the original specimen appears to be a break rather than a foramen. CT scans confirm that the bone structure is interrupted in this area. Although only fragmentarily preserved, a pronounced nuchal crest is absent in Falcarius utahensis (UMNH VP 15000, 15001; Zanno, Citation2010a) and Nothronychus mckinleyi (AZMNH-2117; Kirkland et al., Citation2005a). The rostral-most portion of the supraoccipital is missing in Nothronychus mckinleyi, which leaves the possibility that a more rostrally restricted, or less well-developed, nuchal crest was present.
Rostrally, the supraoccipital of E. andrewsi is rostrocaudally thickened and dorsally raised, forming a broad and vertical articulating surface with the parietal, perpendicular to the nuchal crest. The squamosal processes of the parietal are angled backwards caudally and envelop the lateral margins of the supraoccipital. The lambdoidal crests of the parietal and the nuchal crest of the supraoccipital bound a large flattened area between them, which serves as an attachment area for mm. splenius capitis and transversospinalis capitis (Snively and Russell, Citation2007).
Foramina for the middle cerebral veins appear to be absent. The middle cerebral veins exit the braincase more laterally through a foramen formed by the prootic and the exoccipital/opisthotic. Apparent openings in the respective region on each side constitute breaks and gaps due to preservation or preparation: they do not occur symmetrically or penetrate the bone completely.
The caudal margin of the supraoccipital is nearly horizontal and forms the dorsal border of the large foramen magnum. It projects caudally beyond the exoccipital contact and overhangs the foramen magnum. Ventrolaterally, the supraoccipital contacts the exoccipital/opisthotic along a broad facet. The ventral surface of the supraoccipital is deeply excavated and vaulted dorsally, housing a large cerebellar prominence. It is continuous with the ventral surface of the parietal.
Exoccipital/Opisthotic
The exoccipital and opisthotic (, ) are coossified and cannot be differentiated. They contact the supraoccipital dorsomedially, the quadrate and parietal dorsolaterally, the prootic rostrally, and the basisphenoid and basioccipital ventrally. Medially, the exoccipital forms the lateral margins of the foramen magnum and parts of the caudomedial braincase wall. The margins of the foramen magnum are slightly emarginated. The dorsal margin is formed by the supraoccipital, and the ventral margin is formed by the neck of the occipital condyle. A short, stout paroccipital process extends laterally from the exoccipital/opisthotic. It projects only slightly beyond the quadrate and squamosal laterally and has only a minor inclination caudally, so that it does not extend beyond the base of the occipital condyle. The rostral and caudal surfaces of the paroccipital process are convex. A deep triangular depression marks the caudal surface bounded by the swollen margin of the foramen magnum. The dorsal surface is broad, dorsoventrally flattened, and overlain by the squamosal process of the parietal rostromedially and the quadrate rostrolaterally. Ventrally, the paroccipital process tapers to a thin, gently rounded ridge. Internally, the paroccipital process is hollow, rostrocaudally inflated, and deeply excavated ().
Medially, the exoccipital/opisthotic of E. andrewsi houses the large vagal foramen and the more caudally located hypoglossal foramen (). The rostromedial surface forms the dorsal and caudal portions of the floccular fossa. Internally, the exoccipital/opisthotic houses the caudal semicircular canal. Externally, the vagal canal opens lateroventrally at the base of the paroccipital process, where the latter articulates with the basioccipital and the basisphenoid. The caudal hypoglossal nerve canal exits the braincase caudoventrally, located in a depression ventral to the neck of the occipital condyle at the basioccipital/exoccipital contact.
Although the specimen is damaged in this region on both sides, a delicate groove on the rostroventral surface of the paroccipital process appears to represent the stapedial groove (). A slit-like opening, located externally between the exoccipital/opisthotic, the quadrate, and basisphenoid, connects the stapedial groove with the middle ear cavity.
Basioccipital
The basioccipital (, ) comprises the entire occipital condyle and the floor of the endocranial cavity. Laterally, it contacts the exoccipital/opisthotic and rostrally the prootic. The rostral-most tip contacts the orbitosphenoid. Ventrally, the basioccipital is attached to the basisphenoid via an intricate interdigitating suture. The occipital condyle is prominent and ball-shaped. It is equal in size to the foramen magnum and separated from the main portion of the basioccipital by a thick, horizontal condylar neck. The dorsal surface bears a shallow depression caudal to the margin of the endocranial cavity. A ventral lip as described by Smith et al. (Citation2011) for Falcarius utahensis (UMNH VP 15000) is not developed as prominently in E. andrewsi, but the occipital condyle is distinctly offset from the recessed condylar neck. The occipital condyle and the condylar neck, respectively, merge laterally with the medial portion of the exoccipital/opisthotic and form a confluent, swollen ridge, which is oriented subhorizontally (). It bounds the opening of the vagal canal ventrally.
Ventral to the occipital condyle, a swollen, vertically oriented ridge connects the condylar neck to the inflated basisphenoid. Condylotuberal crests, as found in most other theropods including Falcarius utahensis (UMNH VP 15000, 15001; Smith et al., Citation2011), are absent due to the lack of basitubera. Lateral to the swollen ridge, the basioccipital of E. andrewsi bears a large depression on each side. It is separated from a shallower, laterally located concavity by a small subvertical ridge. The foramen of the rostral hypoglossal nerve canal opens into this depression. The vagal foramen appears to be excluded from the basioccipital or only marginally bordered by it.
The rostral portion of the basioccipital, forming the floor of the endocranial cavity, is smooth and only moderately concave. It maintains a continuous width from the foramen magnum rostrally until tapering at the contact with the orbitosphenoids (). In lateral view, the portion of the basioccipital rostral to the occipital condyle is wedge-shaped. The CT scans show a number of pneumatic pockets within, whereas the occipital condyle is composed of solid cancellous bone.
Basisphenoid
The basisphenoid (, ) is large and inflated, forming a bulbous basisphenoid bulla. It is transversely and rostrocaudally expanded, filling the space between the quadrates entirely. It contacts the latter along the dorsolateral surface, with a large portion of the quadrate flange of the pterygoids wedged between both elements. Dorsally, the basisphenoid attaches to the orbitosphenoid, prootic, exoccipital/opisthotic, and basioccipital. The elongated cultriform process extends from the rostral surface medial to the suture with the pterygoids.
The external surface of the basisphenoid is smooth and convex. Basipterygoid processes and basal tubera are absent, similar to the condition in oviraptorosaurs (Clark et al., Citation2002; Balanoff et al., Citation2009). As a result, the external basisphenoid and basipterygoid recesses, which are common in more basal theropods (Witmer, Citation1997), are absent in E. andrewsi. Ventral to the basioccipital, the basisphenoid is prominently convex along the midline and transversely expanded. Lateral to this expansion, the basisphenoid bears deep, but surficial, recesses (, ), which open caudoventrally. Ventral to the contact with the paroccipital process, respectively, the basisphenoid bears an elongated depression forming the ventral portion of the vagal foramen. It is bounded medially and laterally by thin subvertical ridges. An opening on the right caudal surface tentatively identified as the eustachian tube by Clark et al. (Citation1994) appears to be a break. It is not present on the left side. Rostral to the paroccipital process, the basisphenoid wall is raised dorsally and sutured to the prootic.
Internally, the basisphenoid is extensively invaded by pneumatic pockets (), formed by thin struts and lamina of bone (Clark et al., Citation1994). The full extent of these pneumatic spaces, however, cannot be restored accurately, because only remainders of the original internal laminae are preserved. Most of the latter were probably removed by erosion or preparation, because the right part of the braincase is separated from the rest of the specimen. As preserved, there appears to be a split between a large dorsal space and several smaller ventral cavities within the basisphenoid. Although less transversely expanded, the basisphenoid of Nothronychus mckinleyi exhibits a similar condition to E. andrewsi, in being extensively inflated and internally pneumatized (Kirkland et al., Citation2005a) and subdivided into several pneumatic pockets by delicate struts and laminae (). Several shallow depressions on the caudal and ventral surfaces of the basisphenoid in Nothronychus mckinleyi are the result of preservation and do not represent natural features, as confirmed by the CT data.
Although an exact distinction between the basisphenoid and the parasphenoid is not possible, a transversely thin, blade-like cultriform process is readily identifiable in E. andrewsi (). Unlike the condition found in Troodon formosus (Currie, Citation1985), CT scans show that the cultriform process is unpaired in E. andrewsi. Near its base, where it projects from the midline surface of the basisphenoid, it is slightly inflated and hollow, but narrows in width considerably in the rostral direction, unlike the prominently inflated condition found in ornithomimids (Osmólska et al., Citation1972) and some troodontids (Currie and Zhao, Citation1993a; Xu and Wu, Citation2001). A subsellar recess, normally found at the base of the cultriform process and the articular contact with the basisphenoid (Witmer, Citation1997), is absent in E. andrewsi. The cultriform process is elongated and extends rostrally as far as the preorbital bar. It tapers to a point rostrodorsally and contacts the vomer along its rostroventral margin, fitting into a trough-like depression in the latter element. Similar to the vomer, the dorsal margin of the cultriform process is bifurcated along its entire length, forming a shallow and elongated depression, which housed the trabecular cartilages of the interorbital septum as in various maniraptoriform theropods (Currie, Citation1985).
Prootic
The prootic (, ) forms the lateral (external) and medial (internal) surfaces of the braincase, rostral to the exoccipital/opisthotic. It contacts the exoccipital caudally, the laterosphenoid and the orbitosphenoid rostrally, the parietal dorsally, and the basisphenoid ventrally. The lateral surface of the prootic is smooth and forms a flat, nearly vertical structure, flush with the laterosphenoid. On the medial surface, the prootic is swollen ventrally, accommodating the vestibular eminence. Immediately dorsal to the latter, the prootic contributes to the rostral portion of the floccular fossa. Internally, the prootic houses the rostral and lateral semicircular canals, as well as the cochlear duct. Rostral to the vestibular eminence are the foramina for the vestibulocochlear (VIII) and the facial (VII) nerves (). The vestibulocochlear foramen is small and penetrates the bone caudally. The facial foramen is enlarged and exits the braincase directly laterally. A second foramen for the vestibulocochlear nerve as described by Clark et al. (Citation1994:) might represent the endolymphatic duct (see also Lautenschlager et al., Citation2012), whereas the putative foramen for the endolymphatic duct (see Clark et al., Citation1994) is actually a break, confirmed by the CT scans. On its rostral surface, the prootic bounds the caudal margin of the large internal opening of the trigeminal nerve (V) and the transversely oriented canal for its maxillary branch (V2).
Ventrally, the prootic bears the fenestra vestibuli and fenestra cochleae, which open into the tympanic recess (). Both fenestrae are equal in size and directed laterally. The entire middle ear cavity is walled off by the exoccipital caudally and the prootic and basisphenoid dorsally and laterally. Our restoration of the middle ear regions largely confirms the arrangement depicted in an interpretative drawing by Perle (Citation1981).
Laterosphenoid
The laterosphenoid (, ) consists of a main portion, contributing to the rostromedial braincase wall, and a laterally directed horizontal process. The laterosphenoid is overlain by the parietal dorsally, along its entire length via an intricate and transversely wide suture. It further contacts the prootic caudally and the orbitosphenoid rostrally. Along its rostral margin, the laterosphenoid tapers to a sharp horizontal ridge, which projects laterally paralleling the lateral process. It forms the medioventral demarcation of the cerebral hemispheres in a continuation of the crista cranii of the frontals. The exit of the optic nerve (II) was most likely bound by the medial margin of the laterosphenoids, but a foramen could not be identified or reconstructed due to fragmentary preservation in this region.
The lateral process of the laterosphenoid extends along the caudal and ventrally deflected margin of the frontal. It is overlain by the postorbital process of the parietal for its entire length, and both elements contact the postorbital by attaching to an elongated, oval depression on the medial surface of the latter. The main portion of the laterosphenoid and the lateral process form the rostromedial wall of the adductor chamber.
Orbitosphenoid
The orbitosphenoid (, ) forms the rostral and ventral margins of the braincase and endocranial cavity. It attaches to the laterosphenoid dorsally, the prootic caudally, and basisphenoid ventrally. Both orbitosphenoids contact each other along the braincase midline. Perle (Citation1981) described a sutural contact of the orbitosphenoid with the frontals and depicted it in a reconstruction, which shows the orbitosphenoids enclosing a large foramen for the optic nerve ventrally and a contact with the frontals dorsally. However, such an arrangement could not be confirmed in the specimen or the CT scans.
The rostral surface is swollen and forms a vertical, pillar-like structure. Medial to it, the orbitosphenoid is recessed caudally, bearing the foramina for the ophthalmic branch of the trigeminal nerve (V1) dorsally and the abducens nerve (VI) ventrally. The location of the ophthalmic branch within the orbitosphenoid is unusual in E. andrewsi, because the foramen usually passes through the laterosphenoid (Sampson and Witmer, Citation2007). Both foramina are directed rostrally, with only a minor medial deflection. Rostromedially, the orbitosphenoids bound the internal opening for the trigeminal nerve canal, and the mandibular branch (V3) exits the braincase via a ventrolaterally directed foramen in the lateral surface. Due to the damage in this region, the openings for the oculomotor (III) or trochlear (IV) nerves could not be reconstructed, because these foramina usually tend to be located near the margin of the orbitosphenoid and the laterosphenoid.
Palatal Complex
The palatal complex of E. andrewsi () has been deformed and the individual elements have been displaced. The individual elements, though, are largely complete, and the entire palatal structure could be reconstructed.
Vomer
The vomer (, ) forms a transversely thin, flattened blade, which connects the cultriform process caudally and the premaxilla and maxilla rostromedially. CT scans show that the vomer consists of two coossified counterparts, which are indistinguishable externally, as is typical for theropods. The dorsal margin of the caudal half of the vomer is bifurcated (), receiving the cultriform process and the vomeropalatine rami of the pterygoid in between both arms caudally. Dorsolaterally, the rostral process of the palatine overlies the bifurcated margins on each side, forming a weak, narrow contact. Alongside its lateral surface, the vomer is bordered by the main body of the palatine and a thin pterygoid process on each side, but there appears to be no, or only minor, contact between the individual elements.
Rostrally, the vomer decreases in height. It widens transversely to a dorsoventrally flattened and horizontal process, which connects the palatal shelves of the premaxilla and maxilla. Because the specimen is extensively damaged in this region, the exact extent of this contact could only be approximated. The vomer appears not to contact the premaxilla more rostromedially, leaving an enlarged incisive foramen. The ventral margin of the vomer is slightly curved dorsally towards the rostral tip. It is continuous with the cultriform process. Together, both elements form an extensive, rostrocaudally elongated, straight structure, which traverses nearly the entire length of the skull.
Palatine
The palatine (, ) consists of a rostrally ascending process and a dorsomedial pterygoid process, extending from the main portion of the palatine. The latter is rostrocaudally elongated and has ventrally convex and dorsally concave surfaces. A shallow depression on the dorsal surface possibly represents the palatine pneumatic recess (). The medial surface of the palatine is vertically inclined and gives rise to the dorsally and rostrally ascending process (vomeropterygoid ramus). The process is elongated, spanning nearly the entire length of the antorbital fenestra, and tapers to a point. Its lateral surface is slightly convex, with a pronounced horizontal deflection dorsomedially to meet the opposing palatine. The ventral margin of the process curves dorsoventrally towards the main body of the palatine. At its juncture, a small spur projects from the main body in a rostral direction. Its dorsal surface is markedly concave and bears an elongate facet for reception of the caudal shelf of the maxilla.
The pterygoid process of the palatine is transversely thin and spike-like. It is vertically inclined and projects caudally and slightly dorsally from the caudomedial margin of the palatine. It parallels the vomeropalatine ramus of the pterygoid medially. Due to deformation of the palatal complex, it could not be ascertained whether both elements are in contact in this region. Regardless of a possible contact, the pterygoid process and the vomeropalatine ramus bound a rostrocaudally elongated opening dorsomedially. Its lateral border is formed by the caudal portion of the palatine main body. Clark et al. (Citation1994) identified this fenestra as the subsidiary palatal fenestra.
Pterygoid
The pterygoid (, ) is large and transversely expanded. The dorsal surface of the main portion is concave and the ventral surface respectively convex, similar to the condition in the palatine. Along its rostral margin, it overlies the caudal portion of the palatine in a short overlapping suture. The transversely thin, blade-like vomeropalatine ramus extends medially from the main portion in the rostral direction, where it tapers to a point. It is inclined vertically and borders the cultriform process of the parasphenoid medially and the pterygoid process of the palatine laterally. A prominent ectopterygoid ramus extends from the main portion of the pterygoid rostrolaterally. Its distal part is deflected vertically. It bears a large facet for articulation with the ectopterygoid along its rostral surface.
The quadrate ramus is massive and equal in size to the rest of the pterygoid. It rises vertically from the caudal portion of the main body to contact the basisphenoid. The contact with the latter is extensive and the quadrate ramus covers a large part of the rostral and lateral surfaces of the basisphenoid. Its lateral surface is overlain by the pterygoid flange of the quadrate. As Clark et al. (Citation1994) noted, the contact between the three elements is extensive and akinetic. Evidence from therizinosauroid embryos further indicates that a compact and largely akinetic cranial skeleton developed before hatching (Kundrat et al., Citation2008). The presence of a possible epipterygoid could not be confirmed in either the specimen or CT scans.
Ectopterygoid
The ectopterygoid () is incomplete and its full extent could be restored only tentatively. Its caudal portion is preserved in articulation with the pterygoid. The preserved part forms the rostral portion of the large and vertically inclined transverse flange. A pronounced depression on the lateral surface of the ectopterygoid continues onto the ectopterygoid ramus of the pterygoid. The dorsal surface of the ectopterygoid is oriented horizontally and is slightly convex. The preserved portion tapers rostrally. Clark et al. (Citation1994) suggested that a fragmentary bone on the rostral process of the left jugal represents the rostral part of the ectopterygoid. This element is not preserved on the right side or in any other therizinosaurian. Therefore, the ectopterygoid was restored assuming the fragmentary part forms its rostral extent. As a result, the rostral extent of the ectopterygoid is short and lacks the more delicate, curved morphology found in other theropods (Eddy and Clark, Citation2011).
Sclerotic Ring
Seven complete sclerotic plates from the right eye are preserved in semiarticulation () overlying the pterygoid (Clark et al., Citation1994). Each element has a rectangular to hexagonal outline, with a pronounced pointed end on one side. They are slightly vaulted along the midline. Approximately one-fourth of each plate overlaps the next, with the pointed end lapping onto the adjacent element.
For reconstruction of the complete sclerotic ring (), the three best-preserved and most naturally articulated elements were used. The respective plates were duplicated and arranged radially, following the overlapping pattern as in the preserved elements. This results in a total number of 13 sclerotic plates for the entire ring. This number corresponds well with the number of elements in ornithomimids (Garudimimus brevipes, 11 [Kobayashi and Barsbold, Citation2005a]; Harpymimus okladnikovi, >11 [Kobayashi and Barsbold, Citation2005b]). The internal diameter of the reconstructed sclerotic ring is approximately 20 mm, and the outer diameter approximately 38 mm.
Mandible
Both hemimandibles () are preserved and nearly complete, with the exception of the right articular and surangular.
Dentary
The dentary (, ) has an elongate, wedge-shaped outline in lateral view. The rostral portion is dorsoventrally shortened and increases in height caudally. The tip of the dentary and the symphyseal region are deflected ventrally at an angle of 20–25°, rostral to the fifth tooth position, similar to Segnosaurus galbinensis (Perle, Citation1979), Neimongosaurus yangi (Zhang et al., Citation2001), and, to a lesser degree, Jianchangosaurus yixianensis (Pu et al., Citation2013). In dorsal aspect, the symphysis is approximately perpendicular to the main portion of the dentary and is distinctly ‘U’-shaped, closely reflecting the ventral outline described by the premaxilla. The symphyseal region is completely edentulous and tapers to a thin, sharp dorsal margin. The dorsomedial (lingual or inner) surface of the symphysis is rostroventrally inclined and concave, with a respectively convex and rounded ventrolateral surface.
The dorsal surface of the dentary is dorsoventrally flattened and transversely expanded. It forms a distinct shelf (), which overhangs the lateral surface and gradually merges with the ventrally deflected tip of the dentary (Clark et al., Citation1994). Caudally, near the articulation with the surangular, the dorsolateral shelf twists from a laterally to a dorsomedially facing orientation. The dentary bears 31 alveoli along its entire length, with the exception of the edentulous symphyseal region. The tooth row is distinctly inset and positioned along the medial margin of the dorsal surface. Unlike in the maxilla, the alveoli are arranged in a rostrodorsally straight line as seen in dorsal and rostral views. The alveoli are separated by thin interdental plates, as in Beipiaosaurus inexpectus (Xu and Wang, Citation1999), Alxasaurus elesitaiensis (Russell and Dong, Citation1993), Segnosaurus galbinensis (Perle, Citation1979), and Falcarius utahensis (Zanno, Citation2010a). With 31 dentary teeth, E. andrewsi falls within the range of other therizinosaurians (Falcarius utahensis, >26; Segnosaurus galbinensis, 24–25; Jianchangosaurus yixianensis, >25; Beipiaosaurus inexpectus, >37; Alxasaurus elesitaiensis and Eshanosaurus deguchiianus, 40).
A series of foramina (2–5 mm in diameter) cover the ventral and lateral surfaces of the symphyseal region and continue in a single row along the lateral edge of the dorsolateral shelf. A single large (10 mm diameter) foramen opens rostrally, lateral to the symphyseal suture, whereas an elongated foramen exits the bone caudally to the 20th tooth position. A similar arrangement of foramina, tracing the dorsolateral shelf, is present in Alxasaurus elesitaiensis (Russell and Dong, Citation1993), Jianchangosaurus yixianensis (Pu et al., Citation2013), and partly in the fragmentary dentary of Neimongosaurus yangi (Zhang et al., Citation2001), which shows a single large foramen on the rostrolateral surface. In E. andrewsi, the individual foramina are connected internally by an extensive neurovascular canal (), which parallels the tooth row ventrally and laterally and opens into the Meckelian fossa caudomedially. Prominent neurovascular foramina are absent in the dentary of Falcarius utahensis. A single large canal pervades the bone ventrally (), but appears to lack the intricate network of subsidiary canals found in E. andrewsi.
The Meckelian fossa is a wedge-shaped depression that covers the majority of the medial dentary surface (). It tapers rostrally below the fifth tooth position. Caudally, it increases in height and connects to the mandibular fossa. The Meckelian fossa is enclosed by a thin wall of the dentary laterally and the splenial medially.
Caudally, the dentary forms a transversely thin, tapering process, which extends caudally to articulate with the angular. The dorsolateral shelf of the dentary is slightly convex caudal to the last tooth position and continuous with the rounded dorsal surface of the surangular. The latter contacts the dentary via a bifurcated facet caudal to the tooth row and along the caudodorsal margin of the angular process. As a result, the dentary is largely excluded from the external mandibular fenestra, with the exception of a small part of its rostral border between the surangular and the angular. The ventral surface of the dentary forms a gently rounded margin, which tapers to a thin lamina caudally.
Unlike in the maxillary teeth, and as noted by Clark et al. (Citation1994), there is distinct heterodonty between the rostral and caudal dentary teeth. The rostral four teeth are nearly conical and, on average, twice the length (from root to crown) of the caudal teeth. Caudally, the teeth become more lanceolate and resemble those in the maxilla. The lanceolate teeth are more closely spaced than the conical teeth. The erupted teeth are all exposed to about one-half of their total length. As a result, the tips of the teeth are at approximately the same level, with the elongated caudal teeth compensating for the caudally deflected symphyseal region. Thirteen replacement teeth are preserved in the left dentary (). This accounts for a replacement tooth for every other erupted tooth. Caudally, the number of replacement teeth decreases considerably. Given that this pattern occurs parallel in both dentaries and the otherwise well-preserved condition of the mandibles, it is unlikely to be a preservational artifact. As in the maxillary teeth, wear facets are not visible on any of the dentary teeth.
As far as preserved, the dentaries of Falcarius utahensis (UMNH VP 14528, 14529), each exhibit seven replacement teeth (), in an apparently alternating pattern. A fragmentary dentary (UMNH VP 14527) holds six replacement teeth, with one replacement tooth for every erupted tooth, except for the caudal one (). However, the dentary is only incompletely preserved in this region.
Splenial
The splenial (, ) is transversely flattened and triangular in lateral view. It covers the medial surface of the dentary, tracing the Meckelian fossa. Rostrally, it tapers to a thin bony lamina, which extends to the 13th tooth position, leaving the rostral-most portion of the Meckelian fossa medially exposed. The dorsal tip of the splenial contacts the surangular and dentary at the articulation between the latter elements and then decreases in height caudally. Along its caudodorsal margin, the splenial parallels the rostroventral margin of the prearticular and both elements form a continuous medial surface. Caudally, the splenial articulates with the angular. The latter bears a prominent ridge on its ventromedial surface, which fits between the bifurcated caudal process of the splenial. The medial surface of the splenial bears an elongate, shallow depression rostral to this point. An elongate foramen further penetrates the medial surface along the ventral margin at the level of the caudal-most teeth. A second opening is located along the dorsal margin of the splenial rostral to the aforementioned foramen.
Surangular
The surangular (, ) makes up the dorsal and caudal parts of the mandible and is nearly the same length as the dentary. It articulates with the latter via a complicated facet on its rostrodorsal tip. The tip bifurcates into two small processes. A longitudinal groove separates the processes medially and laterally, to receive the respective processes of the dentary. The lateral process of the surangular articulates with the dentary immediately ventral to the dorsolateral shelf along a large semicircular facet. The rostroventral edge of the surangular and the lateral process, respectively, contact the dentary only loosely. The articulation with the prearticular is restricted to a small area ventral to the dentary facet.
ith the exception of the medial and lateral grooves, the dorsal margin of the surangular is smooth and slightly vaulted transversely. It is deeply excavated ventrally, which gives the rostral part of the surangular an inverted ‘U’-shaped cross-section and roofs the mandibular fossa dorsally. In lateral aspect, it is dorsally convex, reaching its highest point above the external mandibular fenestra and decreasing in height towards the articular contact caudally. At the contact with the articular, the surangular flares laterally, forming a prominent longitudinal buttress. Ventral to it, the surangular is medially inclined and contacts the angular along a rostrocaudally elongated suture. A coronoid eminence (sensu Holliday, Citation2009) is only moderately developed in the form of a slight swelling. It separates the muscle insertion sites for m. adductor mandibulae externus superficialis (m. AMES) laterally, and m. adductor mandibulae externus profundus (m. AMEP) and m. adductor mandibulae externus medialis (m. AMEM) medially (Lautenschlager, Citation2013).
The lateral surface of the surangular is smooth and continuous with that of the dentary, forming the transversely thin lateral wall of the mandibular fossa. Medially, the surangular contributes only dorsally to the medial wall of the mandibular fossa. Three noninvasive foramina pierce the surface dorsal to the external mandibular fenestra. Two rostrally opening foramina are located on the dorsolateral margin of the surangular, the rostral of which (rostral surangular foramen sensu Sampson and Witmer, Citation2007) lies within the lateral groove near the dentary contact. Both foramina open into the mandibular fossa internally.
Caudally, the surangular splits into a medially directed transverse process and a caudal process (), which establish the contact with the articular by embracing the latter rostrolaterally. The caudal process overlies the articular dorsolaterally and forms a large part of the lateral border of the glenoid fossa. The transverse process contacts the articular rostrally, ventral to the prominent rostral buttress of the articular. The dorsal surface of the transverse process further forms the floor of a deeply excavated groove, bound by the rostral buttress of the articular and the caudal process of the surangular. A large foramen (caudal surangular foramen sensu Sampson and Witmer, Citation2007) pierces the lateral surface, ventral to the caudal process. It is connected internally to a small foramen on the medial surface of the surangular, rostroventral to the transverse process ().
Compared with E. andrewsi, the surangular of Falcarius utahensis (UMNH VP 16020) lacks the deep groove on the dorsal surface of the transverse process, which is more continuous with the rostral portion of the surangular. Unlike the condition found in E. andrewsi, the dorsal margin of the surangular bar (sensu Zanno, Citation2010a) tapers to a sharp ridge. It bounds an enlarged surface laterally, which indicates that the attachment site for m. AMES was equally enlarged. The attachment surface is ventrolaterally demarcated by another elongated sharp ridge, which is in the approximately same position as the lateral bulge in E. andrewsi.
Angular
The angular (, ) is an elongate, transversely thin, blade-like element. The rostral half is wedged between the dentary laterally and the splenial medially. On its lateral surface, the angular bears two prominent ridges along the dorsal and caudal margins, which bound a large depression for the reception of the ventrocaudal process of the dentary. The medial surface of the angular is smooth, where it is overlain by the splenial. The latter is bifurcated caudally to form the articulation with a prominent medioventral ridge on the angular. The ridge forms parts of the floor of the mandibular fossa and traces the ventral margin of the angular. Caudally, it becomes less prominent and gradually merges with the slightly medially directed ventral angular surface. The prearticular contacts the angular by overlying the medial surface and the ventral ridge. The angular thus forms the ventral surface of the caudal half of the mandible.
The lateral surface of the angular, caudal to the contact with the dentary, is smooth and slightly convex. Caudodorsally, the angular articulates with the surangular along an elongate, grooved facet in the latter. Both elements bound the rostrocaudally elongated external mandibular fenestra. At its caudal-most extent, the angular overlies the lateral surface articular for a short distance.
Prearticular
The prearticular (, ) is a transversely thin, elongate element. It connects the splenial rostrally with the articular caudally and forms nearly entirely the medial wall of the mandibular adductor fossa. The rostral portion of the prearticular is thin and blade-like, similar to the angular. Its rostral tip is dorsally deflected to meet the ventral margin of the medial surangular surface. Ventrally, the prearticular contacts the splenial along its caudodorsal margin for the entire length. Ventral to the splenial contact, the prearticular rests on the medial ridge of the angular. The dorsal margin of the prearticular is curved and concave between the dentary contact rostrally and the articular contact caudally, except for a moderate convexity at a point halfway along its length.
Caudally, the angular is slightly inflated transversely and overlies the medial surface of the articular just below the glenoid fossa. The lateral surface of the angular is smooth, except for a rostrocaudally elongated depression on the caudal half, which serves as a muscle insertion for m. pterygoideus dorsalis (m. PTd). CT scans confirm that a large opening on the lateral surface, on the left prearticular of the original specimen is due to breakage, as noted by Clark et al. (Citation1994).
Articular
The articular (, ) is firmly ossified to the surangular, and both elements form the rostrocaudally elongated, prominent jaw joint. The articular is only moderately expanded transversely and continuous with the long axis of the hemimandible, except for a slight medial deflection. The glenoid fossa is located comparably low at approximately the same level as the fifth tooth position and the deflected dentary tip. The glenoid fossa is deeply excavated and bordered by prominent, approximately transversely orientated buttresses rostrally and caudally (). Perle (Citation1981) identified the rostral buttress as an antarticular. However, CT scans show that the structure is part of the articular and not a separate ossification, whereas antarticulars appear to be restricted to Allosaurus fragilis (Madsen, Citation1976; Currie and Zhao, Citation1993b; Eddy and Clarke, Citation2011). The interglenoid ridge is located between and parallel to the rostral and caudal articular buttresses, separating the concave facets for the reception of the lateral and medial condyles of the quadrate. The individual glenoid facets are orientated at an angle of approximately 40–45° to the transverse axis, consistent with the orientation of the quadrate condyles. The interglenoid ridge bears a slight concavity on its dorsal surface, which might articulate with the third accessory condyle of the quadrate. On its caudal surface, the interglenoid ridge bears a foramen, which exits the articular caudolaterally (). It is connected to a large foramen on the medial surface of the articular at suture with the prearticular and a more rostrally located foramen opening into the mandibular fossa. Due to its caudolateral position, it was identified as the foramen aerum by Clark et al. (Citation1994). However, the CT scans show no indication of pneumatic chambers in the bone. Rather, the foramen transmitted the chorda tympani.
The lateral glenoid walls are formed by the caudal process of the surangular. The rostral buttress further bounds a deep canal caudally (compare with the surangular). The retroarticular process is short and not distinctly separated from the lateral/caudal glenoid. Ventrally, the articular tapers to a thin ridge. Its lateral surface is medially deflected ventral to the caudal process of the surangular. This results in an enlarged insertion surface for m. pterygoideus ventralis (m. PTv).
DISCUSSION
Therizinosaur Beaks
Keratinous beaks (rhamphothecae) evolved several times within non-avian theropods (Louchart and Viriot, Citation2011) and have been suggested to be present in (amongst others) Ornithomimosauria, Oviraptorosauria, and Therizinosauria. Among these groups, only ornithomimosaurs provide direct evidence of a preserved keratinous sheath (Norell et al., Citation2001; Barrett, Citation2005). A lack of direct evidence notwithstanding, several anatomical characters have been regarded as traits indicative of a rhamphotheca (Zanno and Makovicky, Citation2011), such as an edentulous premaxilla with a thin, tapering ventral margin, successive loss of maxillary and dentary teeth, a mandibular ventral concavity, ventral displacement of the dentary, and a rostral projection of the mandibular symphysis.
Among therizinosaurians, E. andrewsi exhibits all the aforementioned features indicative of a rhamphotheca, whereas the basal-most therizinosaurian Falcarius utahensis has been regarded to represent the basal ‘beak-less’ condition (Zanno, Citation2010a). Although the presence of a rhamphotheca in E. andrewsi is well supported, the extent of its keratinous sheath is less clear. Extant birds exhibit a plethora of sheath morphologies, which cover various portions of the premaxilla, maxilla, and dentary (Hieronymus and Witmer, Citation2010), whereas a rhamphotheca most likely covered the edentulous parts of the jaws in most Mesozoic birds (Louchart and Viriot, Citation2011). For E. andrewsi, the presence of a rhamphotheca on the premaxilla can be deduced by the presence of several neurovascular foramina pitting the rostral and lateral surfaces (Morhardt et al., Citation2009; Hieronymus and Witmer, Citation2010; Louchart and Viriot, Citation2011). Similarly, the rostrolateral surface of the maxilla bears neurovascular foramina, indicating that it might have been covered by the rhamphotheca, with its caudal-most extent marked by the large foramen for the nasopalatine nerve (Hieronymus and Witmer, Citation2010). Clear osteological correlates, such as rhamphothecal grooves, are absent from the premaxilla and maxilla. Soft-tissue preservation of the rhamphotheca in Ornithomimus edmonticus (Norell et al., Citation2001) shows that the keratinous sheath covered the main body of the premaxilla and ventrally overlapped it by a few millimeters. The caudodorsal extent of the rhamphotheca is similarly unknown in this taxon. In extant birds, the rhamphotheca is generally restricted to the premaxilla and maxilla, although it partially covers the nasal in some birds (Knutsen, Citation2007). Thus, it seems likely that the rhamphotheca covered the narial process of the premaxilla in E. andrewsi.
Similarly, the dentary, and in particular the symphyseal region, of E. andrewsi is pitted by numerous foramina, which trace the lateral ridge caudally. The surface ventral and caudal to the symphyseal region and the lateral ridge is distinctly concave and inset. In some extant birds (Hieronymus and Witmer, Citation2010) and Ornithomimus edmonticus (Norell et al., Citation2001; compare also Knutsen, Citation2007), a similar morphology marks the extent of the rhamphotheca.
Two hypothetical rhamphotheca morphologies were reconstructed for the tip of the snout (), based on different interpretations of these osteological correlates. The first covers the premaxilla and maxilla to a large extent and partly covers the external nares. The second reconstruction only covers the rostral and lateral surfaces of the premaxilla, tracing the edentulous ventral margin. The reconstruction of the rhamphotheca on the dentary closely traces the symphyseal region and the lateral ridge. Its caudal extent is demarcated by the caudal-most neurovascular foramen at the 17th tooth position. When occluded, the jaws of E. andrewsi leave a pronounced gap resulting from the ventrally deflected tip of the dentary. Accordingly, the rhamphotheca on the premaxilla and the dentary were each reconstructed overlapping the bone ventrally and dorsally, respectively, to produce a full occlusion of the rhamphothecal tomia.
Homology and Functional Implications of Cranial Modifications in Therizinosauria
Previously, the holotype of E. andrewsi represented the only therizinosaurian with an almost complete, articulated skull. Recent discoveries have not only confirmed its phylogenetic position as a derived therizinosaurian, but have also provided further cranial elements for other taxa, although complete skulls are still rare. A comparison with these other taxa shows that the anatomical specializations of E. andrewsi have a broader distribution among therizinosaurians and some other maniraptoriforms. This is most evident in the edentulous, rhamphotheca-bearing premaxilla and the modification of the dentition. Although the lack of preserved cranial elements, particularly premaxillae and maxillae, makes it difficult to track the presence/absence of rhamphothecae in Therizinosauria, Beipiaosaurus inexpectus (Xu et al., Citation1999, Citation2009a) and Jianchangosaurus yixianensis (Pu et al., Citation2013) both have an edentulous premaxilla and a ventrally deflected dentary bearing a characteristic lateral shelf, indicating that a keratinous rhamphotheca evolved early in the clade. Similarly, a rostral rhamphotheca is assumed to have covered the edentulous elements in Ornithomimosauria, Oviraptorosauria, and Avialae (Louchart and Viriot, Citation2011). However, the development of a complete upper and lower rhamphotheca paralleled by partial or complete edentulism is a derived character that appears within different maniraptoriform lineages (Ornithomimosauria, Oviraptorosauria). Basal taxa of each clade (Perez-Moreno et al., Citation1994; Balanoff et al., Citation2009) are characterized by the possession of teeth and a comparatively unspecialized cranial design, with various stages of partial tooth loss in individual taxa (Ji et al., Citation1998; Kobayashi and Barsbold, Citation2005b). Dentitions are subject to strong functional pressures and are constantly used for foraging, prey capture, or food manipulation. It is therefore assumed that edentulism requires the presence of a preadapted tool preceding tooth loss to co-opt the same or a similar function (Davit- Béal et al., Citation2009). This means that a rhamphotheca must have existed at an early stage, in which teeth were still present. Although this raises the question how two functionally equivalent structures can exist simultaneously, the presence of a rhamphotheca and teeth are not mutually exclusive (O’Connor and Chiappe, Citation2011; Hu et al., Citation2012). A functional shift from the (rostral) teeth to a rhamphotheca would relax the functional constraints on the teeth, permitting their reduction and increased cranial plasticity. Consequently, the presence of a rhamphotheca might constitute a shared feature plesiomorphic for Maniraptoriformes, if not at an even earlier stage, as indicated by the presence of the edentulous ceratosaur Limusaurus (Xu et al., Citation2009b). Davit- Béal et al. (2009) speculated that beaks evolved due to the dedication of the forelimbs to flight, shifting the process of food procurement and manipulation towards the jaw region. However, most maniraptoriform clades exhibit prominently developed forelimbs, which are thought to have specialized paleobiological functions (Nicholls and Russell, Citation1985; Senter and Parrish, Citation2005; Zanno, Citation2006).
CONCLUSIONS
A redescription of E. andrewsi based on digital restoration and reconstruction provides not only a detailed characterization of the cranial anatomy, but also introduces a novel approach for the documentation and illustration of fossil specimens. The combined use of CT scanning, 3D visualization, and reconstruction techniques offers the possibility to describe the individual cranial elements of E. andrewsi on the basis of a largely articulated skull, which previously precluded description of many internal features and intracranial joint morphologies. This is important, because cranial remains are usually rare and fragmentarily preserved, and the description provided herein can thus provide useful comparative information for partial, broken specimens.
The emended diagnosis clarifies the autapomorphic characters of E. andrewsi, which include a mediolaterally expanded antorbital fossa containing three pneumatic pockets, a supraoccipital with a prominent nuchal crest, and a quadrate with an accessory third condyle and an oblique orientation of its mandibular articulation. CT scans reveal internal features such as neurovascular structures in the premaxilla, maxilla, and dentary, the internal pneumatization of the lacrimal and braincase, as well as the number and distribution of replacement teeth.
A review of adaptive changes in the cranial skeleton supports prior proposals that a rhamphotheca appeared early in the therizinosaurian evolution. This development is accompanied by the reduction of (dentary) teeth and replacement teeth, in particular in the caudal part of the jaws.
Lautenschlager et al Supplemental Data
Download Zip (33.9 MB)ACKNOWLEDGMENTS
Thanks are due to A. Ramsey and M. Robinson (Nikon Metrology) for their support with the scanning of Erlikosaurus. M. Getty (Utah Museum of Natural History) made comparative material available for study and for loan to L.M.W. for CT scanning. Thanks also to D. G. Wolfe (Mesa Southwest Museum) for loan of specimens. R. Ridgely and E. Snively (Ohio University) are thanked for insightful discussions. M. Rücklin and J. Bright (University of Bristol) provided helpful advice for Avizo and visualisation questions. Editors P. Barrett (Natural History Museum, London) and J. Clark (George Washington University) and an anonymous reviewer are thanked for their helpful comments and suggestions. This research was supported by a doctoral fellowship to S.L. by the Volkswagen Foundation. L.M.W. acknowledges funding support from the United States National Science Foundation (IBN-9601174, IBN-0343744, IOB-0517257, IOS-1050154) and the Ohio University Heritage College of Osteopathic Medicine. The Ohio Supercomputing Center also provided support.
LITERATURE CITED
- Balanoff, A. M., X. Xu, Y. Kobayashi, Y. Matsufune, and M. A. Norell. 2009. Cranial osteology of the theropod dinosaur Incisivosaurus gauthieri (Theropoda: Oviraptorosauria). American Museum Novitates 3651:1–35.
- Barrett, P. M. 2005. The diet of ostrich dinosaurs (Theropoda: Ornithomimosauria). Palaeontology 48:347–358.
- Barsbold, R. 1983. Carnivorous dinosaurs from the Cretaceous of Mongolia. Joint Soviet-Mongolian Expedition, Transactions 19:5–119. [Russian]
- Barsbold, R., and A. Perle. 1980. Segnosauria, a new infraorder of carnivorous dinosaurs. Acta Palaeontologica Polonica 25:185–195.
- Bever, G. S., and M. A. Norell. 2009. The perinate skull of Byronosaurus (Troodontidae) with observations on the cranial ontogeny of paravian theropods. American Museum Novitates 3657:1–51.
- Brusatte, S. L., and P. C. Sereno. 2007. A new species of Carcharodontosaurus (Dinosauria: Theropoda) from the Cenomanian of Niger and a revision of the genus. Journal of Vertebrate Paleontology 27:902–916.
- Clark, J. M., T. Maryanska, and R. Barsbold. 2004. Therizinosauroidea; pp. 151–164 in D. B. Weishampel, P. Dodson, and H. Osmólska (eds.), The Dinosauria, second edition. University of California Press, Berkeley, California.
- Clark, J. M., M. A. Norell, and T. Rowe. 2002. Cranial anatomy of Citipati osmolskae (Theropoda, Oviraptorosauria), and a reinterpretation of the holotype of Oviraptor philoceratops. American Museum Novitates 3364:1–24.
- Clark, J. M., A. Perle, and M. A. Norell. 1994. The skull of Erlicosaurus andrewsi, a Late Cretaceous “Segnosaur” (Theropoda: Therizinosauridae) from Mongolia. American Museum Novitates 3115:1–39.
- Coria, R. A., and P. J. Currie. 2002. The braincase of Giganotosaurus carolinii (Dinosauria: Theropoda) from the Upper Cretaceous of Argentina. Journal of Vertebrate Paleontology 22:802–811.
- Currie, P. J. 1985. Cranial anatomy of Stenonychosaurus inequalis (Saurischia, Theropoda) and its bearing on the origin of birds. Canadian Journal of Earth Sciences 22:1643–1658.
- Currie, P. J., and X.-J. Zhao. 1993a. A new troodontid (Dinosauria, Theropoda) braincase from the Dinosaur Park Formation (Campanian) of Alberta. Canadian Journal of Earth Sciences 30:2231–2247.
- Currie, P. J., and X.-J. Zhao. 1993b. A new large theropod (Dinosauria, Theropoda) from the Jurassic of Xinjiang, People's Republic of China. Canadian Journal of Earth Sciences 30:2037–2081.
- Davit-Béal, T., A. S. Tucker, and J.-Y. Sire. 2009. Loss of teeth and enamel in tetrapods: fossil record, genetic data and morphological adaptations. Journal of Anatomy 214:477–501.
- Eddy, D. R., and J. A. Clarke. 2011. New information on the cranial anatomy of Acrocanthosaurus atokensis and its implications for the phylogeny of Allosauroidea (Dinosauria: Theropoda). PLoS ONE 6:e17932. doi: 10.1371/journal.pone.0017932.
- Gauthier, J. A. 1986. Saurischian monophyly and the origin of birds; pp. 1–55 in K. Padian (ed.), The Origin of Birds and the Evolution of Flight. Memoirs of the California Academy of Sciences 8.
- Hieronymus, T., and L. M. Witmer. 2010. Homology and evolution of avian compound rhamphothecae. Auk 127:590–604.
- Holliday, C. M. 2009. New insights into dinosaur jaw muscle anatomy. The Anatomical Record 292:1246–1265.
- Hu, D., X. Xu, L. Hou, and C. Sullivan. 2012. A new enantiornithine bird from the Lower Cretaceous of Western Liaoning, China, and its implications for early avian evolution. Journal of Vertebrate Paleontology 32:639–645.
- Huene, F. von. 1914. Das natürliche System der Saurischia. Zentralblatt für Mineralogie, Geologie, und Paläontologie, Series B 1914:154–158.
- Ji, Q., P. J. Currie, M. A. Norell, and S.-A. Ji. 1998. Two feathered dinosaurs from northeastern China. Nature 393:753–761.
- Kirkland, J. I., D. Smith, and D. Wolfe. 2005a. Holotype braincase of Nothronychus mckinleyi Kirkland and Wolfe, 2001 (Theropoda, Therizinosauridae); pp. 87–96 in K. Carpenter (ed.), The Carnivorous Dinosaurs. Indiana University Press, Bloomington and Indianapolis, Indiana.
- Kirkland, J. I., L. E. Zanno, S. D. Sampson, J. M. Clark, and D. D. Deblieux. 2005b. A primitive therizinosauroid dinosaur from the Early Cretaceous of Utah. Nature 435:84–87.
- Knutsen, E. M. 2007. Beak morphology in extant birds with implications on beak morphology in ornithomimids. Masters thesis, University of Oslo, Oslo, Norway, 44 pp.
- Kobayashi, Y., and R. Barsbold. 2005a. Reexamination of a primitive ornithomimosaur, Garudimimus brevipes Barsbold, 1981 (Dinosauria: Theropoda), from the Late Cretaceous of Mongolia. Canadian Journal of Earth Sciences 42:1501–1521.
- Kobayashi, Y., and R. Barsbold. 2005b. Anatomy of Harpymimus okladnikovi Barsbold and Perle 1984 (Dinosauria; Theropoda) of Mongolia; pp 97–126 in K. Carpenter (ed.), The Carnivorous Dinosaurs. Indiana University Press, Bloomington and Indianapolis, Indiana.
- Kobayashi, Y., and J. C. Lü. 2003. A new ornithomimid dinosaur with gregarious habits from the Late Cretaceous of China. Acta Palaeontologica Polonica 48:235–259.
- Kundrat, M., A. R. I. Cruickshank, T. W. Manning, and J. Nudds. 2008. Embryos of therizinosauroid theropods from the Upper Cretaceous of China: diagnosis and analysis of ossification patterns. Acta Zoologica 89:231–251.
- Lautenschlager, S. 2013. Cranial myology and bite force performance of Erlikosaurus andrewsi: a novel approach for digital muscle reconstructions. Journal of Anatomy 222:260–272.
- Lautenschlager, S. 2014. Palaeontology in the third dimension: a comprehensive guide for the integration of three-dimensional content in publications. Paläontologische Zeitschrift 88:111–121.
- Lautenschlager, S., E. J. Rayfield, A. Perle, L. E. Zanno, and L. M. Witmer. 2012. The endocranial anatomy of Therizinosauria and its implications for sensory and cognitive function. PLoS ONE 7:e52289. doi: 10.1111/joa.12000.
- Li, D., C. Peng, H. You, M. C. Lamanna, J. D. Harris, K. J. Lacovara, and J. Zhang. 2007. A large therizinosauroid (Dinosauria: Theropoda) from the Early Cretaceous of Northwestern China. Acta Geologica Sinica 81:539–549.
- Louchart, A., and L. Viriot. 2011. From snout to beak: the loss of teeth in birds. Trends in Ecology and Evolution 26:663–673.
- Madsen, J. H., Jr. 1976. Allosaurus fragilis: a revised osteology. Utah Geological Survey Bulletin 109:1–163.
- Makovicky, P. J., and M. A. Norell. 2004. Troodontidae; pp. 184–195 in D. B. Weishampel, P. Dodson, and H. Osmólska (eds.), The Dinosauria, second edition. University of California Press, Berkeley, California.
- Makovicky, P. J., Y. Kobayashi, and P. J. Currie. 2004. Ornithomimosauria; pp. 137–150 in D. B. Weishampel, P. Dodson, and H. Osmólska (eds.), The Dinosauria, second edition. University of California Press, Berkeley, California.
- Makovicky, P. J., M. A. Norell, J. M. Clark, and T. Rowe. 2003. Osteology and relationships of Byronosaurus jaffei (Theropoda: Troodontidae). American Museum Novitates 3402:1–32.
- Maleev, E. A. 1954. A new turtle-like reptile in Mongolia. Priroda 954:106–108. [Russian]
- Marsh, O. C. 1881. Principal characters of American Jurassic dinosaurs, Part V. American Journal of Science, Series 3 21:417–423.
- Morhardt, A., M. Bonnan, and T. Keillor. 2009. Dinosaur smiles: correlating premaxilla, maxilla, and dentary foramina counts with extra-oral structures in amniotes and its implications for dinosaurs. Journal of Vertebrate Paleontology, Program and Abstracts 29:152A.
- Nicholls, E. L., and A. P. Russell. 1985. Structure and function of the pectoral girdle and forelimb of Struthiomimus altus (Theropoda: Ornithomimidae). Palaeontology 28:643–677.
- Norell, M. A., P. J. Makovicky, and P. J. Currie. 2001. The beak of ostrich dinosaurs. Nature 412:873–874.
- Norell, M. A., J. M. Clark, A. H. Turner, P. J. Makovicky, R. Barsbold, and T. Rowe. 2006. A new dromaeosaurid theropod from Ukhaa Tolgod (Ömnögov, Mongolia). American Museum Novitates 3545:1–51.
- Norell, M. A., P. J. Makovicky, G. S. Bever, A. M. Balanoff, J. M. Clark, R. Barsbold, and T. Rowe. 2009. A review of the Mongolian Cretaceous Dinosaur Saurornithoides (Troodontidae: Theropoda). American Museum Novitates 3654:1–63.
- O’Connor, J. K., and L. M. Chiappe. 2011. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. Journal of Systematic Palaeontology 9:135–157.
- Osmolska, H., E. Roniewicz, and R. Barsbold. 1972. A new dinosaur, Gallimimus bullatus n. gen., n. sp. (Ornithomimidae) from the Upper Cretaceous of Mongolia. Palaeontologia Polonica 27:103–143.
- Owen, R. 1842. Report on British fossil reptiles, Part II. Reports of the British Association for the Advancement of Science 11:60–204.
- Paul, G. S. 1984. The segnosaurian dinosaurs: relics of the prosauropod-ornithischian transition? Journal of Vertebrate Paleontology 4:507–515.
- Perez-Moreno, B. P., J. L. Sanz, A. Buscalioni, J. J. Moratalla, F. Ortega, and D. Rasskin-Gutman. 1994. A unique multitoothed ornithomimosaur dinosaur from the Lower Cretaceous of Spain. Nature 370:363–367.
- Perle, A. 1979. Segnosauridae – a new family of theropods from the Late Cretaceous of Mongolia. Joint Soviet-Mongolian Expedition, Transactions 8:45–55. [Russian]
- Perle, A. 1981. A new segnosaurid from the Upper Cretaceous of Mongolia. Joint Soviet-Mongolian Paleontological Expedition, Transactions 15:59–50. [Russian]
- Pu, H.-Y., Y. Kobayashi, J. Lü, L. Xu, Y. Wu, H. Chang, J. Zhang, and S. Jia. 2013. An unusual basal therizinosaur dinosaur with an ornithischian dental arrangement from northeastern China. PLoS ONE 8:e63423. doi: 10.1371/journal.pone.0063423.
- Russell, D. A. 1972. Ostrich dinosaurs from the Late Cretaceous of Western Canada. Canadian Journal of Earth Sciences 9:375–402.
- Russell, D. A. 1997. Therizinosauria; pp. 729–730 in P. J. Currie and K. Padian (eds.), Encyclopedia of Dinosaurs. Academic Press, San Diego.
- Russell, D. A., and Z.-M. Dong. 1993. The affinities of a new theropod from the Alxa-Desert, Inner Mongolia, People's Republic of China. Canadian Journal of Earth Sciences 30:2107–2127.
- Sampson, S. D., and L. M. Witmer. 2007. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar; pp. 32–102 in S. D. Sampson and D. W. Krause (eds.), Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Society of Vertebrate Paleontology, Memoir 8.
- Seeley, H. G. 1887. On the classification of the fossil animals commonly named Dinosauria. Proceedings of the Royal Society of London 43:165–171.
- Senter, P., and J. M. Parrish. 2005. Functional analysis of the hands of the theropod dinosaur Chirostenotes pergracilis: evidence for an unusual paleoecological role. PaleoBios 25:9–19.
- Shuvalov, V. E. 2000. The Cretaceous stratigraphy and palaeobiogeography of Mongolia; pp. 256–278 in M. J. Benton, M. A. Shishkin, D. M. Unwin, and E. N. Kuroschkin (eds.), The Age of Dinosaurs in Russia and Mongolia. Cambridge University Press, Cambridge, U.K.
- Smith, D. K., L. E. Zanno, R. K. Sanders, D. D. Deblieux, and J. I. Kirkland. 2011. New information on the braincase of the North American therizinosaurian (Theropoda, Maniraptora) Falcarius utahensis. Journal of Vertebrate Paleontology 31:387–404.
- Snively, E., and A. P. Russell. 2007. Functional variation of neck muscles and their relation to feeding style in tyrannosauridae and other large theropod dinosaurs. The Anatomical Record 290:934–957.
- Witmer, L. M. 1997. Craniofacial air sinus systems; pp. 151–159 in P. J. Currie and K. Padian (eds.), The Encyclopedia of Dinosaurs. Academic Press, New York.
- Xu, X., and X.-L. Wang. 1999. A therizinosauroid dinosaur with integumentary structures from China. Nature 399:350–354.
- Xu, X., and X.-C. Wu. 2001. Cranial morphology of Sinornithosaurus millenii Xu et al. 1999 (Dinosauria: Theropoda: Dromaeosauridae) from the Yixian Formation of Liaoning, China. Canadian Journal of Earth Sciences 38:1739–1752.
- Xu, X., X. Zheng, and H. You. 2009a. A new feather type in a nonavian theropod and the early evolution of feathers. Proceedings of the National Academy of Sciences of the United States of America 106:832–834.
- Xu, X., M. A. Norell, X.-L. Wang, P. J. Makovicky, and X.-C. Wu. 2002b. A basal troodontid from the Early Cretaceous of China. Nature 415:780–784.
- Xu, X., X.-H. Zhang, P. C. Sereno, X. Zhao, X.-W. Kuang, J. Han, and L. Tan. 2002a. A new therizinosauroid (Dinosauria, Theropoda) from the Upper Cretaceous Iren Danasu Formation of Nei Mongol. Vertebrata PalAsiatica 3:228–240.
- Xu, X., J. M. Clark, J. Mo, J. Choiniere, C. A. Forster, G. M. Erickson, D. W. E. Hone, C. Sullivan, D. A. Eberth, S. Nesbitt, Q. Zhao, R. Hernandez, C.-K. Jia, F.-L. Han, and Y. Guo. 2009b. A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature 459:940–944.
- Zanno, L. E. 2006. The pectoral girdle and forelimb of the primitive therizinosauroid Falcarius utahensis (Theropoda, Maniraptora): analyzing evolutionary trends within Therizinosauroidea. Journal of Vertebrate Paleontology 26:635–650.
- Zanno, L. E. 2010a. Osteology of Falcarius utahensis (Dinosauria: Theropoda): characterizing the anatomy of basal therizinosaurs. Zoological Journal of the Linnean Society 158:196–230.
- Zanno, L. E. 2010b. A taxonomic and phylogenetic re-evaluation of Therizinosauria (Dinosauria: Maniraptora). Journal of Systematic Palaeontology 8:503–543.
- Zanno, L. E., and P. J. Makovicky. 2011. Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proceedings of the National Academy of Sciences of the United States of America 108:232–237.
- Zanno, L. E., D. D. Gillette, L. B. Albright, and A. L. Titus. 2009. A new North American therizinosaurid and the role of herbivory in ‘predatory’ dinosaur evolution. Proceedings of the Royal Society B 276:3505–3511.
- Zhang, X.-H., X. Xu, X. Zhao, P. C. Sereno, X.-W. Kuang, and L. Tan. 2001. A long-necked therizinosauroid dinosaur from the Upper Cretaceous Iren Dabasu Formation of Nei Mongol, People's Republic of China. Vertebrata PalAsiatica 4:282–290.
- Submitted August 3, 2013; revisions received December 4, 2013; accepted December 8, 2013. Handling editor: Paul Barrett.