ABSTRACT
We describe a new genus and species of extinct ground shark, †Diprosopovenator hilperti, gen. et sp. nov. (Elasmobranchii, Carcharhiniformes), based on a single incomplete skeleton with dentition recovered from basinal marine late Cenomanian (Metoicoceras geslinianum ammonite zone) organic-rich deposits of northern Germany. The new carcharhiniform is characterized by a unique combination of dental morphologies, indicating close architectural resemblance to the family Scyliorhinidae (catsharks). However, the very distinct tooth root morphology readily separates the new taxon from all other scyliorhinids. The extinct Cretaceous carcharhiniform †Pseudoscyliorhinus (represented by †Ps. schwarzhansi and †Ps. reussi) shares tooth root morphologies and vascularization patterns with †Diprosopovenator, gen. nov. We hypothesize that these two sharks form part of an extinct group of carcharhiniforms characterized by a distinct root morphology (viz., low hemiaulacorhize roots with very flat and strongly flared basal faces protruding below the crown labially and mesiodistally and with a well-developed central labiobasal notch). Consequently, we propose a new family of Late Cretaceous carcharhiniforms, †Pseudoscyliorhinidae, fam. nov., to include the new taxon, as well as †Pseudoscyliorhinus. †Pseudoscyliorhinidae, fam. nov., shows a wide European distribution during the Late Cretaceous, ranging from the early Cenomanian to the late Campanian. The longevity of Scyliorhinidae, with a fossil record extending back into the Middle Jurassic, however, remains ambiguous and unresolved; therefore, it may be best to regard the assignment of fossil taxa to Scyliorhinidae as currently uncertain pending further taxonomic work.
INTRODUCTION
Elasmobranchii sensu Maisey (Citation2012) (= Neoselachii Compagno, 1977) form a highly diversified monophyletic group of marine vertebrates encompassing all living sharks, rays, and skates, as well as their fossil relatives, which first appeared in the early Permian (Ivanov, Citation2005). Within the Elasmobranchii, two major shark groups, Galeomorphii and Squalomorphii, are recurrently supported by various molecular studies (e.g., Maisey et al., Citation2004; Winchell et al., Citation2004; Human et al., Citation2006; Mallatt and Winchell, Citation2007; Naylor et al., Citation2012). With more than 280 recognized extant species, the galeomorph order Carcharhiniformes (ground sharks) constitutes the most species-rich and widespread clade of living elasmobranchs (Compagno et al., Citation2005; Weigmann, Citation2016). Predominantly bound to warm tropical latitudes, carcharhiniforms show a nearly worldwide distribution, inhabiting a wide range of marine environments, from marginal marine to offshore epipelagic and even meso- to bathypelagic habitats (Compagno, Citation1984, Citation1988; Compagno et al., Citation2005).
The oldest known fossil occurrences attributable to Carcharhiniformes date back to the Middle Jurassic (Underwood and Ward, Citation2004; Cappetta, Citation2012), and these sharks seemingly became more abundant and diverse during the Cretaceous (e.g., Underwood and Ward, Citation2008a, Citation2008b; Guinot et al., Citation2013, Citation2014) onward to the Cenozoic, when most of the more modern forms had their first appearances (see Cappetta, Citation2012, and Maisey, Citation2012, for summaries). The fossil record of carcharhiniforms (and elasmobranchs in general) is greatly dominated by isolated teeth, which occur frequently in a wide range of marine depositional settings, providing discrete taxonomic features (e.g., Underwood and Ward, Citation2004, Citation2008a; Adnet, Citation2006; Underwood et al., Citation2011; Guinot et al., Citation2013, Citation2014; Carrillo-Briceño et al., Citation2016; Engelbrecht et al., Citation2017; Fuchs et al., Citation2018). Conversely, carcharhiniform skeletal material remains extremely scarce in the fossil record, particularly because their poorly mineralized cartilaginous endoskeletons are subject to specific taphonomic constraints and are, therefore, restricted to a few localities only (e.g., von der Marck, Citation1863; Cappetta, Citation1980; Kriwet and Klug, Citation2004, Citation2015; Marramà et al., Citation2018).
The great majority of described fossil carcharhiniform species have been attributed to the family Scyliorhinidae (catsharks) due to close dental similarities to extant forms traditionally classified within this clade (Underwood and Ward, Citation2008b; Cappetta, Citation2012; Maisey, Citation2012). Nevertheless, controversial issues concerning the allocation of certain taxa to Scyliorhinidae still prevail, especially in the light of recently published molecular phylogenies, which repeatedly found strong support for a nonmonophyletic Scyliorhinidae (e.g., Winchell et al., Citation2004; Iglésias et al., Citation2005; Human et al., Citation2006; Vélez-Zuazo and Agnarsson, Citation2011; Naylor et al., Citation2012), thus rendering the reception of fossil carcharhiniforms and their familial affinities difficult.
The intention of this paper is (1) to describe a new genus and species of carcharhiniform shark based on a partially preserved skeleton with dentition from the lower Upper Cretaceous basinal marine Hesseltal Formation of Hanover-Misburg in northern Germany and (2) to discuss its familial placement within the framework of recently proposed molecular phylogenies of Carcharhiniformes.
Institutional Abbreviation—RE, Stiftung Ruhr Museum, Essen, North Rhine-Westphalia, Germany.
GEOLOGICAL AND STRATIGRAPHIC FRAMEWORK
In Lower Saxony and Westphalia, early Late Cretaceous basinal marine deposits referred to the Hesseltal Formation (Hiss et al., Citation2007a, Citation2007b) crop out at several localities (e.g., Halle-Hesseltal, Lengerich, Sehnde-Höver, Hanover-Misburg, Wunstorf), providing access to thick successions of late Cenomanian to early Turonian organic-rich laminated marlstones with organic carbon (Corg) content of up to 2%, alternating with light and sometimes red limestone and marl beds (see details in Kaplan, Citation1991; Hilbrecht and Dahmer, Citation1994; Lehmann, Citation1999; Hiss et al., Citation2007a; Voigt et al., Citation2008; Richardt, Citation2010). These beds are known to have produced a large number of marine vertebrate remains, including those of bony and cartilaginous fishes (e.g., Freeß, Citation1993; Kriwet and Gloy, Citation1995; Maisch and Lehmann, Citation2000; Müller, Citation2008; Diedrich, Citation2012), followed by rare marine reptile remains (Zawischa, Citation1982; Wittler and Roth, Citation2000), but also marine invertebrates such as ammonites, bivalves, and arthropods (e.g., Neumann and Jagt, Citation2003; Wippich, Citation2005; Hauschke et al., Citation2011; Klug et al., Citation2012).
The Hesseltal Formation itself represents the central infill of a depocenter, which today is mostly eroded due to tectonic inversion of the basin center (see Voigt et al., Citation2008) during the Late Cretaceous compressional regime in central Europe (see Kley and Voigt, Citation2008, for discussion). The base of the Hesseltal Formation represents a significant third-order sequence boundary, which correlates with a prominent and widespread interregional unconformity traceable all over northwestern Europe (e.g., Robaszynski et al., Citation1998; Wilmsen, Citation2003; Gale et al., Citation2005; Voigt et al., Citation2008; Richardt and Wilmsen, Citation2012). This marked facies change corresponds to the onset of the late Cenomanian–early Turonian Oceanic Anoxic Event 2 (OAE 2), a pronounced worldwide episode of carbon cycle perturbation characterized by a major positive δ13C excursion (e.g., Schlanger and Jenkyns, Citation1976; Schlanger et al., Citation1987; Wang et al., Citation2001; Tsikos et al., Citation2004; Erbacher et al., Citation2005; Voigt et al., Citation2008) associated with global warming and widespread and extensive deposition of organic-rich marlstones (e.g., Bice and Norris, Citation2002; Huber et al., Citation2002; Wilson et al., Citation2002; Bice et al., Citation2006), as well as events of marine biotic turnovers (e.g., Schlanger et al., Citation1987; Kerr, Citation1998; Premoli Silva et al., Citation1999; Watkins et al., Citation2005). Notably, the global warming episode associated with the OAE 2 was punctuated by a short-term, but severe cooling pulse in the late Cenomanian (Metoicoceras geslinianum ammonite zone) that is referred to as the Plenus Cold Event, with a drop of paleowater temperatures from ca. 22°C to 14°C (Zheng et al., Citation2013).
MATERIALS AND METHODS
The fossil shark material described herein consists of an incomplete skeleton preserved on two slabs, RE A 4872/1 and RE A 4872/2, which were collected from late Cenomanian organic-rich marlstones (Metoicoceras geslinianum ammonite zone) referred to the Hesseltal Formation and which was formerly accessible in the now abandoned open-cast quarry HPCF (Hannoversche Portland Cementfabrik) II of Hanover-Misburg (52°23′14.7″N, 9°52′04.1″E, Lower Saxony, Germany) (). The specimen therefore was derived from the short-term cooling phase called the Plenus Cold Event. It was originally found by the private collector Karl-Heinz Hilpert (Datteln, Germany), who also prepared the specimen and subsequently donated it to the former Ruhrlandmuseum, now Ruhr Museum (Essen, North Rhine-Westphalia, Germany).
FIGURE 1. Geographic and stratigraphic information. A, B, maps of geographic location. C, Cenomanian to Turonian lithostratigraphic subdivision in Lower Saxony (compiled after Niebuhr et al., Citation2007, and Ogg et al., Citation2016). D, lithological section of the upper Cenomanian–lower Turonian transition formerly exposed in the HPCF II quarry of Hanover-Misburg (after Ernst et al., Citation1984; Maisch and Lehmann, Citation2000), with stratigraphic position of †Diprosopovenator hilperti, gen. et sp. nov., indicated by the star.
Digital photographs presented in the text were obtained using digital macro- and microphotography and a scanning electron microscope (SEM). Photographs under ultraviolet (UV) light (, ) were produced following the UV technique of Tischlinger and Arratia (Citation2013). The single tooth shown in K–O was removed from RE A 4872/2 and subsequently coated with gold/palladium for SEM analysis.
Terminology—Descriptive terms used for the endoskeletal morphology correspond to those of Compagno (Citation1999), and terms for the dental morphology largely follow Cappetta (Citation2012).
SYSTEMATIC PALEONTOLOGY
CHONDRICHTHYES Huxley, Citation1880
ELASMOBRANCHII Bonaparte, Citation1838, sensu Maisey, Citation2012
GALEOMORPHII Compagno, Citation1973
CARCHARHINIFORMES Compagno, Citation1973
†PSEUDOSCYLIORHINIDAE, fam. nov.
Diagnosis—†Pseudoscyliorhinidae, fam. nov., includes extinct carcharhiniform sharks exhibiting the following combination of dental characters: multicuspidate crowns with distinct main cusp; low roots not forming ‘V’-shaped lobes, with very flat and flared basal faces protruding labially and mesiodistally below the crown, root vascularization pattern of hemiaulacorhize type; and labial root face with well-developed central and basally open notch.
Content—†Pseudoscyliorhinus schwarzhansi Müller and Diedrich, Citation1991, †Pseudoscyliorhinus reussi (Herman, Citation1977), and †Diprosopovenator hilperti, gen. et sp. nov.
Temporal and Spatial Distribution—Early Cenomanian to late Campanian of Europe (Germany, France, England, and Ireland) (Müller and Diedrich, Citation1991; Underwood and Ward, Citation2008a; Guinot et al., Citation2013; Fischer et al., Citation2017; this study).
Etymology—In reference to the first described genus within this new family, Pseudoscyliorhinus Müller and Diedrich, Citation1991, from the lower to middle Cenomanian of Germany, which is here designated as the type genus.
†DIPROSOPOVENATOR, gen. nov.
Type Species—†Diprosopovenator hilperti, sp. nov.
Diagnosis—†Pseudoscyliorhinid shark showing the following unique combination of morphological characters: presence of a supraorbital crest above the orbit; possession of an antorbital and postorbital wall delimiting the orbit anteriorly and posteriorly, respectively; presence of a small postorbital process and a distinct sphenopterotic ridge; Meckel’s cartilage rather slender and anteroposteriorly elongated; vertebrae of cyclospondylic type and strongly hourglass-shaped without ornamentation; orthodont-type teeth; tooth crown with prominent, lingually inclined main cusp that is flanked by a single pair of short, pointed lateral cusplets; cusp and lateral cusplets with continuous, weakly developed cutting edges; absence of tooth crown ornamentation; teeth of lateral and posterior positions with distally inclined main cusp forming oblique cutting edges that reach about one-third of the way to the apex of the cusp; oblique cutting edges are associated with a pair of vertical depressions or grooves on the labial face of the crown; root low with hemiaulacorhize vascularization pattern; basal face of the root very flat and strongly flared, exhibiting a roughly semicircular to oval outline in basal view; lingual protuberance of the root rounded and weakly developed; and labial face of the root with grooves, furrows, and small foramina, especially along the labial basal edge of the root, and with a central basally open notch that gives rise to a slit-like, deeply incised mediolabial groove on the basal face of the root.
Differential Diagnosis—Teeth of †Diprosopovenator, gen. nov., differ from those of all other carcharhiniforms in the presence of unornamented crowns, combined with the presence of a main cusp with oblique cutting edges and associated labial depressions in teeth of lateral and posterior positions, and the presence of low roots with very flat and flared basal faces forming no ‘V’-shaped lobes.
Etymology—The genus name is derived from the Greek nouns di (δίς) and prósopo (πρόσωπο) meaning ‘two’ and ‘face,’ respectively, and the Latin noun venator meaning ‘hunter’ in reference to the holotype specimen, which includes portions of the skull preserved on two slabs, and its presumed predaceous lifestyle.
†DIPROSOPOVENATOR HILPERTI, gen. et sp. nov.
(–)
Paraorthacodus sp.: Diedrich, Citation2012:fig. 9e–g.
Paraorthacodus: Diedrich, Citation2014:fig. 3s.
FIGURE 2. †Diprosopovenator hilperti, gen. et sp. nov., RE A 4872/1, holotype. A, interpretative composite drawing combining RE A 4872/1 (dark gray) and RE A 4872/2 (light gray; see ). B–E, RE A 4872/1. B, under normal light; C, close-up view of placoid scales; D, interpretative drawing; E, under ultraviolet light. Abbreviations: ao.w, antorbital wall; d, dermis; hym, hyomandibula; l, left (in parentheses, e.g., ‘M.c(l)’); M.c, Meckel’s cartilage; nc, neurocranium; ot.cap, otic capsule; pl.sc; placoid scales; po.pr, postorbital process; po.w, postorbital wall; pq, palatoquadrate; r, right (in parentheses, e.g., ‘M.c(r)’); so.cr, supraorbital crest; spt.r, sphenopterotic ridge; t, teeth; v.c, vertebral centra.
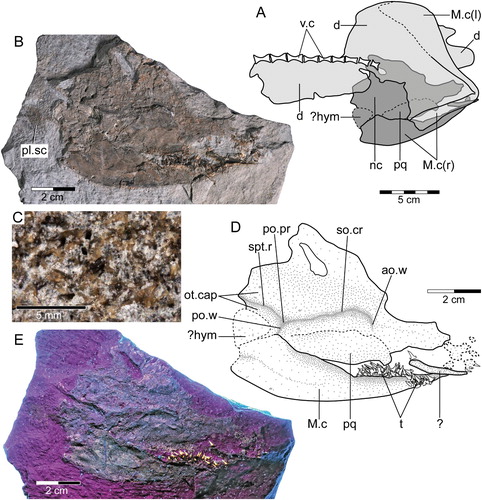
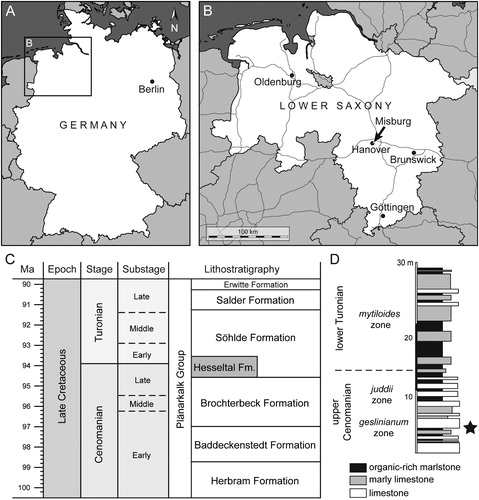
FIGURE 3. †Diprosopovenator hilperti, gen. et sp. nov., RE A 4872/2, holotype. A, under normal light; B, close-up view of vertebral column; C, under ultraviolet light. Abbreviations: l, left (in parentheses); M.c, Meckel’s cartilage; r, right (in parentheses).
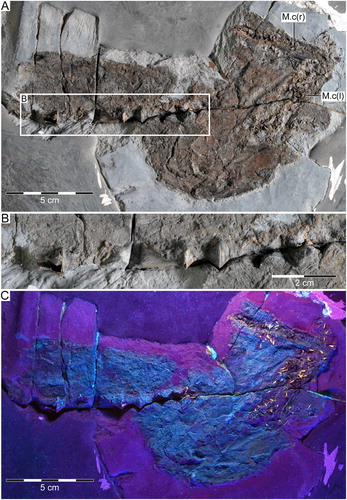
FIGURE 4. †Diprosopovenator hilperti, gen. et sp. nov., RE A 4872/1 and RE A 4872/2, holotype. Close-up views of dentitions preserved in A, RE A 4872/1 (anterior to right) and B, RE A 4872/2 (anterior to top). Abbreviations: d.gr, dental groove; l, left (in parentheses, e.g., ‘M.c(l)’); M.c, Meckel’s cartilage; pq, palatoquadrate; r, right (in parentheses, e.g., ‘M.c(r)’).
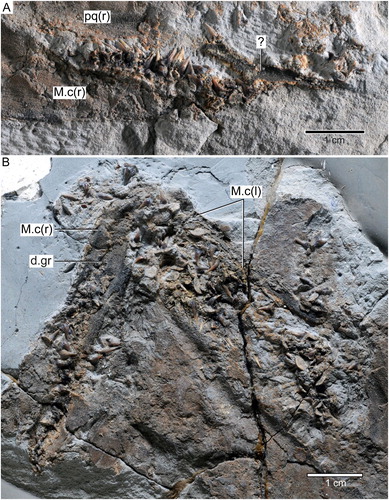
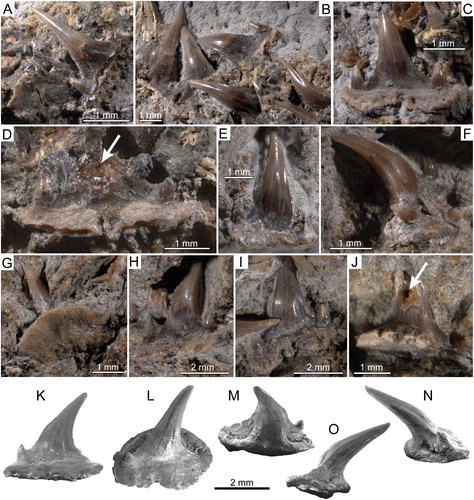
FIGURE 5. †Diprosopovenator hilperti, gen. et sp. nov., RE A 4872/1 and RE A 4872/2, holotype. Close-up views of selected teeth preserved in A–D, RE A 4872/1 and E–J, RE A 4872/2 under normal light. A, two anterolateral teeth in basal and labial views, respectively. B, lateral teeth in labial views. C, posterior tooth in labial view. D, posterior tooth with broken main cusp in labial view. E, anterior tooth in labial view. F, anterolateral tooth in oblique view. G, lateral tooth in basal view. H, lateral tooth in oblique view. I, posterolateral tooth in lingual view. J, lateral tooth in lingual view with broken main cusp. K–O, SEM pictures of lateral tooth extracted from RE A 4872/2 in K, labial, L, occlusal, M, labial, N, mesial, and, O, distal views. Pulp cavities marked by arrows.
Diagnosis—As for genus (by monotypy).
Holotype—RE A 4872/1 and RE A 4872/2, an incomplete articulated skeleton preserved on two slabs including part of the skull with dentition and part of the anterior vertebral column.
Type Locality and Horizon—Abandoned open-cast marl HPCF II quarry of Misburg (Hanover, Lower Saxony, Germany); Metoicoceras geslinianum ammonite zone (late Cenomanian, Late Cretaceous, Hesseltal Formation).
Etymology—Species name in honor of Karl-Heinz Hilpert, who found the fossil specimen and kindly donated it to the Ruhr Museum, Essen, Germany.
DESCRIPTION
The holotype and single specimen of †Diprosopovenator hilperti, gen. et sp. nov., is represented by an incompletely articulated and highly compressed skeleton preserved on two slabs, RE A 4872/1 and RE A 4872/2 (, ). The first slab, RE A 4872/1, comprises part of the neuro- and splanchocranium, including part of the upper and lower mandibular arch with associated dentition, as well as a patch of densely packed placoid scales posterior to the cranium (, ). The endocranial elements preserved in RE A 4872/1 are mostly exposed in right lateral view, except for the neurocranium, which is obliquely compressed so that part of the cranial roof is accessible.
The second slab, RE A 4872/2, also encompasses remains of the lower mandibular arch plus associated dentition, part of the anterior vertebral column comprising nine vertebral centra, as well as part of the dermis, which is visible from the inside yielding no further information (, ). A tentative composite drawing of the holotype specimen combining the fossil remains preserved on both slabs is given in A.
Due to the strong compression of the specimen, endoskeletal morphologies are difficult to discern, except for more robust parts, which still show some relief suitable for identifying discrete morphological features. In addition, the specimen shows, unfortunately, a few tool marks caused during preparation.
The description of †Diprosopovenator hilperti, gen. et sp. nov., provided below falls into two separate parts: the first relates to the endoskeleton, with RE A 4872/1 and RE A 4872/2 being addressed separately in order to avoid confusion, and the second covers the dentition of the new taxon based on both parts, RE A 4872/1 and RE A 4872/2.
RE A 4872/1
Neurocranium—The neurocranium of †Diprosopovenator hilperti, gen. et sp. nov., is accessible in RE A 4872/1 only (), whereas no parts are preserved in RE A 4872/2 (). The orbital region and part of the cranial roof are visible in right lateral and dorsal aspects, respectively, with the lower orbital region being obscured by the right palatoquadrate. The occipital region and the nasal capsule are missing. The otic capsule is for the most part covered by the supposed right hyomandibula.
The neurocranium possesses a low supraorbital crest above the orbit. The supraorbital crest is extended posteriorly to form a very small, inconspicuous postorbital process. The orbit is delimited anteriorly by the antorbital wall and posteriorly by the postorbital wall. Posteriorly, the neurocranium displays the sphenopterotic ridge that extends far backward from the rear of the postorbital process. As mentioned above, the otic capsule is mostly inaccessible, because it is obscured by the right hyomandibula ventrally.
The cranial roof is partially preserved, lacking its left lateral portion. There is a large, elongated foramen penetrating the cranial roof posteriorly. This feature, however, is here interpreted as a preservational artifact, because the parietal foramen, which anteriorly houses the paired endolymphatic foramina and posteriorly the paired perilymphatic foramina, would be expected to be much smaller and positioned further posteriorly. Cranial nerve openings penetrating the neurocranium could not be identified.
Mandibular Arch—The mandibular arch, which forms the anterior part of the splanchocranium, is partially preserved in RE A 4872/1 and includes the right palatoquadrate and the right Meckel’s cartilage, both being exposed in labial view (A, B, D, E). In addition, an elongated broken piece of cartilage is overlying the right Meckel’s cartilage anteriorly. It might be part of the right palatoquadrate or a labial cartilage.
The palatoquadrate is incompletely preserved and is slightly overlain by the right Meckel’s cartilage posteriorly. Both the dorsal and the ventral margins are imperfect and hardly to discern. Teeth are not preserved.
The right Meckel’s cartilage, as preserved, is almost complete, except for its anterior-most tip, which is missing. It is anteroposteriorly elongated and measures ca. 85 mm in maximum length. At its maximum height, the right Meckel’s cartilage measures about 23 mm and tapers anteriorly. The ventral margin of the Meckel’s cartilage is gently convex. The posterior margin is incomplete. The right Meckel’s cartilage displays abundant teeth of lateral and posterior positions arranged along its dorsal margin. The teeth are slightly disarticulated but still are closely associated. In addition, there are a few isolated teeth close to the elongated broken piece of cartilage that overlies the right Meckel’s cartilage anteriorly.
Hyoid Arch—In RE A 4872/1 a very badly preserved cartilage overlying part of the otic capsule is visible, which is here tentatively identified as the right hyomandibula (A, B, D, E).
Squamation—Posterior to the supposed hyomandibula, the rock matrix bears a patch of very small, densely arranged placoid scales (B, E). The placoid scales are poorly preserved and do not provide any useful morphological information (C).
RE A 4872/2
Mandibular Arch—Endoskeletal elements present in RE A 4872/2 include parts of the paired Meckel’s cartilages and part of the anterior vertebral column only (, ).
The right and left Meckel’s cartilage form an angle of ca. 75°. The symphysis could not be discerned with certainty. The right Meckel’s cartilage is incomplete posteriorly, measuring ca. 60 mm in maximum length. It displays the dental groove, which becomes slightly wider posteriorly. The left Meckel’s cartilage appears to be almost complete, although its precise outline could not be determined due to poor preservation. Both the right and left Meckel’s cartilage preserve abundant teeth. They are disarticulated and occur on the Meckel’s cartilages but are also randomly arranged adjacent to it.
Axial Skeleton—RE A 4872/2 preserves part of the anterior vertebral column, incorporating nine cyclospondylic vertebral centra (, ). Of these, only one centrum is fairly complete, whereas the remaining centra are broken or only preserved as imprints. The centra are longer than wide, strongly hourglass-shaped, and devoid of any ornamentation, increasing in length posteriorly.
Dentition
The dentition of †Diprosopovenator hilperti, gen. et sp. nov., includes teeth that can be differentiated into those coming from anterior, lateral, and posterior positions (, ). Symphysial and parasymphysial teeth could not be identified. Whereas RE A 4872/1 preserves ca. 30 teeth (A), RE A 4872/2 displays ca. 70 teeth (B). Most teeth accessible in the holotype specimen, if not all, certainly belong to the lower dentition, pending discovery of more completely articulated material. Consequently, it is impossible to determine the precise type of heterodonty (mono- or dignathic heterodonty) in †Diprosopovenator hilperti, gen. et sp. nov. In addition, some teeth display broken tooth crowns, providing information about their histology (see below).
Anterior Teeth—Anterior teeth of †Diprosopovenator hilperti, gen. et sp. nov., are up to 4 mm high and characterized by a symmetrical tooth crown with an upright, slender, and slightly lingually inclined central cusp that is flanked by a single pair of small lateral cusplets (E). The main cusp exhibits a semicircular cross-section that becomes slightly broader basally. It displays continuous and moderately well-developed cutting edges and lacks any ornamentation. The lateral cusplets are upright, pointed, and positioned close to the main cusp. They also are devoid of any ornamentation and display continuous, but weakly developed cutting edges.
The root is low and displays a very flat basal face with an oval outline in basal view. In addition, a few tiny, irregularly arranged foramina may occur along the basal root face. The root is flared labially and mesiodistally and protrudes below the crown. The labial face of the root exhibits a somewhat rugose surface texture with grooves and furrows. The lingual root face could not be assessed, because it is not exposed in teeth of anterior positions.
Lateral Teeth—Lateral teeth are up to 4.5 mm high and closely resemble those of anterior positions, in particular in displaying a prominent, lingually inclined central cusp, a single pair of small lateral cusplets, and continuous, but weakly well-developed cutting edges (A, B, F–O). In addition, the tooth crowns lack evidence of any surface ornamentation on the labial and lingual faces as in teeth of anterior positions. Otherwise, unlike anterior teeth, those of lateral positions are characterized by broader and even more lingually inclined central cusps, which strongly overhang the root lingually. The main cusp generally also is inclined distally and exhibits a subcircular to oval cross-section. It becomes broader toward its base, where it forms oblique cutting edges that extend upward about one-third the height of the cusp (A, B, H–O). In addition, the labial face of the main cusp creates a slight vertical groove or depression on either side close to the oblique cutting edges. The paired vertical depressions or grooves on the labial crown face are more prominent in teeth of posterolateral (I) than anterolateral (A) position.
Teeth of lateral positions usually bear larger and more widely spaced lateral cusplets when compared with anterior teeth. Lateral teeth usually exhibit deep notches between the cusplets and the oblique cutting edges connecting them to the main cusp. The lateral cusplets are slender, pointed, and slightly inclined lingually. They are either straight or divergent in labial and lingual views and display weak cutting edges. A few teeth preserve broken crowns, showing a hollow pulp cavity that is surrounded by orthodentine (J).
The root of lateral teeth of †Diprosopovenator hilperti, gen. et sp. nov., is low and has a very flat basal face that is strongly flared mesiodistally and labiolingually (A, G, K–O). It protrudes below the crown mesiodistally and labially, and in basal view it displays a roughly oval outline, with a strong convex lingual margin and a rather straight to weak concave labial margin. The lingual face of the root is smoothly rounded and bears a rather prominent lingual protuberance in profile view. The labial face is uneven, with grooves and furrows as well as a few very small, irregularly arranged foramina. There is a basally open notch in the central part of the labial root face that gives rise to a narrow, deeply incised labiomedial groove that extends lingually to about the middle of the basal root face (A). In some teeth, the labial face of the root includes a few additional basally open notches close to the central one, which gives rise to the mediolabial groove (I). The root vascularization pattern is of the hemiaulacorhize type and includes a very small lingual foramen just above the lingual protuberance and a single pair of marginolingual foramina (M).
Posterior Teeth—There are at least two posterior teeth accessible in RE A 4872/1 (C, D), whereas in RE A 4872/2 no posterior teeth could be identified with certainty due to poor preservation. The teeth, as preserved, are exposed in labial view, but only one of them remains fairly intact (C). However, the principal cusp of the latter is still apically embedded in rock matrix; therefore, its precise morphology as well as the height of the tooth could not be determined. In the incomplete one, the main cusp remains broken basally, showing a hollow pulp cavity that is encased in compact, well-mineralized orthodentine (D).
The posterior teeth of †Diprosopovenator hilperti, gen. et sp. nov., seemingly are characterized by an upright and slightly distally inclined central cusp and a pair of small, pointed, and widely spaced lateral cusplets. Unlike in teeth of lateral positions, the main cusp is less lingually inclined and wider at its base. It possesses weakly developed cutting edges and forms prominent oblique cutting edges with associated but weak paired vertical depressions on the lower part of the labial face. The lateral cusplets are straight in labial view and show an only slight lingual bending with weak cutting edges. There is no evidence of any surface ornamentation along the labial face of the crown.
The root is rather high and protrudes below the crown mesiodistally and labially, but it is less spread out than in lateral teeth. The labial face of the root bears a distinct central notch and is slightly thickened along its basal edge, with small grooves and ridges and a few irregularly arranged foramina.
DISCUSSION
The single specimen RE A 4872/1 and RE A 4872/2, which is here described as a new genus and species, †Diprosopovenator hilperti, gen. et sp. nov., was previously figured by Diedrich (2012:fig. 9e–g, 2014:fig. 3s), who erroneously referred it to the Early Jurassic to Paleocene paraorthacodontid synechodontiform †Paraorthacodus Glikman, 1975 (ascribed to Hexanchiformes by Cappetta, Citation2012), which is otherwise characterized by tooth roots displaying the pseudopolyaulacorhize vascularisation pattern and a labial root depression (Klug et al., Citation2009; Klug, Citation2010). In addition, lateral and posterior teeth of †Paraorthacodus possess at least two pairs of lateral cusplets, unlike in †Diprosopovenator hilperti, gen. et sp. nov. This, combined with the pseudoosteodont tooth histology present in †Paraorthacodus (i.e., pulp cavity filled with osteodentine and encased by orthodentine; P. Jambura, pers. comm., 2019), readily distinguishes the latter from †Diprosopovenator hilperti, gen. et sp. nov., as well as other carcharhiniforms, which are generally characterized by teeth exhibiting the orthodont histotype (i.e., hollow pulp cavity surrounded by orthodonetine; Cappetta, Citation2012), except for extinct and extant species of the hemigaleid Hemipristis Agassiz, 1833–1844, which exhibit the pseudoosteodont tooth crown histology (Jambura et al., Citation2018).
Teeth of †Diprosopovenator hilperti, gen. et sp. nov., are highly distinctive among carcharhiniforms, indicating close morphological resemblance to those of extant forms traditionally included within the family Scyliorhinidae. Generally, this clade is considered to be the most speciose one within Carcharhiniformes, comprising more than 170 extant species (in 17 genera) of small to medium size and characterized by elongated bodies with the origin of the first dorsal fin over or behind the pelvic bases (e.g., Compagno, Citation1984, Citation1988), and new species are continuously being discovered and described (e.g., McCosker et al., Citation2012; Ebert and Clerkin, Citation2015; Fahmi and White, Citation2015; Kaschner et al., Citation2015; Soares et al., Citation2015; Weigmann et al., Citation2016; Weigmann and Kaschner, Citation2017).
The stratigraphic range of Scyliorhinidae is traditionally given as extending back to the Bathonian (Middle Jurassic), where the earliest tooth-based carcharhiniforms with dental morphologies similar to those of extant scyliorhinids occurred (Underwood and Ward, Citation2004). However, controversial issues concerning the taxonomic content and phylogenetic relationships of Scyliorhinidae, as well as their longevity, still prevail because the family Scyliorhinidae is repeatedly assumed to represent an assemblage of unrelated taxa based on molecular data (e.g., Winchell et al., Citation2004; Iglésias et al., Citation2005; Human et al., Citation2006; Vélez-Zuazo and Agnarsson, Citation2011; Naylor et al., Citation2012), thus rendering the familial allocation of fossil carcharhiniforms characterized by scyliorhinid-like dentitions difficult (Maisey, Citation2012). In addition, our knowledge of extant carcharhiniforms (and elasmobranchs in general) is strongly biased toward external body anatomy and proportions as well as color patterns, whereas detailed dental and endoskeletal information often is omitted from species diagnoses provided by elasmobranch neontologists, resulting in significant negative effects in assessing the taxonomic and systematic affinities of fossil species properly (Guinot et al., Citation2018).
Molecular Data
Although the monophyly of Carcharhiniformes is strongly supported by both morphological and molecular evidence (e.g., Shirai, Citation1996; Douady et al., Citation2003; Winchell et al., Citation2004; Naylor et al., Citation2012; Gkafas et al., Citation2015), the phylogenetic relationships within the clade, especially among the basal and more generalized members, still remain ambiguous because some taxa may be para- or polyphyletic, as inferred from morphological and molecular data (Maisey, Citation1984; Winchell et al., Citation2004; Iglésias et al., Citation2005; Human et al., Citation2006; Lopéz et al., Citation2006; Vélez-Zuazo and Agnarsson, Citation2011; Naylor et al., Citation2012). For instance, based on nuclear and mitochondrial ribosomal genes, Iglésias et al. (Citation2005) provided strong evidence that the family Scyliorhinidae represents a paraphyletic arrangement, with the type genus Scyliorhinus de Blainville, Citation1816, and Cephaloscyllium Gill, Citation1862, forming a basal group within Carcharhiniformes distinct from other putative scyliorhinids (i.e., Apristurus Garman, Citation1913, Asymbolus Whitley, Citation1939, Cephalurus Bigelow and Schroeder, Citation1941, Galeus Rafinesque, Citation1810, and Parmaturus Garman, Citation1906), which were found to form a group more closely related to other carcharhiniforms. A similar taxonomic grouping was already proposed by Springer (Citation1979), who provided a dichotomous key for separating two groups of scyliorhinids based on a distinct neurocranial feature: one uniting all genera characterized by the presence of a supraorbital crest on the neurocranium above the orbit (i.e., Scyliorhinus, Cephaloscyllium, Poroderma Smith, Citation1838, Atelomycterus Garman, Citation1913, Aulohalaelurus Fowler, Citation1934, and Schroederichthys Springer, Citation1966) and a second group that included genera characterized by the absence of a supraorbital crest on the neurocranium (i.e., Apristurus, Asymbolus, Cephalurus, Galeus, Parmaturus, Halaelurus Garman, Citation1913, Haploblepharus Garman, Citation1913, Holohalaelurus Fowler, Citation1934, and Pentanchus Fowler, Citation1934). Following the taxonomic scheme provided by Springer (Citation1979), Iglésias et al. (Citation2005) proposed a redefinition of Scyliorhinidae for the group containing genera characterized by the possession of a supraorbital crest, and for the second group, seemingly being a more derived group, to resurrect the family Pentanchidae, which was initially placed within Hexanchiformes by Smith and Radclive in Smith (Citation1912) to include their monospecific genus Pentanchus. However, more recent molecular phylogenies published by Vélez-Zuazo and Agnarsson (Citation2011) and Naylor et al. (Citation2012) indicate an even more complex picture of the phylogenetic relationships within basal carcharhiniforms, with Scyliorhinidae found to be a paraphyletic assemblage consisting of three distinct lineages, which disproves the phylogenetic significance of the presence or absence of a supraorbital crest on the neurocranium for better understanding the interrelationships of carcharhiniforms previously referred to Scyliorhinidae. In both studies, the most basal grouping was recovered to include the genera Scyliorhinus, Cephaloscyllium, and Poroderma; therefore, Scyliorhinidae sensu stricto should be restricted to these taxa to the exclusion of most fossil forms previously referred to Scyliorhinidae, which are best left as Carcharhiniformes incertae familiae until their phylogenetic relationships are resolved.
Fossil Record and Comparison
The oldest fossil records attributable to Carcharhiniformes are from the Middle Jurassic, where three valid species are recognized based on isolated teeth described by Underwood and Ward (Citation2004) from Bathonian-aged, near-coastal to lagoonal strata of England, incorporating their new species †Palaeoscyllium tenuidens, which the authors referred to Scyliorhinidae, and their new species †Praeproscyllium oxoniensis and †Eypea leesi, which they tentatively assigned to Proscylliidae (note that teeth of †Eypea also were reported from the Bathonian of Morocco by Cappetta, Citation2012). These three species are readily distinguished from †Diprosopovenator hilperti, gen. et sp. nov., by their distinct dental morphologies, particularly in displaying ornamented tooth crowns and distinct and clearly ‘V’-shaped root lobes. In addition, Underwood and Ward (Citation2004) reported a few strongly abraded teeth of a new, as yet unnamed carcharhiniform, which in fact might represent a form closely related to †Thiesus concavus Guinot et al., Citation2014, from the Lower Cretaceous of France (see below).
The Late Jurassic carcharhiniform fossil record incorporates abundant material described from different European localities of predominantly near-coastal to lagoonal origin, including dental and disarticulated skeletal remains, as well as holomorphic specimens of exceptional preservation (e.g., Candoni, Citation1993; Thies and Candoni, Citation1998; Kriwet, Citation1998; Underwood, Citation2002; Kriwet and |Klug, Citation2004, Citation2008, Citation2015; Thies and Leidner, Citation2011; Klug and Kriwet, Citation2013). Currently, three carcharhiniform species are recognized from the Late Jurassic of Europe, †Palaeoscyllium formosum Wagner, Citation1857, †Bavariscyllium tischlingeri Thies, Citation2005, and †Corysodon cerinensis Saint-Seine, Citation1949, and as with most other Jurassic carcharhiniforms, †Pa. formosum and †B. tischlingeri have been generally classified within Scyliorhinidae up to now, particularly based on sharing similar body shapes and proportions, as well as similar dental morphologies to extant carchariniforms traditionally attributed to Scyliorhinidae. In general, teeth of †Palaeoscyllium formosum include a single pair of lateral cusplets, thus separating them from teeth of the Middle Jurassic †P. tenuidens, which are characterized by up to three pairs of lateral cusplets and more obtuse-angled root lobes. However, the distinction between the two species remains problematic and unresolved, as exemplified by teeth described by Leuzinger et al. (Citation2017) from Kimmeridgian strata of Switzerland, which the authors tentatively identified as †Palaeoscyllium cf. formosum based on the presence of two pairs of lateral cusplets reminiscent of †Pa. tenuidens. Consequently, more material is needed in order to determine more discrete characters for use in specific distinction between †Pa. tenuidens and †Pa. formosum.
The teeth of †Bavariscyllium tischlingeri are characterized by a principal cusp with convex heels and inconspicuous, rudimentary lateral cusplets, a distinct lingual protuberance that overhangs the root, and two lateral ridges on the labial face of the crown. The exact tooth root morphology of †Bavariscyllium tischlingeri, however, remains unknown. Teeth of †Corysodon cerinensis markedly differ from those of other carcharhiniforms, particularly in having a low and rather blunt tooth crown, thus rendering the perception of its phylogenetic placement difficult (Thies and Candoni, Citation1998; Kriwet and Klug, Citation2004); therefore, a detailed species revision is needed to clarify its taxonomic affinities.
†Macrourogaleus hassei (Woodward, Citation1889) from the Tithonian of Germany, which is known from a few holomorphic specimens, has generally been considered to be a basal carcharhiniform (e.g., Kriwet and Klug, Citation2004; Underwood and Ward, Citation2008b), but a detailed reexamination of †M. hassei conducted by Klug (Citation2008) revealed that this taxon in fact belongs to Synechodontiformes, which represent a phylogenetically well-supported lineage of stem-group elasmobranchs ranging from the late Permian to the Paleocene (Klug, Citation2010).
By the Early Cretaceous, carcharhiniforms seemingly became more diverse, as they witnessed the first appearance of members of the families Triakidae and Carcharhinidae (Underwood et al., Citation1999; Guinot et al., Citation2014), and obviously, it was the time when carcharhiniforms became common in a wide variety of depositional environments ranging from marginal marine to even deep water settings (e.g., Biddle and Landemaine, Citation1988; Landemaine, Citation1991; Biddle, Citation1993; Kriwet, Citation1999; Underwood and Mitchell, Citation1999; Underwood, Citation2004; Rees, Citation2005; Sweetman and Underwood, Citation2006; Underwood and Ward, Citation2008b; Guinot et al., Citation2014; Fuchs et al., Citation2018). Most carcharhiniform species recorded from the Lower Cretaceous, which in fact are all known from isolated teeth only, have been allocated to Scyliorhinidae. They comprise †Cadierra camboensis Guinot et al., Citation2014, and †Thiesus concavus from the Valanginian of France, †Protoscyliorhinus lamaudi Biddle and Landemaine, Citation1988, from the Barremian and Albian of France and the Barremian of Spain, †Pteroscyllium speetonensis Underwood, Citation2004, and †Pt. ornatum Underwood and Mitchell, Citation1999, from the English Aptian and Albian, and †Cretascyliorhinus destombesi (Cappetta, Citation1977b) from the Albian of France and England. In addition, teeth of †Palaeoscyllium also have been reported from different European localities, including indeterminate species from the Valanginian of Poland (Rees, Citation2005) and the Barremian of England (Underwood and Ward, Citation2008b; identified as †Palaeoscyllium aff. formosum by Sweetman and Underwood, Citation2006) and †Pa. reticularis (Underwood and Mitchell, Citation1999) from the Albian of England, which represents the youngest species assigned to †Palaeoscyllium. The English Albian also has yielded the oldest fossil record referred to Scyliorhinus, consisting of a single fragmentary tooth crown ascribed to †Sc. dubius Woodward, Citation1889, by Underwood and Mitchell (Citation1999). All these forms can be clearly separated from †Diprosopovenator hilperti, gen. et sp. nov., by different dental morphologies, in particular by the presence of a tooth crown ornamentation, which includes weak to strongly developed ridges and folds on the labial and/or lingual face of the crown and rather massive roots with more or less well-developed and divergent root lobes. In addition, the teeth of †Protoscyliorhinus lamaudi, †Pteroscyllium speetonensis, and †Pteroscyllium ornatum also differ from those of †Diprosopovenator hilperti, gen. et sp. nov., in sharing a holaulacorhize root vascularization pattern. The specific generic affiliation of †Protoscyliorhinus lamaudi and even its phylogenetic placement within Galeomorphii remains unresolved and controversial, because it may represent a lamniform rather than a carcharhiniform shark (Landemaine, Citation1991; Biddle, Citation1993; Bernárdez, Citation2018). Similarly, lamniform affinities also were discussed for †Pteroscyllium (Maisey, Citation1984; Underwood, Citation2004, Citation2006; Underwood and Ward, Citation2008b), pending further studies.
The Late Cretaceous carcharhiniform fossil record displays high diversities of species, the teeth of which resemble those of extant carcharhiniforms traditionally included in Scyliorhinidae, and incorporates both dental and articulated remains (e.g., Cappetta, Citation1980; Halter, Citation1990, Citation1995; Case and Cappetta, Citation1997; Noubhani and Cappetta, Citation1997; Cappetta and Case, Citation1999; Underwood and Mitchell, Citation1999; Vullo, Citation2005; Underwood and Ward, Citation2008a, Citation2008b; Hübner and Müller, Citation2010; Guinot et al., Citation2013). Described species with scyliorhinid-like dentitions assigned to extinct genera include †Cretascyliorhinus destombesi from the Cenomanian of Germany, France, and England, as well as Coniacian deposits of Ireland (Müller and Diedrich, Citation1991; Guinot et al., Citation2013); †Crassescyliorhinus germanicus (Herman, Citation1982) from the Santonian to Campanian of England and Campanian and Maastrichtian of Germany; †Prohaploblepharus riegrafi (Müller, Citation1989) from the German, English, and Irish Campanian; †Sigmoscyllium striatum (Underwood and Ward, Citation2008a) from Santonian to Campanian deposits of England; and †Si. acuspidatum Guinot et al., Citation2013, from the French Turonian. All of these species are readily distinguished from †Diprosopovenator hilperti, gen. et sp. nov., by different crown and root characteristics, including lower, less prominent main cusps, crown ornamentation, and rather massive roots exhibiting more or less well-developed lateral lobes.
Numerous records of Scyliorhinus were described from the Late Cretaceous, occurring almost worldwide (e.g., Case, Citation1987; Cappetta, Citation1977a, Citation1980; Halter, Citation1990, Citation1995; Case and Cappetta, Citation1997; Noubhani and Cappetta, Citation1997; Cappetta and Case, Citation1999; Underwood and Mitchell, Citation1999; Vullo, Citation2005; Underwood and Ward, Citation2008a; Guinot et al., Citation2013), and nominal species include †Sc. antiquus (Agassiz, 1833–1844), †Sc. arambourgi Cappetta, Citation1980, †Sc. arlingtonensis Cappetta and Case, Citation1999, †Sc. biddlei Halter, Citation1995, †Sc. bloti Cappetta, Citation1980, †Sc. cepaeformis Halter, Citation1990, †Sc. dubius Woodward, Citation1989, †Sc. elongatus (Davis, Citation1887), †Sc. entomodon Noubhani and Cappetta, Citation1997, †Sc. ivagrantae Case and Cappetta, Citation1997, †Sc. monsaugustus Guinot et al., Citation2013, †Sc. muelleri Guinot et al., Citation2013, †Sc. reyndersi Halter, Citation1995, †Sc. sulcidens Noubhani and Cappetta, Citation1997, and †Sc. taylorensis Cappetta and Case, Citation1999. Of these, †Sc. arambourgi, †Sc. bloti, and †Sc. elongatus are known by skeletal remains from the Santonian of Lebanon (Cappetta, Citation1980).
The distinction between fossil species referred to Scyliorhinus, however, remains in many cases difficult because of very similar dental characteristics, which commonly relate to differences in crown ornamentation combined with the number of lateral cusplets and their development. In addition, the type species of Scyliorhinus, Sc. canicula de Blainville, Citation1816, is known to exhibit a strong gynandric heterodonty (Ellis and Shackley, Citation1995; Cappetta, Citation2012:fig. 252), which renders identification of fossil species assigned to Scyliorhinus, but also those placed in closely related fossil genera, difficult.
As with reported records of Scyliorhinus, Late Cretaceous occurrences of both †Protoscyliorhinus and †Pteroscyllium are documented almost worldwide (e.g., Herman, Citation1977; Cappetta, Citation1980; Landemaine, Citation1991; Müller and Diedrich, Citation1991; Kriwet, Citation1999; Bardet et al., Citation2000; Antunes and Cappetta, Citation2002; Guinot et al., Citation2013). Currently recognized species include †Protoscyliorhinus bettrechiensis Herman, Citation1977, and †Pr. magnus Landemaine, Citation1991, as well as †Pteroscyllium dubertreti Cappetta, Citation1980, †Pt. hermani Underwood and Ward, Citation2008a, †Pt. lamranii Noubhani and Cappetta, Citation1997, †Pt. nolfi Müller and Diedrich, Citation1991, †Pt. ornatum Underwood and Mitchell, Citation1999, and †Pt. signeuxi Cappetta, Citation1980. The species †Protoscyliorhinus magnus from the Cenomanian of France recently was transferred to the new lamniform Truyolsodontos from the Cenomanian of Spain by Bernádez (Citation2018), for which he erected the family Truyolsodontidae, and further research may help to resolve the phylogenetic relationships of the remaining species assigned to †Protoscyliorhinus and †Pteroscyllium.
Despite the uncertain familial relationships of various Late Cretaceous carcharhiniforms with overall dental morphologies reminiscent of recent species that are generally included in Scyliorhinidae, there are also some European Late Cretaceous carcharhiniform species that are readily united with †Diprosopovenator hilperti, gen. et sp. nov., in sharing teeth characterized by low roots with very flat and strongly flared basal faces, including †Pseudoscyliorhinus schwarzhansi Müller and Diedrich, Citation1991, †Ps. reussi (Herman, Citation1977), †Platyrhizodon gracilis Guinot et al., Citation2013, and †Pl. barbei Guinot et al., Citation2013. Likewise, a comparable root morphology is also characteristic of the monotypic genus †Platyrhizoscyllium jaegeri Adnet, Citation2006, from the Eocene (Lutetian) of France, separating this taxon, together with †Pseudoscyliorhinus, †Platyrhizodon, and †Diprosopovenator, gen. nov., from all other extinct as well as extant carcharhiniforms, suggesting that they form a distinct species group of extinct carcharhiniform sharks characterized by low roots with flat and flared basal faces. This grouping, however, remains inconsistent with the root vascularization pattern found in these taxa, which in fact groups †Pseudoscyliorhinus and †Diprosopovenator, gen. nov., together, because they share the hemiaulacorhize type. Conversely, †Platyrhizodon and †Platyrhizoscyllium share tooth roots exhibiting the holaulacorhize and anaulacorhize types, respectively, and therefore may represent distinct but closely related genera pending further taxonomic work.
When initially introducing their new genus †Pseudoscyliorhinus, Müller and Diedrich (Citation1991) tentatively referred it to Scyliorhinidae based on minor dental differences from recent forms traditionally kept within this family. This taxonomic scheme was followed by Underwood and Ward (Citation2008a) and Guinot et al. (Citation2013), particularly because the characteristic low roots with flat and strongly flared basal faces were shared by †Ps. schwarzhansi and †Ps. reussi. Underwood and Ward (Citation2008a), however, argued that the small size of teeth of †Pseudoscyliorhinus, in combination with their peculiar roots, might also be attributable to early ontogenetic stages and hence morphological paedomorphism. Otherwise, the high abundance and wide environmental distribution of †Pseudoscyliorhinus teeth in both shelf as well as deeper, open marine deposits (see Guinot, Citation2013) makes it unlikely that †Pseudoscyliorhinus teeth can be unequivocally referred to those shed by young individuals only.
Consequently, given the obvious overlap in overall root morphology and root vascularization pattern of †Diprosopovenator, gen. nov., and †Pseudoscyliorhinus, we assign these two genera to a new family of Late Cretaceous carcharhiniform sharks, †Pseudoscyliorhinidae, to the exclusion of all other carcharhiniforms characterized by distinct catshark-like dentitions, whose familial affinities remain still ambiguous and unresolved.
Dental features for use in distinction between †Diprosopovenator, gen. nov., and †Pseudoscyliorhinus include (1) teeth of †Diprosopovenator, gen. nov., are larger, displaying a more prominent main cusp; (2) teeth of †Diprosopovenator, gen. nov., are devoid of any ornamentation, with lateral and posterior teeth being characterized by main cusps with oblique cutting edges and associated vertical depressions on the labial crown face, whereas the teeth of †Pseudoscyliorhinus are characterized by displaying distinct crown ornamentation patterns occurring on both the labial and the lingual crown face; (3) the main cusp is flanked by a single pair of lateral cusplets in †Diprosopovenator, gen. nov., contrasting with †Pseudoscyliorhinus, which is characterized by exhibiting up to two pairs of lateral cusplets; (4) the root is roughly semicircular to oval in basal aspect and more flared in †Diprosopovenator, gen. nov., whereas the root is less flared and more mesiodistally elongated in †Pseudoscyliorhinus, with a convex lingual margin and a straight to concave labial margin when viewed basally.
As revealed by the fossil record, †Pseudoscyliorhinus formed a common and widely distributed component within the European Late Cretaceous elasmobranch communities, and reported occurrences of this genus are known from northern Ireland, the Anglo-Paris Basin (i.e., England and France), and the North German Basin (i.e., northern and eastern Germany), ranging from the early Cenomanian to the late Campanian (Müller and Diedrich, Citation1991; Underwood and Ward, Citation2008a; Guinot et al., Citation2013; Fischer et al., Citation2017). In addition, the presence of †Pseudoscyliorhinus in both shelf and deeper, open marine facies suggests a rather wide tolerance range to specific ecological and/or environmental constraints in this taxon (see Guinot, Citation2013). In contrast, based on the current data available, †Diprosopovenator, gen. nov., seems to have been limited in its facies distribution toward open marine, offshore environments, probably in response to specific limiting factors, but more material is needed to test this hypothesis.
CONCLUSIONS
There is strong molecular and morphological evidence that Scyliorhinidae, which are considered the most basal group of carcharhiniform sharks, represent a nonmonophyletic grouping. Nevertheless, numerous extinct carcharhiniform taxa have been assigned to Scyliorhinidae, with a fossil record extending back into the Middle Jurassic. The unifying argument for inclusion of fossil taxa in this group is based on rather generalized tooth morphologies that, however, show distinct characters that could help to better understand their systematic placement within carcharhiniforms. However, a better understanding of dental patterns in extant carcharhiniform sharks also is mandatory (see also Guinot et al., Citation2018). The discovery of a new carcharhiniform shark, †Diprosopovenator hilperti, gen. et sp. nov., comprising a partial skeleton preserving large parts of the dentition and also cranial as well as postcranial remains from early Late Cretaceous open marine sediments allows the recognition of a distinct clade of carcharhiniform sharks based on dental traits (e.g., low roots characterized by a hemiaulacorhize vascularization pattern, with distinctly flat and strongly flared basal faces protruding below the crown labially and mesiodistally, and with a well-developed central labiobasal notch). This distinct root morphology is shared with the European Late Cretaceous carcharhiniform †Pseudoscyliorhinus (represented by †Ps. schwarzhansi and †Ps. reussi) only, despite minor morphological differences. Consequently, we introduce a new family, †Pseudoscyliorhinidae, fam. nov., for †Diprosopovenator, gen. nov., and †Pseudoscyliorhinus, which we assume to be closely related to scyliorhinids. The new taxon not only allows the identification of a new, monophyletic group within carcharhiniforms but also adds to the particularly rare endoskeletal fossil record of carcharhiniforms and elasmobranchs in general. †Pseudoscyliorhinus, which is represented by isolated dental material only, is known from numerous Cenomanian to Campanian localities throughout Europe that represent open marine to even shallow marine depositional settings, which might be attributable to different ecological and/or environmental tolerance ranges. This indicates that this group of small sharks likely were characteristic elements of Late Cretaceous marine elasmobranch associations. The reasons for the disappearance of this group in the Late Cretaceous, before the end-Cretaceous extinction event, remain ambiguous. A reevaluation of fossil elasmobranch material from the latest Cretaceous of continental northern Europe (e.g., northern Germany) might provide new insights into the stratigraphic distribution of this group. Moreover, a detailed study of fossil material represented by skeletal material from the Upper Jurassic (e.g., Solnhofen area, Germany) and Upper Cretaceous (e.g., Lebanon), combined with study of extant taxa, will provide additional skeletal information that will contribute to our understanding of the systematic content of Scyliorhinidae.
ACKNOWLEDGMENTS
We are deeply indebted to K.-H. Hilpert (Datteln, Germany) who recovered the fossil specimen and kindly donated it to the Ruhr Museum, Essen, Germany. Rudolf Gold (Department of Palaeontology, University of Vienna) is acknowledged for helping with the UV photographs. We also thank A. Engelbrecht (Department of Palaeontology, University of Vienna) for preparing the SEM photos of the extracted tooth. The manuscript has been greatly improved by the critical reviews of two anonymous referees.
ORCID
Sebastian Stumpf http://orcid.org/0000-0002-1945-2387
Jürgen Kriwet http://orcid.org/0000-0002-6439-8455
LITERATURE CITED
- Adnet, S. 2006. Nouvelles faunes de Sélaciens (Elasmobranchii, Neoselachii) de L’Eocène moyen des Landes (Sud-Ouest, France)—implications dans la connaissance des communautés de sélaciens d’eaux profundes. Palaeo Ichthyologica 10:1–128.
- Agassiz, L. J. R. 1833–1844. Recherches sur les Poissons fossiles, Volume 3. Petitpierre, Neuchâtel, 390 + 92 pp.
- Antunes, M. T., and H. Cappetta. 2002. Sélaciens du Crétacé (Albien–Maastrichtien d’Angola). Palaeontographica, Abteilung A 264:85–146.
- Bardet, N., H. Cappetta, X. P. Suberbiola, and M. Mouty. 2000. The marine vertebrate faunas from the Late Cretaceous phosphates of Syria. Geological Magazine 137:269–290.
- Bernárdez, E. 2018. Truyolsodontos estauni n. gen., n. sp., Truyolsodontidae, a new family of lamniform sharks from the Cenomanian of northern Spain. Annales de Paléontologie 104:175–181.
- Bice, K. L., and R. D. Norris. 2002. Possible atmospheric CO2 extremes of the Middle Cretaceous (late Albian-Turonian). Paleoceanography 17:1070. doi: 10.1029/2002PA000778.
- Bice, K. L., D. Birgel, P. A. Meyers, K. A. Dahl, K.-U. Hinrichs, and R. D. Norris. 2006. A multiple proxy and model study of the Cretaceous upper ocean temperatures and atmospheric CO2 concentrations. Paleoceanography 21:PA2002. doi: 10.1029/2005PA001203.
- Biddle, J. P. 1993. Les elasmobranches de l’Albien inférieur et moyen (Crétacé inférieur) de la Marne et de la Haute-Marne (France). Belgian Geological Survey, Professional Paper 246:191–240.
- Biddle, J. P., and O. Landemaine. 1988. Contribution à l’étude des Sélaciens du Crétacé du Bassin de Paris—découverte de quelques nouvelles espèces associées à une faune de type wealdien dans le Barrêmien supérieur (Crétacé inférieur) des environs de Troyes (Aube). Musée de Saint Dizier, Cahiers 2:1–22.
- Bigelow, H. B., and W. C. Schroeder. 1941. Cephalurus, a new genus of scyliorhinid shark with rediscription of the genotype, Catulus cephalus Gilbert. Copeia 1941:73–76.
- Blainville, H. M. D. de. 1816. Prodrome d’une distribution systématique du regne animal. Bulletin de la Société Philomatique de Paris 8:105–124.
- Bonaparte, C. L. 1838. Synopsis vertebratorum systematis. Nuovi Annali delle Scienze Naturali Bologna 2:105–133.
- Candoni, L. 1993. Découverte de Parasymbolus octevillensis gen. et sp. nov. (Scyliorhinidae–Elasmobranchii) dans le Kimméridgien de Normandie, France. Belgian Geological Survey, Professional Paper 264:147–156.
- Cappetta, H. 1977a. Observations sur quelques sélaciens du Crétacé supérieur d’Angleterre avec la description d’un genre nouveau. Geobios 10:479–485.
- Cappetta, H. 1977b. Sélaciens nouveaux de l’Albien supérieur de Wissant (Pas-de-Calais). Geobios 10:967–973.
- Cappetta, H. 1980. Les sélaciens du Crétacé supérieur du Liban 1—Requins. Palaeontographica Abteilung A 168:69–148.
- Cappetta, H. 2012. Chondrichthyes—Mesozoic and Cenozoic Elasmobranchii. Handbook of Paleoichthyology 3E. Verlag Dr. Friedrich Pfeil, Munich, Germany, 512 pp.
- Cappetta, H., and G. R. Case. 1999. Additions aux faunes de sélaciens du Crétacé du Texas (Albien supérieur-Campanien). Palaeo Ichthyologica 9:5–111.
- Carrillo-Briceño, J. D., O. A. Aguilera, C. de Gracia, G. Aguirre-Fernández, R. Kindlimann, and M. R. Sánchez-Villagra. 2016. An Early Neogene Elasmobranch fauna from the southern Caribbean (Western Venezuela). Palaeontologica Electronica 19(2):27A. doi: 10.26879/664.
- Case, G. R. 1987. A new selachian fauna from the Late Campanian of Wyoming (Teapot Sandstone Member, Mesaverde Formation, Big Horn Basin). Palaeontographica Abteilung A 197:1–37.
- Case, G. R., and H. Cappetta. 1997. A new selachian fauna from the Late Maastrichtian of Texas (Upper Cretaceous/Navarro Group; Kemp Formation). Münchner Geowissenschaftliche Abhandlungen A 34:131–189.
- Compagno, L. J. V. 1973. Interrelationships of living elasmobranches; pp. 15–61 in P. H. Greenwood, R. S. Miles, and C. Patterson (eds.), Interrelationships of Fishes. Zoological Journal of Linnean Society 53(1, Supplement).
- Compagno, L. J. V. 1977. Phylogenetic relationships of living sharks and rays. American Zoologist 17:303–322.
- Compagno, L. J. V. 1984. Sharks of the world–An annotated and illustrated catalogue of shark species known to date, Part 2, Carcharhiniformes. FAO Fisheries Synopsis 125:251–655.
- Compagno, L. J. V. 1988. Sharks of the Order Carcharhiniformes. Princeton University Press, Princeton, New Jersey, 486 pp.
- Compagno, L. J. V. 1999. Endoskeleton; pp. 69–92 in W. C. Hamlett (ed.), Sharks, Skates, and Rays—The Biology of Elasmobranch Fishes. The Johns Hopkins University Press, Baltimore, Maryland.
- Compagno, L. J. V., M. Dando, and S. Fowler. 2005. A Field Guide to the Sharks of the World. Harper Collins, London, 368 pp.
- Davis, J. W. 1887. The fossil fishes of the chalk of Mount Lebanon, in Syria. Scientific Transactions of the Royal Dublin Society 2:457–636.
- Diedrich, C. 2012. Stomach and gastrointestinal tract contents in Late Cenomanian (Upper Cretaceous) teleosts from Black Shales of Germany and analysis of fish mortality and food chains in the upwelling influenced pre-North Sea Basin of Europe. New Mexico Museum of Natural History and Science 57:241–254.
- Diedrich, C. 2014. Skeleton of the fossil shark Isurus denticulatus from the Turonian (Late Cretaceous) of Germany—ecological coevolution with prey of mackerel sharks. Paleontology Journal. doi: 10.1155/2014/934235.
- Douady, C. J., M. Dosay, M. S. Shivji, and M. J. Stanhope. 2003. Molecular phylogenetic evidence refuting the hypothesis of Batoidea (rays and skates) as derived sharks. Molecular Phylogenetics and Evolution 26:215–221.
- Ebert, D. A., and P. J. Clerkin. 2015. A new species of deep-sea catshark (Scyliorhinidae: Bythaelurus) from the southwestern Indian Ocean. Journal of the Ocean Science Foundation 15:53–63.
- Ellis, J. R., and S. E. Shackley. 1995. Ontogenetic changes and sexual dimorphism in the head, mouth and teeth of the lesser spotted dogfish. Journal of Fish Biology 47:155–164.
- Engelbrecht, A., T. Mörs, M. A. Reguero, and J. Kriwet. 2017. New carcharhiniform sharks (Chondrichthyes, Elasmobranchii) from the early to middle Eocene of Seymour Island, Antarctic Peninsula. Journal of Vertebrate Paleontology. doi: 10.1080/02724634.2017.1371724.
- Erbacher, J., O. Friedrich, P. A. Wilson, H. Birch, and J. Mutterlose. 2005. Stable organic carbon isotope stratigraphy across Oceanic Anoxic Event 2 of Demerara Rise, western tropical Atlantic. Geochemistry, Geophysics, Geosystems 6:Q06010. doi: 10.1029/2004GC000850.
- Ernst, G., C. J. Wood, and H. Hilbrecht. 1984. The Cenomanian-Turonian boundary problem in NW-Germany with comments on the north-south correlation to the Regensburg Area. Bulletin of the Geological Society of Denmark 33:103–113.
- Fahmi, and W. T. White. 2015. Atelomycterus erdmanni, a new species of catshark (Scyliorhinidae: Carcharhiniformes) from Indonesia. Journal of the Ocean Science Foundation 14:14–27.
- Fischer, J., I. Kogan, E. Popov, N. Janetschke, and M. Licht. 2017. The Late Cretaceous chondrichthyan fauna of the Elbtal Group (Saxony, Germany). Research & Knowledge 3:13–17.
- Fowler, H. W. 1934. Descriptions of new fishes obtained 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Proceedings of the Academy of Natural Sciences of Philadelphia 85:233–367.
- Freeß, W. B. 1993. Über Palaeospinax (Pisces, Neoselachii) aus der Kreide von Niedersachsen. Arbeitskreis Paläontologie Hannover 21:78–82.
- Fuchs, I., A. Engelbrecht, A. Lukeneder, and J. Kriwet. 2018. New Early Cretaceous sharks (Chondrichthyes, Elasmobranchii) from deep-water deposits of Austria. Cretaceous Research 84:245–257.
- Gale, A. S., W. J. Kennedy, S. Voigt, and I. Walaszczyk. 2005. Stratigraphy of the Upper Cenomanian-Lower Turonian chalk succession at Eastbourne, Sussex, UK—ammonites, inoceramid bivalves and stable carbon isotopes. Cretaceous Research 26:460–487.
- Garman, S. 1906. New Plagiostomia. Memoirs of the Museum of Comparative Zoölogy at Harvard College 46:203–208.
- Garman, S. 1913. The Plagiostomia (Sharks, Skates, and Rays). Memoirs of the Museum of Comparative Zoölogy at Harvard College 36:1–528.
- Gill, T. 1862. Analytical analysis of the order of Squali and revision and nomenclature of genera. Annals of the Society of Natural History of New York 7:367–408.
- Gkafas, G. A., P. Megalofonou, G. Batzakas, A. P. Apostolidis, and A. Exadactylos. 2015. Molecular phylogenetic convergence within Elasmobranchii revealed by cytochrome oxidase subunits. Biochemical Systematics and Ecology 61:510–515.
- Glikman, L. S. 1957. [On the relationships between the families Lamnidae and Odontaspidae and on new lamnid genera from the Late Cretaceous]. Trudy Geologicheskogo Muzeja ‘A. P. Karpinskogo’, Akademia Nauk SSSR 1:110–117. [Russian]
- Guinot, G. 2013. Late Cretaceous elasmobranch palaeoecology in NW Europe. Palaeogeography, Palaeoclimatology, Palaeoecology 388:23–41.
- Guinot, G., H. Cappetta, and S. Adnet. 2014. A rare elasmobranch assemblage from the Valanginian (Lower Cretaceous) of southern France. Cretaceous Research 48:54–84.
- Guinot, G., C. J. Underwood, H. Cappetta, and D. Ward. 2013. Sharks (Elasmobranchii: Euselachii) from the Late Cretaceous of France and the UK. Journal of Systematic Palaeontology 11:589–671.
- Guinot, G., S. Adnet, K. Shimada, C. J. Underwood, M. Siversson, D. J. Ward, J. Kriwet, and H. Cappetta. 2018. On the need of providing tooth morphology in descriptions of extant elasmobranch species. Zootaxa 4461:118–126.
- Halter, M. C. 1990. Additions to the fish fauna of N.W. Europe. 2. Two new species of Scyliorhinus from the Late Cretaceous (Maastrichtien) of the Limburg area (Belgium and The Netherlands). Mesozoic Research 2:219–236.
- Halter, M. C. 1995. Additions to the Fish Fauna of N.W. Europe 3—three new species of the genus Scyliorhinus from the Late Cretaceous (Campanian and Maastrichtian) of the Limburg area (Belgium and The Netherlands) with a reassignment of four additional species to the genus Scyliorhinus sensu stricto. Belgian Geological Survey, Professional Paper 278:65–109.
- Hauschke, N., L. Schöllmann, and H. Keupp. 2011. Oriented attachment of a stalked cirripede on an orthoconic heteromorph ammonite—implications for the swimming position of the latter. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 262:199–212.
- Herman, J. 1977. Les Sélachians néocrétacés et paléocénes de Belgique et des contrées limitrophes eléments d’une biostratigraphie intercontinentale. Mémoires pour servir à l’Explication des Cartes Géologiques et Minières de la Belgique 15:1–450.
- Herman, J. 1982. Die Selachier-Zähne aus der Maastricht-Stufe von Hemmoor, Niederelbe (NW-Deutschland). Geologisches Jahrbuch, Reihe A 61:129–159.
- Hilbrecht, H., and D.-D. Dahmer. 1994. Sediment dynamics during the Cenomanian-Turonian (Cretaceous) Oceanic Anoxic Event in northwestern Germany. Facies 30:63–84.
- Hiss, M., U. Kaplan, and F. Wiese. 2007a. Hesseltal-Formation; pp. 37–38 in B. Niebuhr, M. Hiss, U. Kaplan, K.-A. Tröger, S. Voigt, T. Voigt, F. Wiese, and M. Wilmsen (eds.), Lithostratigraphie der norddeutschen Oberkreide. Schriftenreihe der Deutschen Gesellschaft für Geowissenschaften 55. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, Germany.
- Hiss, M., U. Kaplan, and F. Wiese. 2007b. Hesseltal-Formation. LithoLex. Available at litholex.bgr.de/pages/Einheit.aspx?ID = 2008036. Accessed November 22, 2018.
- Huber, B. T., R. D. Norris, and K. G. MacLeod. 2002. Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology 30:123–126.
- Hübner, T., and A. Müller. 2010. Selachian teeth from Campanian sediments (Upper Cretaceous) of the Münsterland Cretaceous Basin (NW-Germany). Paläontologische Zeitschrift 84:437–455.
- Human, B. A., E. P. Owen, L. J. V. Compagno, and E. H. Harley. 2006. Testing morphologically based phylogenetic theories within the cartilaginous fishes with molecular data, with special reference to the catshark family (Chondrichthyes; Scyliorhinidae) and the interrelationships within them. Molecular Phylogenetics and Evolution 39:384–391.
- Huxley, T. H. 1880. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proceedings of the Scientific Meetings of the Zoological Society of London 1880:649–661.
- Iglésias, S. P., G. Lecointre, and D. Y. Sellos. 2005. Extensive paraphylies within sharks of the order Carcharhiniformes inferred from nuclear and mitochondrial genes. Molecular Phylogenetics and Evolution 34:569–583.
- Ivanov, A. 2005. Early Permian chondrichthyans of the Middle and South Urals. Revista Brasileira de Paleontologia 8:127–138.
- Jambura, P. L., C. Pfaff, C. J. Underwood, D. J. Ward, and J. Kriwet. 2018. Tooth mineralization and histology patterns in extinct and extant snaggletooth sharks, Hemipristis (Carcharhiniformes, Hemigaleidae)—evolutionary significance or ecological adaptation? PLoS ONE 13:e0200951. doi: 10.1371/journal.pone.0200951.
- Kaplan, U. 1991. Zur Stratigraphie der tiefen Oberkreide im Teutoburger Wald (NW-Deutschland), Teil 2—Turon und Coniac im Steinbruch des Kalkwerks Foerth, Halle/Westfalen. Bericht des naturwissenschaftlichen Vereins für Bielefeld und Umgegend 32:125–159.
- Kaschner, C. J., S. Weigmann, and R. Thiel. 2015. Bythaelurus tenuicephalus n. sp., a new deep-water catshark (Carcharhiniformes, Scyliorhinidae) from the western Indian Ocean. Zootaxa 4013:120–138.
- Kerr, A. C. 1998. Oceanic plateau formation—a cause of mass extinction and black shale deposition around the Cenomanian-Turonian boundary? Journal of the Geological Society 155:619–626.
- Kley, J., and T. Voigt. 2008. Late Cretaceous intraplate thrusting in central Europe: effect of Africa-Iberia-Europe convergence, not Alpine collision. Geology 36:839–842.
- Klug, S. 2008. The Late Jurassic neoselachian Macrourogaleus Fowler, 1947 is a palaeospinacid shark (Elasmobranchii; Synechodontiformes). Acta Geologica Polonica 58:229–234.
- Klug, S. 2010. Monophyly, phylogeny and systematic position of the †Synechodontiformes (Chondrichthyes, Neoselachii). Zoologica Scripta 39:37–49.
- Klug, S., and J. Kriwet. 2013. An offshore fish assemblage (Elasmobranchii, Actinopterygii) from the Late Jurassic of NE Spain. Paläontologische Zeitschrift 87:235–257.
- Klug, C., W. Riegraf, and J. Lehmann. 2012. Soft-part preservation in heteromorph ammonites from the Cenomanian-Turonian Boundary Event (OAE 2) in north-west Germany. Palaeontology 55:1307–1331.
- Klug, S., J. Kriwet, R. Böttcher, G. Schweigert, and G. Dietl. 2009. Skeletal anatomy of the extinct shark Paraorthacodus jurensis (Chondrichthyes; Palaeospinacidae), with comments on synechodontiform and palaeospinacid monophyly. Zoological Journal of the Linnean Society 157:107–134.
- Kriwet, J. 1998. Late Jurassic elasmobranch and actinopterygian fishes from Portugal and Spain. Cuadernos de Geologia Ibérica 24:241–260.
- Kriwet, J. 1999. Neoselachier (Pisces, Elasmobranchii) aus der Unterkreide (unteres Barremium) von Galve und Alcaine (Spanien, Provinz Teruel). Palaeo Ichthyologica 9:113–142.
- Kriwet, J., and U. Gloy. 1995. Zwei mesopelagische Raubfische (Actinopterygii: Euteleostei) aus dem Unterturon der Kronsberg-Mulde bei Hannover/Misburg (NW-Deutschland). Berliner geowissenschaftliche Abhandlungen E 16:335–355.
- Kriwet, J., and S. Klug. 2004. Late Jurassic selachians (Chondrichthyes, Elasmobranchii) from southern Germany: re-evaluation on taxonomy and diversity. Zitteliana, Reihe A 44:67–95.
- Kriwet, J., and S. Klug. 2008. Diversity and biogeography patterns of Late Jurassic neoselachians (Chondrichthyes, Elasmobranchii); pp. 55–69 in L. Cavin, A. Longbottom, and M. Richter (eds.), Fishes and the Break-up of Pangaea. Geological Society, London, Special Publications 295.
- Kriwet, J., and S. Klug. 2015. Knorpelfische (Chondrichthyes); pp. 334–359 in G. Arratia, H.-P. Schultze, H. Tischlinger, and G. Viohl (eds.), Solnhofen—Ein Fenster in die Jurazeit 2. Verlag Dr. Friedrich Pfeil, Munich, Germany.
- Landemaine, O. 1991. Sélaciens nouveaux du Crétacé Supérieur du Sud-Ouest de la France—quelques apports à la systématique des élasmobranches. SAGA Information 1:1–145.
- Lehmann, J. 1999. Integrated stratigraphy and palaeoenvironment of the Cenomanian–Lower Turonian (Upper Cretaceous) of northern Westphalia, North Germany. Facies 40:25–70.
- Leuzinger, L., G. Cuny, E. Popov, and J.-P. Billon-Bruyat. 2017. A new chondrichthyan fauna from the Late Jurassic of the Swiss Jura (Kimmeridgian) dominated by hybodonts, chimaeroids and guitarfishes. Papers in Palaeontology 3:471–511.
- López, J. A., Ryburn, O. Fedrigo, and G. J. Naylor. 2006. Phylogeny of sharks of the family Triakidae (Carcharhiniformes) and its implications for the evolution of carcharhiniform placental viviparity. Molecular Phylogenetics and Evolution 40:50–60.
- Maisch, M. W., and J. Lehmann. 2000. Tselfatia formosa Arambourg, 1943 (Teleostei) from the Upper Cretaceous of Lower Saxony (Northern Germany). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 2000:499–512.
- Maisey, J. G. 1984. Higher elasmobranch phylogeny and biostratigraphy. Zoological Journal of the Linnean Society 82:33–54.
- Maisey, J. G. 2012. What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution. Journal of Fish Biology 80:918–951.
- Maisey, J. G., G. J. P. Naylor, and D. J. Ward. 2004. Mesozoic elasmobranchs, neoselachian phylogeny and the rise of modern elasmobranch diversity; pp. 17–56 in G. Arratia and A. Tintori (eds.), Mesozoic Fishes 3—Systematics, Paleoenvironments and Biodiversity. Verlag Dr. Friedrich Pfeil, Munich, Germany.
- Mallatt, J., and C. J. Winchell. 2007. Ribosomal RNA genes and deuterostome phylogeny revisited: more cyclostomes, elasmobranchs, reptiles, and a brittle star. Molecular Phylogenetics and Evolution 43:1005–1022.
- Marramà, G., G. Carnevale, A. Engelbrecht, K. M. Claeson, R. Zorzin, M. Fornasiero, and J. Kriwet. 2018. A synoptic review of the Eocene (Ypresian) cartilaginous fishes (Chondrichthyes: Holocephali, Elasmobranchii) of the Bolca Konservat-Lagerstätte, Italy. Paläontologische Zeitschrift 92:283–313.
- McCosker, J. E., D. J. Long, and C. C. Baldwin. 2012. Description of a new species of deepwater catshark, Bythaelurus giddingsi sp. nov., from the Galápagos Islands (Chondrichthyes: Carcharhiniformes: Scyliorhinidae). Zootaxa 3221:48–59.
- Müller, A. 1989. Selachier (Pisces: Neoselachii) aus dem höheren Campanium (Oberkreide) Westfalens (Nordrhein-Westfalen, NW-Deutschland). Geologie und Paläontologie in Westfalen 14:1–161.
- Müller, A. 2008. Ein artikulierter Fund von Ptychodus aus dem Obercenoman von Westfalen. Geologie und Paläontologie in Westfalen 70:55–63.
- Müller, A. and C. Diedrich. 1991. Selachier (Pisces, Chondrichthyes) aus dem Cenomanium von Ascheloh am Teutoburger Wald (Nordrhein-Westfalen, NW-Deutschland). Geologie und Paläontologie in Westfalen 20:5–105.
- Naylor, G. J. P., J. N. Caira, K. Jensen, K. A. M. Rosana, N. Straube, and C. Lakner. 2012. Elasmobranch phylogeny: a mitochondrial estimate based on 595 species; pp. 31–56 in J. C. Carrier, J. A. Musick, and M. R. Heithaus (eds.), The Biology of Sharks and Their Relatives. CRC Press, Boca Raton, Florida.
- Neumann, C., and J. W. M. Jagt. 2003. A ?carcineretid crab from Lower Turonian (Cretaceous) black shales of Misburg, Hannover area (Germany). Contributions to Zoology 72:161–163.
- Niebuhr, B., M. Hiss, U. Kaplan, K.-A. Tröger, S. Voigt, T. Voigt, F. Wiese, and M. Wilmsen. 2007. Lithostratigraphie der norddeutschen Oberkreide. Schriftenreihe der Deutschen Gesellschaft für Geowissenschaften 55:1–136.
- Noubhani, A., and H. Cappetta. 1997. Les Orectolobiformes, Carcharhiniformes et Myliobatiformes (Elasmobranchii, Neoselachii) des Bassins à phosphate du Maroc (Maastrichtien-Lutétien basal)—systématique, biostratigraphie, évolution et dynamique des faunes. Palaeo Ichthyologica 8:1–327.
- Ogg, J. G., G. M. Ogg, and F. M. Gradstein. 2016. A Concise Geologic Time Scale 2016. Elsevier, Amsterdam, The Netherlands, 240 pp.
- Premoli Silva, I., E. Erba, G. Slavini, C. Locatelli, and D. Verga. 1999. Biotic changes in Cretaceous oceanic anoxic events of the Tethys. Journal of Foraminiferal Research 29:352–370.
- Rafinesque, C. S. 1810. Caratteri di alcuni nuovi generi e nuove specie di animali e pinate della Sicilia, con varie osservazioni sopra i medisimi, lère partie—part 1. Stamperia Sanfilippo, Palermo, 69 pp.
- Rees, J. 2005. Neoselachian shark and ray teeth from the Valanginian, Lower Cretaceous, of Wa˛wał, central Poland. Palaeontology 48:209–221.
- Richardt, N. 2010. Das Cenoman im Teutoburger Wald bei Halle/Westfalen (NW-Deutschland)—eine integrierte stratigraphisch-sedimentologische, mikrofazielle und geophysikalische Analyse. Geologie und Paläontologie in Westfalen 78:5–60.
- Richardt, N., and M. Wilmsen. 2012. Lower Upper Cretaceous standard section of the southern Münsterland (NW Germany)—carbon stable-isotopes and sequence stratigraphy. Newsletters on Stratigraphy 45:1–24.
- Robaszynski, F., A. Gale, P. Juignet, F. Amédro, and J. Hardenbol. 1998. Sequence stratigraphy in the Cretaceous of the Anglo-Paris Basin, exemplified by the Cenomanian stage; pp. 363–385 in T. Jaquin, P. de Graciamsky, and J. Hardenbol (eds.), Mesozoic and Cenozoic Sequence Stratigraphy of European Basins. SEPM Special Publication 60. Society of Sedimentary Geology, Tulsa, U.S.A.
- Saint-Seine, P. de. 1949. Les poissons des calcaires lithographiques de Cerin (Ain). Nouvelles Archives du Muséum d’histoire naturelle de Lyon 2:159–276.
- Schlanger, S. O., and H. C. Jenkyns. 1976. Cretaceous oceanic anoxic events: causes and consequences. Geologie en Mijnbouw 55:179–184.
- Schlanger, S. O., M. A. Arthur, H. C. Jenkyns, and P. A. Scholle. 1987. The Cenomanian-Turonian oceanic anoxic event, I. Stratigraphy and distribution of organic carbon-rich beds and the marine δ13 excursion; pp. 371–399 in J. Brooks and A. J. Fleet (eds.), Marine Petroleum Source Rocks. Geological Society, London, Special Publications 26.
- Shirai, S. 1996. Phylogenetic interrelationships of neoselachians (Chondrichthyes: Euselachii); pp. 9–34 in M. Stiassny, L. Parenti, and D. Johnson (eds.), Interrelationships of Fishes. Academic Press, New York.
- Smith, A. 1838. On the necessity for a revision of the groups included in the Linnean genus Squalus. Proceedings of the Zoological Society of London 5:85–86.
- Smith, H. M. 1912. Description of a new notidanoid shark from the Philippine islands, representing a new family. Proceedings of the United States National Museum 41:489–491
- Soares, K. D. A., O. F. B. Gadig, and U. L. Gomes. 2015. Scyliorhinus ugoi, a new species of catshark from Brazil (Chondrichthyes: Carcharhiniformes: Scyliorhinidae). Zootaxa 3937:347–361.
- Springer, S. 1966. A review of Western Atlantic cat sharks, Scyliorhinidae, with descriptions of a new genus and five new species. Fishery Bulletin 65:581–624.
- Springer, S. 1979. A revision of catsharks, family Scyliorhinidae. NOAA Technical Report NMFS Circular 422:1–152.
- Sweetman, S. C., and C. J. Underwood. 2006. A neoselachian shark from the non-marine Wessex Formation (Wealden Group: Early Cretaceous, Barremian) of the Isle of Wight, southern England. Palaeontology 49:457–465.
- Thies, D. 2005. A catshark (Neoselachii, Carcharhiniformes, Scyliorhinidae) from the Late Jurassic of Germany. Paläontologische Zeitschrift 79:339–348.
- Thies, D., and L. Candoni. 1998. Corysodon Saint-Seine 1949—a valid genus of Mesozoic neoselachian sharks. Geologica et Palaeontologica 32:221–233.
- Thies, D., and A. Leidner. 2011. Sharks and guitarfishes (Elasmobranchii) from the Late Jurassic of Europe. Palaeodiversity 4:63–184.
- Tischlinger, H., and G. Arratia. 2013. Ultraviolet light as a tool for investigating Mesozoic fishes, with a focus on the ichthyofauna of the Solnhofen archipelago; pp. 549–560 in G. Arratia, H.-P. Schultze, and M. V. H. Wilson (eds.), Mesozoic Fishes 5—Global Diversity and Evolution. Verlag Dr. Friedrich Pfeil, Munich, Germany.
- Tsikos, H., H. C. Jenkyns, B. Walsworth-Bell, M. R. Petrizzo, A. Forster, S. Kolonic, E. Erba, I. Premoli Silva, M. Baas, T. Wagner, and J. S. Sinninghe Damsté. 2004. Carbon-isotope stratigraphy recorded by the Cenomanian-Turonian Oceanic Anoxic Event: correlation and implications based on three key-locations. Journal of the Geological Society of London 161:711–719.
- Underwood, C. J. 2002. Sharks, rays and a chimaeroid from the Kimmeridgian (Late Jurassic) of Ringstead, Southern England. Palaeontology 45:297–325.
- Underwood, C. J. 2004. Barremian and Aptian (Cretaceous) sharks and rays from Speeton, Yorkshire, north-east England. Proceedings of the Yorkshire Geological Society 55:107–118.
- Underwood, C. J. 2006. Diversification of the Neoselachii (Chondrichthyes) during the Jurassic and Cretaceous. Paleobiology 32:215–235.
- Underwood, C. J., and S. Mitchell. 1999. Albian and Cenomanian selachian assemblages from north east England. Special Papers in Palaeontology 60:9–56.
- Underwood, C. J., and D. J. Ward. 2004. Neoselachian sharks and rays from the British Bathonian (Middle Jurassic). Palaeontology 473:447–501.
- Underwood, C. J., and D. J. Ward. 2008a. Sharks of the order Carcharhiniformes from the British Coniacian, Santonian and Campanian (Upper Cretaceous). Palaeontology 51:509–536.
- Underwood, C. J., and D. J. Ward. 2008b. A review of the Mesozoic record of the Carcharhiniformes; pp. 433–442 in G. Arratia, H.-P. Schultze, and M. V. H. Wilson (eds.), Mesozoic Fishes 4—Homology and Phylogeny, Verlag Dr. Friedrich Pfeil, Munich, Germany.
- Underwood, C. J., S. M. Mitchell, and K. J. Veltkamp. 1999. Shark and ray teeth from the Hauterivian (Lower Cretaceous) of north-east England. Palaeontology 42:287–302.
- Underwood, C. J., D. J. Ward, C. King, S. M. Antar, I. S. Zalmout, and P. D. Gingerich. 2011. Shark and ray faunas in the Middle and Late Eocene of the Fayum Area, Egypt. Proceedings of the Geologists’ Association 122:47–66.
- Véles-Zuazo, X., and I. Agnarsson. 2011. Shark tales: a molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes). Molecular Phylogenetics and Evolution 58:207–217.
- Voigt, S., J. Erbacher, J. Mutterlose, W. Weiss, T. Westerhold, F. Wiese, M. Wilmsen, and T. Wonik. 2008. The Cenomanian–Turonian of the Wunstorf section (North Germany): global stratigraphic reference section and new orbital time scale for Oceanic Anoxic Event 2. Newsletters on Stratigraphy 43:65–89.
- von der Marck, W. 1863. Fossile Fische, Krebse und Pflanzen aus dem Plattenkalk der jüngsten Kreide in Westphalen. Palaeontographica 11:1–83.
- Vullo, R. 2005. Selachians from the type Campanian area (Late Cretaceous), Charentes, western France. Cretaceous Research 26:609–632.
- Wagner, J. A. 1857. Charakteristik neuer Arten von Knorpelfischen aus den lithographischen Schiefern der Umgegend von Solnhofen. Gelehrter Anzeiger der Bayerischen Akademie der Wissenschaften 44:288–293.
- Wang, C. S., X. M. Hu, L. Jansa, X. Q. Wan, and R. Tao. 2001. The Cenomanian-Turonian anoxic event in southern Tibet. Cretaceous Research 22:481–490.
- Watkins, D. K., M. J. Cooper, and P. A. Wilson. 2005. Calcareous nannoplankton response to late Albian oceanic anoxic event 1d in the western North Atlantic. Paleoceanography 20:PA2010. doi: 10.1029/2004PA001097.
- Weigmann, S. 2016. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. Journal of Fish Biology 88:837–1037.
- Weigmann, S., and C. J. Kaschner. 2017. Bythaelurus vivaldii, a new deep-water catshark (Carcharhiniformes, Scyliorhinidae) from the northwestern Indian Ocean off Somalia. Zootaxa 4263:97–119.
- Weigmann, S., D. A. Ebert, P. J. Clerkin, M. F. W. Stehmann, and G. J. P. Naylor. 2016. Bythaelurus bachi n. sp., a new deep-water catshark Carcharhiniformes, Scyliorhinidae) from the southwestern Indian Ocean, with a review of Bythaelurus species and a key to their identification. Zootaxa 4208:401–432.
- Whitley, G. P. 1939. Taxonomic notes on sharks and rays. Australian Zoologist 9:227–262.
- Wilmsen, M. 2003. Sequence stratigraphy palaeoceanography of the Cenomanian Stage in northern Germany. Cretaceous Research 24:525–568.
- Wilson, P. A., R. D. Norris, and M. J. Cooper. 2002. Testing the Cretaceous greenhouse hypothesis using glassy foraminiferal calcite from the core of the Turonian tropics on the Demerara Rise. Geology 30:607–610.
- Wippich, M. G. E. 2005. Ammonoideen-Kiefer (Mollusca, Cephalopoda) aus Schwarzschiefern des Cenoman/Turon-Grenzbereichs (Oberkreide) im nördlichen Westfalen. Geologie und Paläontologie in Westfalen 65:77–93.
- Winchell, C. J., A. P. Martin, and J. Mallatt. 2004. Phylogeny of elasmobranchs based on LSU and SSU ribosomal RNA genes. Molecular Phylogenetics and Evolution 31:214–224.
- Wittler, F. A., and R. Roth. 2000. Platypterygius (Reptilia, lchthyosauria) aus dem oberen Untercenoman des Teutoburger Waldes (Oberkreide, Nordwestdeutschland). Geologie und Paläontologie in Westfalen 65:7–24.
- Woodward, A. S. 1889. Catalogue of the Fossil Fishes in the British Museum (Natural History)—Part I. British Museum (Natural History), London, 474 pp.
- Zawischa, D. 1982. Saurierzähne aus Wunstorf. Arbeitskreis Paläontologie Hannover 10:16–17.
- Zheng, X.-Y., H. C. Jenkyns, A. S. Gale, D. J. Ward, and G. M. Henderson. 2013. Changing ocean circulation and hydrothermal inputs during Ocean Anoxic Event 2 (Cenomanian–Turonian)—evidence from Nd-isotopes in the European shelf sea. Earth and Planetary Science Letters 375:338–348.
