ABSTRACT
The larger species in many mammalian clades have relatively longer faces than their smaller relatives. This has been shown to be true for extant kangaroos (Macropodinae), who follow the CREA rule of positive facial allometry; but the extinct short-faced kangaroos (Sthenurinae) have not so far been examined. Using linear measurements, rather than CREA techniques, we show here that sthenurine face lengths scale with negative allometry, thus differing from the trend seen in their extant relatives.
INTRODUCTION
It has long been observed that larger mammals tend to have proportionally longer faces than their smaller relatives. While this appears to be true for extant kangaroos (Macropodidae, Macropodinae) (Cardini et al., Citation2015), the extinct short-faced kangaroos, the sthenurines (Macropodidae, Sthenurinae), have not been examined in this regard, although the extremely short faces of the largest taxa (e.g., Procoptodon goliah) led us to think that positive allometry of facial length might not be the case in this lineage.
Cranio-Facial Allometry in Mammals
Radinsky (Citation1985) examined trends in facial length in a diversity of placental families, distinguishing between the “facial skull” (the jaw apparatus, measured as the tooth-bearing portion of the skull, his Tooth Row Length—TRL, see ), and the “cerebral skull” (the portion of the skull containing the neurocranium, estimated by him as BCAL, the basicranial axis length, the length of the basioccipital plus basisphenoid bones). Radinsky (Citation1985) showed that for most families the scaling of facial length (TRL) against basicranial axis length (BCAL) was strongly positively allometric and that the scaling of the jaw length (JL, from the anterior tip of the jaw to the posterior tip of the mandibular angle) to the basicranial length was almost identical. However, Radinsky (Citation1985) cautioned that allometric pattern is mainly due to the negative allometric scaling of basicranial length with body mass, related in part (but not entirely) to the negative allometry of brain size (see also discussion of this issue in Mitchell et al. [Citation2024]). This implies that facial length actually scales isometrically, but that large animals appear to have longer faces due to a change in cranial proportions.
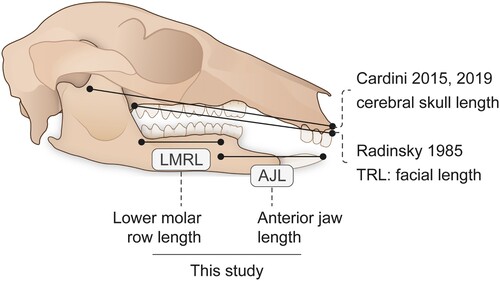
FIGURE 1. Kangaroo skull and lower jaw showing the estimates of facial length used by other authors and the linear measurements used in the investigation of face length scaling in this contribution. LMRL + AJL = our measure of facial length, TRL. Based on the extant short-eared rock wallaby, Petrogale brachyotis. Figure by www.sciencegraphicdesign.com.
A more recent examination of facial allometric scaling is the notion of the CREA rule (craniofacial evolutionary allometry), first proposed by Cardini (Cardini & Polly, Citation2013). This is the proposed evolutionary trend that larger species of a particular mammalian lineage have relatively longer faces and smaller braincases, similar in essence to Radinksy’s (Citation1985) proposal, but using scans of skulls and a complex of morphometric measurements rather than the simple linear measurements of Radinsky (Citation1985), and with a slightly different estimation of facial length (“cerebral skull length,” see ). Positive facial allometry may represent the evolutionary “line of least resistance” and is a highly conserved evolutionary pattern in many groups of mammals (e.g., Cardini, Citation2019; Rhoda et al., Citation2023). CREA measures the covariation between the braincase and the rostrum rather than specifically measuring facial length, but note that there is no proposed explanation for this pattern. Although many mammal lineages do show facial elongation with increasing size (e.g., Bibi & Tyler, Citation2022 [bovids]; Cardini & Polly, Citation2013 [antelope, bats, mongooses and squirrels]; Chemisquy et al., Citation2021; Ferreira-Cardoso et al., Citation2020 [pangolins]; Marcy et al., Citation2020 [murid rodents]; Magnus et al., Citation2018 [glirids]; Maroig, Citation2007 [primates]; Mitchell et al., Citation2018 [kangaroos]); Tamagnini et al., Citation2017 [felids]; Rhoda et al., Citation2023 [bovids]). A number of exceptions exist (e.g., Cardini & Polly [hominins]; Flores et al., Citation2018 [didelphids]; Hautier et al., Citation2014 [sloths]; Law et al., Citation2018 [musteloids]; Marcucio et al., Citation2011 [hominins]; Tamagnini et al., Citation2023 [saber-toothed felids]).
A CREA type of facial scaling is also not the rule in non-mammalian synapsids, and in particular non-mammalian cynodonts show no such pattern, either in interspecific allometry or in ontogenetic allometry (Krone et al., Citation2019). Thus, although CREA-like intraspecific allometry may be apparent in other groups of tetrapods, among synapsids mammalian facial scaling may have unique causes, possibly related to the ontogenetic change in skull shape common to mammals (and probably related to the mammalian type of reproduction) (Cardini et al., Citation2015; Krone et al., Citation2019). Mitchell et al. (Citation2024) provide a thorough review of the literature on craniofacial allometry in mammals, and conclude that there is no simple allometric rule: facial elongation (or lack thereof) relates to a number of different factors relating to cranial ecomorphological specializations, and may arise by different mechanisms.
Extant kangaroos (Macropodidae: Macropodinae) follow the CREA rule (Cardini et al., Citation2015). Cardini et al. (Citation2015) considered the issue of the extinct short-faced kangaroos (Macropodidae: Sthenurinae), and speculated that they might show the same trends as their longer-faced relatives, despite their absolutely shorter faces. However, Cardini et al. (Citation2015) did not include sthenurines in their investigation, in large part because there is insufficient fossil data for their mode of analysis. Here we use a different method to investigate face length in macropodines and sthenurines, and show that face lengths of these two subfamilies exhibit very different allometric trends.
Kangaroo Diversity and Facial Anatomy
Macropodidae (Diprotodonta: Marsupialia) includes three subfamilies of kangaroos; Macropodinae (kangaroos, wallabies, and tree-kangaroos), Lagostrophinae (the Banded Hare-wallaby being the sole extant species, not considered here) and the extinct Sthenurinae (“short-faced kangaroos,” including many of the largest macropod species, the largest ones being more than double the mass of the largest extant macropodine species: Helgen et al. [Citation2006]; Johnson & Prideaux [Citation2004]; Prideaux [Citation2004]). Both the cranial and postcranial morphologies of sthenurine kangaroos are distinct from those of macropodines, generally being more robust (Prideaux, Citation2004; Wells & Tedford, Citation1995). The earliest sthenurine is known from the Middle Miocene and the oldest macropodines from the early Late Miocene. Among the Macropodinae, only tree-kangaroos (dendrolagines) have relatively short faces, but not as short as in many sthenurines (Mitchell & Wroe, Citation2019). Dendrolagines are medium-sized macropodines, and their face length likely relates to their browsing diet rather than to allometry. In contrast, the large, grazing species of extant kangaroos (genera Macropus and Osphranter) have relatively long faces, which likely reflects diet as well as allometric scaling (Mitchell et al., Citation2018).
The Miocene sthenurines have faces of similar lengths to extant macropodines, but the Plio–Pleistocene forms divided into two distinct shorter-faced lineages of different face lengths: one (the “Sthenurus lineage”) includes species in the genus Sthenurus, which had relatively long faces (termed dolicocephalic), although they were still shorter-faced than the macropodines; the other (the “Procoptodon lineage”) includes the genera Archaeosimus, Metasthenurus, Simosthenurus, and Procoptodon, which had extremely short faces (termed brachycephalic) (Prideaux, Citation2004). The Plio-Pleistocene sthenurines’ distinct differences in face lengths are possibly reflective of differences in diet and ecology (Prideaux, Citation2004; Wells & Tedford, Citation1995). Did these shorter-faced lineages still maintain the CREA rule of positive allometry of face length, as speculated by Cardini et al. (Citation2015)? Could it be the case that the shorter face of sthenurines is simply an illusion related to their deeper skulls, as noted for the “short-faced” bear Arctodus by Figuerido et al. (Citation2010)? We show here that this is not the case for sthenurines.
Facial Length, the Cranium, and the Jaws
Although the notion of “facial length” is usually associated with the cranium, in mammals where the upper and lower incisors precisely meet, as in macropodids (and, indeed, all diprotodont marsupials), the jaw must be the same length as the anterior cranium, and the tooth row length measured along the lower jaw must perforce be the same as that in the skull, to allow for dental occlusion. Mandibles, especially fragments containing the tooth row and the anterior jaw, are much more available in the fossil record than even that corresponding portion of the skull.
As noted by Radinsky (Citation1985), the position of the tooth row in the jaw and cranium, is generally very stable across mammals due to the biomechanics of loading forces for chewing, and the inhibitory cascade of tooth development also limits the variation of the length of the entire molar row (Kavanagh et al., Citation2007). Furthermore, lower molar row length correlates well with body mass in herbivores, and so is a relatively stable measure of cranial proportions, and is not highly correlated with diet in either ungulates or macropodoids (Janis, Citation1990a, Citationb). In contrast, the premolar row length varies considerably, seen in both ungulates and macropodoids (where there is only single premolar) (Janis, Citation1990a, Citationb). The molar/premolar boundary thus appears to be a suitable point from which to estimate the length of the anterior jaw (= facial length). Thus, lengthening of the face essentially equates to the lengthening of the diastema, without the complication of variation in the size of the lower premolar.
Using the molar/premolar boundary as the point from which to define the posterior border of the face avoids issues in facial length estimation such as changes in the position of the orbit (see Mitchell et al., Citation2024). While the rostrum (the length of the cranium anterior to the orbits: e.g., Devillers et al., Citation1984) is often considered to represent face length, the position of the orbit can vary: in larger mammals the orbit is smaller, due to allometric scaling of the eye, and often is placed more posteriorly in the skull relative to the tooth row (Mitchell et al., Citation2024). Many ungulates (e.g., horses) appear to have extremely long faces, but this is due in part to the posterior position of the orbit.
MATERIALS AND METHODS
Materials
Linear morphometric measurements of tooth row length (TRL and its components, see below) were obtained from photographs and expressed in millimeters. Availability and completeness of adult specimens limited the total number of taxa investigated, but our total data set comprised 38 macropodines (including four extinct species) and 17 sthenurines (see Table S1). Photographs were deemed appropriate provided that: the specimen represented an adult individual (m4 present); the lower incisor was intact; no dental deformities were observed; and a measure of scale was included.
Photographs of extant taxa were sourced primarily from the online databases of The Natural History Museum (NHM data.nhm.ac.uk/dataset/) plus some photographs taken by CMJ at the American Museum of Natural History. Measurements on jaws were made from the lateral view. Sthenurine jaw photographs were mostly sourced from published literature (Cooke, Citation1999; Marshall, Citation1973; Kerr & Prideaux, Citation2022; Prideaux, Citation2004), with additional photographs taken by the CMJ (see Table S1). In a few cases (detailed in Table S1), when a suitable jaw (preserving the incisors) was not available, these measures were taken from photographs of partial skulls.
Body mass averages (in kg) for macropods were taken from Animal Diversity Web (https://animaldiversity.org/) for extant taxa and from Butler et al. (Citation2017), Helgen et al. (Citation2006), Johnson and Prideaux (Citation2004), and Wagstaffe et al. (Citation2022) for extinct taxa (see Table S1 for further details).
Methods
We used linear measurements () from the lower jaw. Radinsky (Citation1985) showed the length of the lower jaw to have similar allometric patterns to his tooth row length estimates of facial length; see Discussion. These data are unlike the estimations of CREA, a methodology which requires scans of skulls. Complete undamaged skulls of sthenurines are available for only a few taxa. Our method is thus more similar to that of Radinsky (Citation1985), except we are only considering the length of the face, not a comparison with the basicranial portions of the skull. We consider that this is the best that can be done for study of these two closely related clades, given the rarity of cranial material.
In order to investigate changing proportions of regions of the lower jaw, we broke up the jaw length (TRL) into two components: the lower molar row length (MRL), and the anterior part of the jaw (AJL, essentially the diastema length, measured from the molar/premolar boundary to the tip of the lower jaw). As diprotodontid marsupials have long protruding lower incisors that match up with a considerable length of the skull above the jaw, we took this measurement to the tip of the first incisor (see ), rather than to the anterior incisor alveolus (as in Radinsky, Citation1985).
We plotted two sets of linear variables (measured in mm) against body mass (in kg): the tooth row length (TRL, as per Radinsky [Citation1985] = AJL + MRL), and a measure of the ratio between the anterior jaw length versus the molar row (jaw length ratio, or JLR, = AJL/MRL). The scaling of facial length () was shown by creating a scatterplot of the log of JLR (jaw length ratio) against the log of body mass. The original plot, created in R (R Core Team, Citation2021), was further edited using Adobe Illustrator 27.0 (Adobe, Citation2022).
The significance of any differences in slope values between these two regressions was evaluated using base R (ver. 4.1.1) (R Core Team, Citation2021). A linear model (OLS) was built on the full dataset using JLR, body mass, and a “dummy” variable as an interaction (that is, in this case, the grouping variable that identifies the taxon to which each data point belongs). Then an ANOVA on the model’s output allowed extraction of the separate regressions and a significance value for any differences in slopes. (The R code is included in the supplementary information.)
It is worth noting that the question we are addressing is whether two slopes are different from each other, and not whether regression slopes are different from zero (which is the default for most statistical software). We are interested here in the linear trends, but not for the purpose of prediction. Thus, zero is just another slope value and not critical or even particularly interesting information. Also, we are not seeking an estimate of the “true” value of the structural relationship between any two variables, and thus OLS is fine for our purposes; there is no reason to replace it with methods that make specific assumptions about the error variances in the variables, such as Major Axis or Reduced Major Axis.
We did not attempt to phylogenetically correct our data, especially as we are considering two sister lineages of similar cranial anatomy, not a diversity of different mammalian forms. Mitchell et al. (Citation2024) have noted that size often co-varies with phylogeny, as would be expected from Cope’s Rule. shows a phylogeny of the macropodid taxa included in this study, and it is clear that, separately in the macropodines, and in the two lineages of sthenurines, larger sizes are not equally distributed along the phylogeny but cluster in the more derived portions of the tree. It becomes impossible to statistically distinguish between shape variation related to size and shape variation due to evolutionary divergence if size and phylogeny co-vary. Phylogenetic correction in such cases would merely remove the allometric signal. It has been noted that inclusion of phylogenetic relatedness in tests should not be used in cases where the predictor trait (here = cranial size) itself carries a phylogenetic signal (Rohlf, Citation2001, Citation2006; Uyeda et al., Citation2018) (see further discussion in Mitchell et al., Citation2024).
FIGURE 2. Phylogeny of the Macropodidae showing the relationship of body size with phylogenetic position. Macropodine phylogeny taken from Westerman et al. (Citation2022). Sthenurine phylogeny taken from Prideaux (Citation2004). Figure by www.sciencegraphicdesign.com.
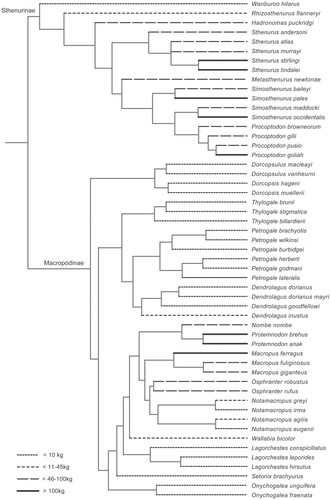
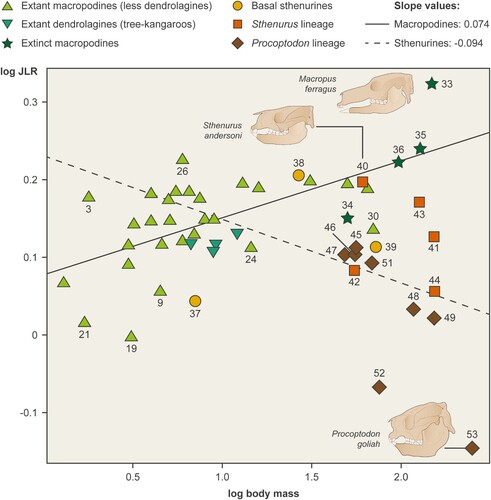
FIGURE 3. Scaling of log jaw length ratio (JLR) against log body mass. Macropodines scale positively with slope 0.074 and sthenurines scale negatively with slope –0.094. Difference in slopes is highly significant (p = 0.0012). The numbers identifying the taxa are as follows (only extinct taxa and outlier extant taxa are identified here). Extant outliers: 3 = Dorcopsulus vanheurni, 9 = Petrogale brachyotis, 21 = Lagorchestes hirsutus, 24 = Wallabia bicolor, 26 = Notamacropus eugenii, 30 = Osphranter rufus. Extinct taxa: 33 = Macropus ferragus, 34 = Nombe nombe (protemnodontid), 35 = Protemnodon sp., 36 = Protemnodon brehus, 37 = Wanburoo hilarus, 38 = Rhizosthenurus flanneryi, 39 = Hadronomas puckridgi, 40 = Sthenurus andersoni, 41 = Sthenurus atlas, 42 = Sthenurus murrayi, 43 = Sthenurus tindalei, 44 = Sthenurus stirlingi, 45 = Metasthenurus newtoni, 46 = Simosthenurus baileyi, 47 = Simosthenurus maddocki, 48 = Simosthenurus occidentalis, 49 = Procoptodon baileyi, 50 = Procoptodon browneorum, 51 = Procoptodon gilli, 52 = Procoptodon pusio, 53 = Procopotodon goliah. Figure by www.sciencegraphicdesign.com.
RESULTS
Radinsky’s tooth row length (TRL) plotted against body mass showed a virtually identical isometric relationship for both macropodines (slope = 0.318, intercept = 1.502) and sthenurines (slope = 0.310, intercept = 1.496). This is what Radinsky (Citation1985) might have predicted (TRL scales with isometry in his plots), and is in contrast to the CREA findings in macropodids of overall cranial proportions (Cardini et al., Citation2015). However, plotting JLR against body mass confirms the primary hypothesis of this paper: the facial lengths of macropodines scale with positive allometry, while sthenurines exhibit strong negative allometry, and the difference in slopes is highly significant (p = 0.012; see , ).
TABLE 1. Regression of log jaw length ratio against log body mass. Abbreviations: df, degrees of freedom; lower CL, lower confidence limit; SE, standard error; upper CL, upper confidence limit; x. trend, slope of the trend line.
As macropodines increase in size, AJL is an increasing proportion of TRL. In contrast, as sthenurines increase in size, AJL is a decreasing proportion of TRL. This contrast in slopes is strong enough not to have been influenced by minor inaccuracies in body mass estimation or observational error.
If the entire jaw length (i.e., TRL) scales isometrically, as we have shown, then the relationship between JLR and body mass should be zero. However, it does not for either macropodid group, showing differences in facial proportions diverging from a condition of isometry. The positive slope for macropodines is highly significant from zero, while the negative slope for sthenurines is not. The weaker linear trend in the sthenurines is likely due to a small sample size and a wide dispersal of points: the important issue here is that they are significantly different from the macropodines.
For both lineages the slope of the regression line is substantially influenced by the extreme values of the largest taxa: Macropus ferragus for the macropodines and Procoptodon goliah for the sthenurines. Within the macropodines, tree-kangaroos have relatively short faces (all placed below the regression line). But dendrolagines do not have the lowest values here: those belong to the hare wallabies Lagorchestes conspiculatus (#19) and L. hirstus (#21), and the rock wallaby Petrogale brachyotis (#14) (all the rock wallabies plot below the macropodine regression line). The low value for the red kangaroo (Osphranter rufus, #30) is not easily explained.
Within the early diverging sthenurines, the Late Miocene Rhizosthenurus flanneryi (#38) plots with the macropodines, while both the Middle Miocene Wanburoo hilarus (#37) and the latest Miocene Hadronomas puckridgi (#39) have lower values than macropodines of similar size, H. puckridgi clustering with the smaller basally branching members of the Procoptodon lineage. Sthenurus andersoni (#40), the earliest diverging member of the Sthenurus lineage, plots with similarly sized macropodines (large species of Macropus), but all of the other derived sthenurines plot a long way below the macropodine regression line. It is clear that the longer-faced members of the Sthenurus lineage (with the exception of S. murrayi, #42) have higher values of JLR than similarly sized members of the Procoptodon lineage, plotting on or above the general sthenurine regression line, while the converse is true for the shorter-faced members of the Procoptodon lineage. However, the sample size is too low and data points too scattered to demonstrate this as a linear trend with body size that differs from that of the Sthenurus lineage (see Discussion).
DISCUSSION
This investigation reveals intriguing results with respect to facial allometry. Macropodines show positive allometry of facial length, reflecting the results of Cardini et al. (Citation2015); in contrast, sthenurines (primarily the larger Plio–Pleistocene species) show clear negative allometry. The Procoptodon lineage evidently represents the more derived condition, with shorter face lengths in response to an increased specialization towards browsing on tough materials, where nipping and cropping movements are necessary in food acquisition (Mitchell, Citation2019; Mitchell & Wroe, Citation2019).
Could it be the case that these differences in the scaling of facial proportions relate primarily to different diets in the two clades rather than any developmental influences? As previously noted, grazers tend to have longer anterior jaw lengths than browsers in both ungulates and extant macropodoids, and the long faces of the large grazing extant kangaroos may be related to diet rather than to developmental or genetic constraints (see Mitchell et al., Citation2018). However, the two species of Protemnodon included in this study (larger than any extant macropodine) also have extremely long faces, and their brachydont teeth are indicative of an intermediate feeding or browsing diet, as also supported by isotopic data (DeSantis et al., Citation2017; Koutamanis et al., Citation2023). A short face might benefit a browser: the original sthenurine diet was most likely some form of browsing (as in the Miocene brachydont forms Wanburoo hilarus and Rhizosthenurus flanneryi, whose craniodental form is indicative of a browsing diet [Janis et al., Citation2016]), but these sthenurines cluster with the longer-faced macropodines (see ). Negative allometry of facial length might be explained by the need to have an extremely powerful incisor bite in larger forms feeding on tough browse (Mitchell, Citation2019; Mitchell & Wroe, Citation2019), but see the discussion below on how this might also relate to forelimb mobility.
Alongside these observations, it is important to consider the forelimb morphologies of these kangaroos and how this relates both to their gaits and to the likely use of the forelimbs in feeding. Extant macropods all employ some form of quadrupedal (or pentapedal) locomotion as a slow gait, necessitating the use of the forelimbs in locomotion and so limiting their utility for use in other activities, such as feeding. Forelimb mobility in kangaroos in comparison to other mammals of similar size is reflected in the morphology of both the proximal (Janis et al., Citation2020) and distal (Jones et al., Citation2022) humerus. Macropodines have a humeral morphology resembling that of semiarboreal mammals, suggestive of adaptation for a degree of stability while also permitting some flexibility in movement for other things, such as aiding in food prehension (Janis et al., Citation2020; Jones et al., Citation2022). Tree-kangaroos use their forelimbs for both feeding and climbing, and have humeral features more similar to arboreal mammals (Jones et al., Citation2022; Warburton et al., Citation2011).
In contrast, sthenurines (at least the Plio–Pleistocene species, the relevant anatomy of Miocene species is unknown) show a humeral morphology indicative of a greater extent of forelimb mobility, similar to that seen in extant suspensory primates, which would also imply a lesser extent of weight-bearing on the forelimbs (Janis et al., Citation2020; Jones et al., Citation2022). This accords both with the hypotheses of sthenurine locomotion (bipedal striding rather than a quadrupedal gait at slow speeds; Janis et al., Citation2014) and feeding (extensive use of the forelimbs for prehension of food; Wells & Tedford, Citation1995). Sthenurine forelimbs also feature a relatively longer manus but a relatively shorter ulna and radius than macropodines, a morphology that likely also reflects this proposed feeding behavior (Wells & Tedford, Citation1995). Interestingly, the species of Protemnodon considered here had a humeral morphology more typical of a committedly terrestrial mammal than extant macropodines (Janis et al., Citation2020; Jones et al., Citation2022).
We propose that greater ability for forearm mobility and the use of the hand in feeding allowed sthenurines to adopt shorter faces, diverging from the positive allometric macropodine trend typical of many other mammals. While a short face may be advantageous for use of the incisors in browsing, allowing for a stronger bite (Mitchell, Citation2019; Mitchell & Wroe, Citation2019; Mitchell et al., Citation2024), a longer face may aid in food prehension. If sthenurines had lacked this forelimb mobility, and had to use their faces for food prehension (like macropodines), then a longer snout would have been necessary. Thus, it may be the case that it was this forelimb mobility of sthenurines that allowed them to not only have absolutely shorter faces, but facial proportions that scaled with strong negative allometry.
Two other observations from our results are interesting in this regard. One is that the single Plio–Pleistocene sthenurine with macropodine-like facial proportions, Sthenurus andersoni, is also the only sthenurine studied to have a macropodine-like proximal humeral morphology (Janis et al., Citation2020). The other is that the Protemnodon species, which have proportionally longer faces than do extant large macropodines (see ), also have a humeral morphology indicative of a lesser degree of forearm mobility, perhaps necessitating greater use of the face in feeding (although the forelimb mobility in the much larger and very long-faced Macropus ferragus is not known).
CONCLUSIONS
While other authors have commented on the differences in craniofacial length between macropodine and sthenurine kangaroos (e.g., Mitchell, Citation2019; Mitchell & Wroe, Citation2019; Prideaux, Citation2004; Wells & Tedford, Citation1995), here we demonstrate this quantitatively, and note differences in the scaling of facial length, represented here by the ratio of the anterior portion of the jaw to the molar row length.
While macropodine facial proportions scale with positive allometry, sthenurine facial proportions differ and scale with negative allometry. Within the sthenurines, basally branching forms cluster with the macropodines, and species of Sthenurus appear to have a lesser degree of negative allometry to species within the Procoptodon lineage, although this cannot be demonstrated statistically due to small sample size and lack of a discernible linear trend. We hypothesize that sthenurines were able to have short faces because their forelimbs were mobile enough to handle food, and thus they did not need a longer snout for food manipulation. However, what is remarkable is not that sthenurines simply had short faces, but that their faces were proportionally shorter at larger sizes, thus differing not only from their extant relatives but also from the majority of mammalian lineages.
The proposed links between forelimb mobility and face lengths highlighted in this study pose an interesting question about mammalian morphology in general. Whether or not greater degrees of forelimb mobility are correlated with facial lengths in other mammal families will be the subject of further investigation.
AUTHOR CONTRIBUTIONS
CMJ and NMM-G conceived of the project. WR made the measurements, performed the initial analyses, and wrote the original version of the MS. WR located most of the photographs and CMJ provided some of them. JD and SS performed the statistical analyses. NMM-G did the artwork. CMJ and JD wrote the final version of the MS with input from the other authors.
Supplemental Material
Download Zip (254.8 KB)DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
ACKNOWLEDGMENTS
We thank the following museums and museum curators for access to specimens in their collections: S. Pappa at the Natural History Museum, London, U.K.; M-A. Binnie at the South Australian Museum, Adelaide, Australia; K. Spring and S. Hocknull at the Queensland Museum, Brisbane, Australia; J. Meng and J. Galkin (Vertebrate Paleontology) and E. Westwig and R. McPhee (Mammalogy) at the American Museum of Natural History, New York, U.S.A.; K. Roberts at Museum Victoria, Melbourne, Australia. Also thanks to V. Weisenbecker, and an anonymous reviewer, whose comments resulted in a greatly improved contribution.
DATA AVAILABILITY STATEMENT
All of the relevant data are in the supplementary files.
SUPPLEMENTARY FILES
Supplementary File 1.docx: table of specimens measured, including measurements, body masses, and museum numbers.
Supplementary File 2.docx; R code for the analyses
LITERATURE CITED
- Adobe (2022). Adobe Illustrator (Version 27.0) [Computer software]. https://www.adobe.com/uk/products/illustrator.html
- Bibi, F., & Tyler, J. (2022). Evolution of the bovid cranium: morphological diversification under allometric constraint. Communications Biology, 5(1), 1–12. doi:10.1038/s42003-021-02877-6
- Butler, K., Travouillon, K. J., Price, G. J., Archer, M., & Hand, S. J. (2017). Species abundance, richness, and body size evolution of kangaroos (Marsupialia: Macropodiformes) through the Oligo-Miocene of Australia. Palaeogeography, Palaeoclimatology, Palaeoecology, 487, 25–36. doi:10.1016/j.palaeo.2017.08.016
- Cardini, A. (2019). Craniofacial allometry is a rule in evolutionary radiations of placentals. Evolutionary Biology, 46(3), 239–248. doi:10.1007/s11692-019-09477-7
- Cardini, A., & Polly, P. D. (2013). Larger mammals have longer faces because of size-related constraints on skull form. Nature Communications, 4(1), 1–7. doi:10.1038/ncomms3458
- Cardini, A., Polly, D., Dawson, R., & Milne, N. (2015). Why the long face? Kangaroos and wallabies follow the same ‘rule’ of cranial evolutionary allometry (CREA) as placentals. Evolutionary Biology, 42(2), 169–176. doi:10.1007/s11692-015-9308-9
- Chemisquy, M. A., Tarquini, S. D., Romano Muñoz, C. O., & Prevosti, F. J. (2021). Form, function and evolution of the skull of didelphid marsupials (Didelphimorphia: Didelphidae). Journal of Mammalian Evolution, 28(1), 23–33. doi:10.1007/s10914-019-09495-4
- Cooke, B. N. (1999). Wanburoo hilarus gen. et sp. nov., a lophodont bulungamayine kangaroo (Macropodoidiea: Bulungamayinae) from the Miocene of Riversleigh, northwestern Queensland. Records of the Western Australian Museum, Suppl., 57, 239–253.
- DeSantis, L. R. G., Field, J. H., Wroe, S., & Dodson, J. R. (2017). Dietary responses of Sahul (Pleistocene Australia – New Guinea) megafauna to climate and environmental change. Paleobiology, 43(2), 181–195. doi:10.1017/pab.2016.50
- Devillers, C., Mahe, J., Ambroise, D., Bauchot, R., & Chatelain, E. (1984). Allometric studies on the skull of living and fossil Equidae (Mammalia: Perissodactyla). Journal of Vertebrate Paleontology, 4(3), 471–480. doi:10.1080/02724634.1984.10012023
- Ferreira-Cardoso, S., Billet, G., Gaubert, P., Delsuc, F., & Hautier, L. (2020). Skull shape variation in extant pangolins (Pholidota: Manidae): allometric patterns and systematic implications. Zoological Journal of the Linnean Society, 188(1), 255–275.
- Figueirido, B., Pérez-Claros, J. A., Torregrosa, V., Martín-Serra, A., & Palmqvist, P. (2010). Demythologizing Arctodus simus, the ‘short-faced’long-legged and predaceous bear that never was. Journal of Vertebrate Paleontology, 30(1), 262–275. doi:10.1080/02724630903416027
- Flores, D. A., Giannini, N., & Abdala, F. (2018). Evolution of post-weaning skull ontogeny in New World opossums (Didelphidae). Organisms Diversity & Evolution, 18(3), 367–382. doi:10.1007/s13127-018-0369-3
- Hautier, L., Billet, G., Eastwood, B., & Lane, J. (2014). Patterns of morphological variation of extant sloth skulls and their implication for future conservation efforts. The Anatomical Record (Hoboken), 297(6), 979–1008. doi:10.1002/ar.22916
- Helgen, K. M., Wells, R. T., Kear, B. P., Gerdtz, W. R., & Flannery, T. F. (2006). Ecological and evolutionary significance of sizes of giant extinct kangaroos. Australian Journal of Zoology, 54(4), 293–303. doi:10.1071/ZO05077
- Janis, C. M. (1990a). Correlation of cranial and dental variables with body size in ungulates and macropodoids. In J. Damuth, & B. F. MacFadden (Eds.), Body size in mammalian paleobiology: estimation and biological implications (pp. 255–300). Cambridge University Press.
- Janis, C. M. (1990b). Correlation of cranial and dental variables with dietary preferences: a comparison of macropodoid and ungulate mammals. Memoirs of the Queensland Museum, 28(1), 349–366.
- Janis, C. M., Buttrill, K., & Figueirido, B. (2014). Locomotion in extinct giant kangaroos: were sthenurines hop-less monsters? PLoS ONE, 9(10), e10988. doi:10.1371/journal.pone.0109888
- Janis, C. M., Damuth, J., Travouillon, K. J., Figueirido, B., Hand, S. J., & Archer, M. (2016). Palaeoecology of Oligo-Miocene macropodoids determined from craniodental and calcaneal data. Memoirs of Museum Victoria, 74, 209–232. doi:10.24199/j.mmv.2016.74.17
- Janis, C. M., Napoli, J. G., Billingham, C., & Martín-Serra, A. (2020). Proximal humerus morphology indicates divergent patterns of locomotion in extinct giant kangaroos. Journal of Mammalian Evolution, 27(4), 627–647. doi:10.1007/s10914-019-09494-5
- Johnson, C. N., & Prideaux, G. J. (2004). Extinctions of herbivorous mammals in the late Pleistocene of Australia in relation to their feeding ecology: no evidence for environmental change as cause of extinction. Austral Ecology, 29(5), 553–557. doi:10.1111/j.1442-9993.2004.01389.x
- Jones, B., Martín-Serra, A., Rayfield, E. J., & Janis, C. M. (2022). Distal humeral morphology indicates locomotory divergence in extinct giant kangaroos. Journal of Mammalian Evolution, 29(1), 27–41. doi:10.1007/s10914-021-09576-3
- Kavanagh, K. D., Evans, A. R., & Jernvall, J. (2007). Predicting evolutionary patterns of mammalian teeth from development. Nature, 449(7161), 427–432. doi:10.1038/nature06153
- Kerr, I. A., & Prideaux, G. J. (2022). A new genus of kangaroo (Marsupialia, Macropodidae) from the late Pleistocene of Papua New Guinea. Transactions of the Royal Society of South Australia, 146(2), 295–318. doi:10.1080/03721426.2022.2086518
- Koutamanis, D., McCurry, M., Tacail, T., & Dosseto, A. (2023). Reconstructing Pleistocene Australia megafauna herbivore diet using calcium and strontium isotopes. Royal Society Open Science, 10(11), 230991. doi:10.1098/rsos.230991
- Krone, I. W., Kammerer, C. F., & Angielczyk, K. D. (2019). The many faces of synapsid cranial allometry. Paleobiology, 45(4), 531–545. doi:10.1017/pab.2019.26
- Law, C. J., Duran, E., Hung, N., Richards, E., Santillan, I., & Mehta, R. S. (2018). Effects of diet on cranial morphology and biting ability in musteloid mammals. Journal of Evolutionary Biology, 31(12), 1918–1931. doi:10.1111/jeb.13385
- Magnus, L. Z., Machado, R. F., & Caceres, N. (2018). Ecogeography of South-American Rodentia and Lagomorpha (Mammalia, Glires): roles of size, environment, and geography on skull shape. Zoologischer Anzeiger, 277, 33–41. doi:10.1016/j.jcz.2018.06.002
- Marcucio, R. S., Young, N. M., Hu, D., & Hallgrimsson, B. (2011). Mechanisms that underlie co-variation of the brain and face. Genesis, 49(4), 177–189. doi:10.1002/dvg.20710
- Marcy, A. E., Guillerme, T., Sherratt, E., Rowe, K. C., Phillips, M. J., & Weisbecker, V. (2020). Australian rodents reveal conserved Cranial Evolutionary Allometry across 10 million years of murid evolution. The American Naturalist, 196(6), 755–768. doi:10.1086/711398
- Marroig, G. (2007). When size makes a difference: allometry, life-history and morphological evolution of capuchins (Cebus) and squirrels (Saimiri) monkeys (Cebinae, Platyrrhini). BMC Evolutionary Biology, 7(1), 20–26. doi:10.1186/1471-2148-7-20
- Marshall, L. G. (1973). Fossil vertebrate faunas from the Lake Victoria region, S.W. New South Wales, Australia. Memoirs of the National Museum of Victoria, 34, 151–171. doi:10.24199/j.mmv.1973.34.03
- Mitchell, D. R. (2019). The anatomy of a crushing bite: The specialised cranial mechanics of a giant extinct kangaroo. PLoS One, 14(9), e0221287.
- Mitchell, D. R., & Wroe, S. (2019). Biting mechanics determines craniofacial morphology among extant diprotodont herbivores: dietary predictions for the giant extinct short-faced kangaroo, Simosthenurus occidentalis. Paleobiology, 45(1), 167–181. doi:10.1017/pab.2018.46
- Mitchell, D. R., Sherratt, M., & Weisbecker, V. (2024). Facing the facts: Adaptive trade-offs along body size ranges determine mammalian craniofacial scaling. Biological Reviews, 99(2), 496–524. doi: 10.1101/2023.09.28.560051
- Mitchell, D. R., Sherratt, E., Ledogar, J. A., & Wroe, S. (2018). The biomechanics of foraging determines face length among kangaroos and their relatives. Proceedings of the Royal Society of London, B, 285, 21080845.
- Prideaux, G. (2004). Systematics and evolution of the sthenurine kangaroos. University of California Press, 146, 1–604.
- R Core Team (2021). R: A language and environment for statistical computing (version 4.1.1) [Computer software]. R Foundation for Statistical Computing, https://www.R-project.org/.
- Radinsky, L. B. (1985). Approaches in morphology: a search for patterns. Annual Review of Ecology and Systematics, 16(1), 1–14. doi:10.1146/annurev.es.16.110185.000245
- Rhoda, D. P., Haber, A., & Angielczyk, K. D. (2023). Diversification of the ruminant skull along an evolutionary line of least resistance. Science Advances, 9(9), eade8929. doi:10.1126/sciadv.ade8929
- Rohlf, F. J. (2001). Comparative methods for the analysis of continuous variables: geometric interpretations. Evolution, 55(11), 2143–2160.
- Rohlf, F. J. (2006). A comment on phylogenetic correction. Evolution, 60(7), 1509–1515. doi:10.1554/05-550.1
- Tamagnini, D., Meloro, C., & Cardini, A. (2017). Anyone with a long-face? Craniofacial evolutionary allometry (CREA) in a family of short-faced mammals, the Felidae. Evolutionary Biology, 44(4), 476–495. doi:10.1007/s11692-017-9421-z
- Tamagnini, D., Michaud, M., Meloro, C., Raia, P., Soibelzon, L., Tambusso, P. S., Varela, L., & Maiorano, L. (2023). Conical and sabertoothed cats as an exception to craniofacial evolutionary allometry. Scientific Reports, 13(1), 13571. doi:10.1038/s41598-023-40677-6
- Uyeda, J. C., Zenil-Ferguson, R., & Pennell, M. W. (2018). Rethinking phylogenetic comparative methods. Systematic Biology, 67(6), 1091–1109. doi:10.1093/sysbio/syy031
- Wagstaffe, A. Y., O’Driscoll, A. M., Kunz, C. J., Rayfield, E. J., & Janis, C. M. (2022). Divergent locomotor evolution in “giant” kangaroos: Evidence from foot bone bending resistances and microanatomy. Journal of Morphology, 283(3), 313–332. doi:10.1002/jmor.21445
- Warburton, N. M., Harvey, K. J., Prideaux, G. J., & O'Shea, J. E. (2011). Functional morphology of the forelimb of living and extinct tree-kangaroos (Marsupialia: Macropodidae). Journal of Morphology, 272(10), 1230–1244. doi:10.1002/jmor.10979
- Wells, R. T., & Tedford, R. H. (1995). Sthenurus (Macropodidae, Marsupialia) from the Pleistocene of Lake Callabonna, South Australia. Bulletin of the American Museum of Natural History, 225, 1–111.
- Westerman, M., Loke, S., Tan, M. H., & Kear, B. P. (2022). Mitogenome of the extinct Desert ‘rat-kangaroo’ times the adaptation to aridity in macropodoids. Scientific Reports, 12(1), 5829. doi:10.1038/s41598-022-09568-0
