Abstract
The abundance of Bull Trout Salvelinus confluentus in the Metolius River and Lake Billy Chinook, a hydroelectric reservoir, increased dramatically from 1998 to 2004, following implementation of restrictive fishery regulations. However, both adult Bull Trout and kokanee Oncorhynchus nerka (prey) populations have declined in more recent years. We investigated the relationships between Bull Trout spawner densities, emerging fry densities, and numbers of juveniles migrating into the reservoir to determine the potential role of juvenile production in this decline. We hypothesized that age-1 and older juvenile production is now limited by natal habitat capacity and that excess fry production is lost to density-dependent mortality. Bull trout redd densities in surveyed spawning reaches ranged from 14 to 39 redds/km during recent years. The estimated abundance of emerging Bull Trout fry in monitored spawning reaches has varied from about 1.0–2.5 million annually since 2005, while mean estimated densities of newly emerged fry varied from over 35/m2 in Roaring Creek during 2005 to less than 1/m2 in Jefferson Creek during 2009. Fry capture numbers from high versus low escapement broods at the Metolius River downstream trap varied by a factor of four, but capture of age-1 and age-2 juveniles did not differ substantially. These findings indicate natal Bull Trout habitats are seeded at spawner densities of 14 redds/km and higher. Fry in excess of natal habitat capacity are probably lost to density-dependent mortality factors. We estimate natural recruitment of age-1 and older Bull Trout into Lake Billy Chinook of at least 9 individuals/ha annually since 2001. Recent population bottlenecks limiting adult Bull Trout abundance occurred after juvenile recruits entered the lake and are probably related to prey availability.
Received February 23, 2015; accepted July 8, 2015
Lake Billy Chinook, a 1,619-ha hydroelectric reservoir on the Deschutes River in central Oregon (), supports important fisheries for wild kokanee (nonanadromous Sockeye Salmon Oncorhynchus nerka) (Thiesfeld et al. Citation1995; Thiede et al. Citation2002) and Bull Trout Salvelinus confluentus (Stuart et al. Citation1997; Ratliff Citation2012). Both species follow adfluvial life histories, spawning in the Metolius River and tributaries, with all kokanee fry and most juvenile Bull Trout migrating downstream into the rearing habitats provided by Lake Billy Chinook. During initial population status assessments, Bull Trout in the Metolius River were rated as having a low risk of extinction (Ratliff and Howell Citation1992; Buchanan et al. Citation1997). Bull Trout in the western USA were federally listed under the U.S. Endangered Species Act (ESA) as a threatened species in 1998 and are classified as a sensitive species in Alberta, British Columbia, and the Yukon Territory, Canada (ASRD and ACA 2009). Metolius River Bull Trout populations were considered healthy enough that a limited take fishery for large adults was allowed to continue at Lake Billy Chinook under federal rules adopted during the ESA listing processes.
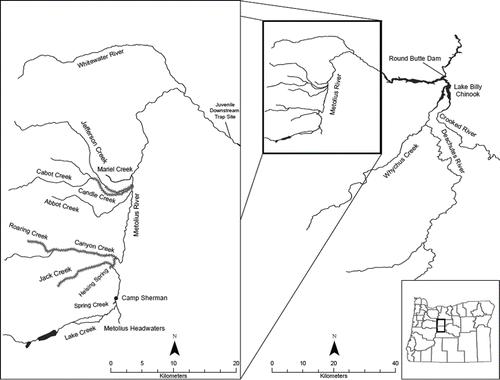
FIGURE 1. Location of the Metolius River and Lake Billy Chinook within the Deschutes River basin of Central Oregon. The lower Metolius River downstream-migrant fish trap, and monitored Bull Trout spawning reaches (crosshatched) are shown on the enlarged map.
The abundance of Bull Trout and their major prey species in Lake Billy Chinook have changed dynamically over time. Bull Trout spawner abundance increased in the years following listing and peaked from 2003 through 2006, but then declined by 60% from 2006 to 2008 (). Kokanee, the primary limnetic prey of Bull Trout in Lake Billy Chinook (Beauchamp and Van Tassell Citation2001), peaked in 2000 at an estimated (±95% confidence interval[CI]) 569,201 (±237,713) spawners (Thiede et al. Citation2002), but declined to 27,958 (±59,076) spawners in 2004. Estimated kokanee escapement peaked again at 349,920 (±26,473) spawners in 2009 but declined to 24,057 (±4,175) spawners in 2013 (Oregon Department of Fish and Wildlife [ODFW] and Confederated Tribes of the Warm Springs Reservation of Oregon [CTWSRO], unpublished data). The kokanee harvest limit, historically 25/d, was decreased to 5/d in 2008 due to the low population numbers. Pronounced sequential changes in the abundance of Bull Trout and kokanee within Lake Billy Chinook prompted questions of what factors now control abundance of the two species within this ecosystem.
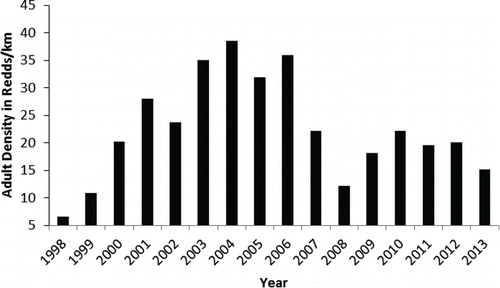
FIGURE 2. Density of spawning adult Bull Trout from 1998 through 2013 in the 27.1 km of spawning streams within the Metolius River basin inventoried annually.
Bull Trout in the Metolius River–Lake Billy Chinook ecosystem may no longer be limited by spawner abundance or juvenile recruitment. We examined three questions regarding factors that may limit abundance of this population: (1) is production of age-1 and older juveniles now limited by natal habitat capacity, (2) do fry that migrate into the lake contribute to adult abundance, and (3) does current annual recruitment of age-1 and older juvenile Bull Trout into the lake exceed recommended stocking densities for large salmonid predators?
We use several long-term data sets to describe the adult spawner to juvenile production relationships, juvenile population dynamics, and important Bull Trout habitat variables in this ecosystem. Objectives of this study were: (1) to use spawner (redd) densities in monitored reaches, estimated fecundity, and recent embryo survival estimates to examine the potential range of fry numbers and densities recruited into natal habitats during recent years, (2) to describe estimated natural annual recruitment of age-1 and older juvenile Bull Trout into the lake to compare recruitment with recommended stocking densities of large salmonid predators into kokanee waters, (3) to compare fry and age-1 and age-2 capture rates at the lower Metolius River downstream-migrant trap for evidence of density-dependent population limitations. Fisheries managers are challenged with determining at what point in population recovery Lake Billy Chinook Bull Trout should be actively managed to maintain a healthy, diverse, and stable ecosystem.
STUDY AREA
The Metolius River starts as several large springs (9.5–10.8°C) near the base of Black Butte and flows north then east around Green Ridge, approximately 45 km into Lake Billy Chinook (). It has a stable hydrograph due to strong groundwater dominance. Mean monthly flow varies from 38.2 m3/s during October to 46.4 m3/s during June (U.S. Geological Survey [USGS] gauge 14091500). Four tributary streams with cold (about 4.5°C; Riehle Citation1993) groundwater sources, Jefferson, Candle, Canyon/Roaring, and Jack creeks () are used by Bull Trout for spawning and natal rearing, and redd counts in these streams were used as an index of spawner density for this study. These streams have very constant base flow from year to year. In 1989 measured flows for the four creeks were 2.77, 0.82, 1.33, and 1.70 m3/s, respectively (Riehle Citation1993). In 2010, Bowerman et al. (Citation2014) measured base flows of Jefferson, Canyon, and Jack creeks at 2.8, 1.3, and 1.6 m3/s, respectively.
Bull Trout are the only fish species in most reaches of these tributaries (Ratliff Citation1992; Riehle et al. Citation1997), hence, allopatric. With the input from these cold tributaries, the main-stem Metolius River cools downstream with temperatures at its discharge into Lake Billy Chinook exceeding 12°C only during hot, midsummer afternoons. Genetic data indicate that the Metolius basin contains three relatively distinct Bull Trout population groups, each spawning in different groundwater-driven tributary complexes, including Jack/Canyon creeks, Candle/Jefferson creeks, and Whitewater River (DeHann et al. Citation2008; ).
During 2003, the Bear Butte and Booth Forest Fire complex (B and B Fire) burned both banks of nearly all of Jack and Canyon/Roaring creeks, 70% of Candle Creek, and 50% of Jefferson Creek. The fire increased solar input and significantly increased the density and complexity of fire-induced woody structure within affected stream reaches (M. Riehle, U.S. Forest Service [USFS], Sisters Ranger District, personal communication). We believe that the increased structural complexity in these natal habitat reaches has likely increased their rearing capacity for juvenile Bull Trout since the B and B Fire.
Lake Billy Chinook was formed in 1964 when Round Butte Dam was completed on the Deschutes River 179 km upstream from where it enters the Columbia River (). At full pool, the reservoir has a surface elevation of 593 m AMSL, a wetted surface of 1,619 ha, and 95 km of shoreline. Lake Billy Chinook is considered a productive kokanee lake (Thiede et al. Citation2002). Through November 2009, all discharge water was withdrawn from an unscreened deep intake (midline 73 m) at the dam. New selective water withdrawal and downstream fish passage facilities were installed in December 2009. Nearly all discharged water is now withdrawn from the upper 14 m of the reservoir from November through May, and over half from June through October (Corson Citation2012). Surface withdrawal removes warmer surface water, resulting in a significantly cooler reservoir with the photic zone normally extending below the seasonal thermocline, and summer hypolimnic temperatures of 8–12°C. Due to the cooler temperatures, subadult Bull Trout, historically confined to cool tributary influence zones during midsummer (D. Ratliff, personal observations), now occur throughout the reservoir and are captured year-round at the dam. The only downstream emigration route from Lake Billy Chinook is through the new fish facility, where all fish are counted (except during very infrequent spill) because both the surface and deep intakes are fully screened. Efforts are underway to establish populations of Sockeye Salmon, spring-run Chinook Salmon O. tshawytscha, and summer-run steelhead (anadromous Rainbow Trout O. mykiss) upstream of Round Butte Dam, in part to increase and diversify the prey base available to Bull Trout.
METHODS
Estimated Fry Recruited into Natal Habitats
Spawner abundance
Bull Trout spawner abundance for the Jack/Canyon, Candle, and Jefferson populations has been monitored annually since 1986 by counting redds in the majority of their spawning habitats. Redds in this system are easy to identify due to large female size and stable stream discharge during late summer. Studies during the 1990s of adult numbers passed over tributary weirs versus subsequent redd counts yielded a relatively consistent ratio of 2.3 adults/redd, including early maturing males (Ratliff et al. Citation1996), which ODFW and CTWSRO fish managers continue to use to estimate adult spawner abundance. For our study, 27.1 km of stream reaches used for spawning by these two populations were surveyed twice annually from 1998 through 2013 (). We estimated that monitored reaches represent over 70% of the annual spawning activity above Lake Billy Chinook. Redd surveys were conducted during the second or third weeks of September and October by interagency teams supervised by the ODFW; at least one member of each team was an experienced redd surveyor. Redds identified during September surveys were flagged to avoid recounting during October. To convert the redd numbers tallied annually to redd densities (redds/km), total redds counted were divided by the total length of the stream reaches surveyed (27.1 km).
Egg deposition and swim-up fry abundance
We estimated egg deposition within monitored spawning reaches by multiplying the numbers of redds by the mean fecundity value of 3,722 eggs/female (as reported from four Bull Trout populations by McPhail and Baxter Citation1996); minus 5% to account for average egg retention (Martin Citation1985; reported in Goetz Citation1989). Mean total length of all females sampled from these four populations was 561 mm, slightly smaller than the spawners in the Metolius basin. Mean total length of about 127 female spawners captured entering Jack Creek from 1990 to 1993 was 578 mm (Riehle et al. Citation1997). Maturing females entering traps on Canyon and Candle creeks in 2009 and 2010 (N = 387) averaged 583 mm (B. Hodgson, ODFW, personal communication). To bound the range of egg numbers deposited in monitored spawning habitats during post-B and B Fire brood years (2003–2013), we expanded the count of adult redds in each reach from the highest escapement (2004) and the lowest escapement (2008) spawning year-classes () by estimated average egg deposition. We assumed that each redd represented a successful spawning event and that all eggs were deposited within individual redds.
Embryo survival to emergence was estimated based on previous studies by Bowerman et al. (Citation2014). They measured mean survival to emergence from green eggs placed within artificial redds constructed in three Metolius River tributaries with different hydrographs and sediment conditions: Jack Creek = 87% (SD, 9%), Canyon Creek = 51% (SD, 34%), and Jefferson Creek 27% (SD, 28%). Mean rates of survival to emergence for these three streams were applied to other Metolius basin Bull Trout spawning streams included in this study, based on similarity of hydrographs and substrate embeddedness. We applied the mean embryo survival observed for Jack Creek with its stable hydrograph to Roaring Creek and Heising Spring, which are also very stable. We applied the survival rate for Canyon Creek to Candle Creek and the short spawning reach of the main-stem Metolius River between the mouths of Canyon and Jack creeks because these two reaches also experience occasional high-flow events and some sediment transport most years, albeit probably less than Canyon Creek. Among monitored spawning reaches, Jefferson Creek is unique, having abundant glacial sediment input and a large watershed that adds the variability of seasonal runoff to a baseline of groundwater-driven low flows.
To compare estimated mean densities of fry emerging in each monitored reach, we calculated wetted stream area of each monitored reach by multiplying survey reach lengths that adult Bull Trout access by average widths measured during stream surveys (N. Dachtler, USFS, Sisters Ranger District, personal communication). From these highest and lowest escapement years, we estimated total fry emerging within all the monitored streams, and for each reach (excluding small side channels) we estimated number and mean density of emerging fry/m2 of wetted stream bottom.
Recruitment of Age-1 and Older Juvenile Bull Trout into Lake Billy Chinook
Trapping of juvenile downstream migrants
A 2.4-m downstream-migrant, rotary screw trap has been operated annually at the same site on the river since 1999. The trap site is located adjacent to the Metolius River gauge (USGS 14091500) 1.3 km upstream from Lake Billy Chinook (). The trap has been operated from March 1 through May 31 each year and during other months in some years. Young Bull Trout captured at the lower Metolius trap were assigned to age-classes based on scale and otolith aging and linear regression of length at annulus formation for Metolius basin Bull Trout (Pratt Citation1991, Citation2000). Bull Trout <55 mm were classified as age 0, 55–111 mm as age 1, 112–191 mm as age 2, and >191 mm as age 3 or older (all total lengths). Age-0 and age-1 cohorts are distinctly separated by length frequency in spring sampling (Pratt Citation1991, Citation2000). Age-2 and age-3 cohorts overlapped 191 mm, approximately equal proportions being larger or smaller (Pratt Citation1991, Citation2000). We assumed that any age-3 fish included in the 112–191-mm group were offset by equal numbers of age-2 fish >191 mm. The age-3 and older fish exhibit diverse and overlapping size ranges, and could not be assigned to a brood year (Pratt Citation1991).
Mark-recapture population estimates
To determine trap efficiency, age-1 and older juvenile Bull Trout (>55 mm TL) were anaesthetized with tricaine methanesulfonate (MS-222), weighed, and measured (TL), and given either a partial fin clip (1999–2008) or a 12-mm full duplex PIT tag (2009–2013). After recovery from anesthesia, marked fish were released 150 to 300 m upstream of the screw trap; exact release locations were varied daily to reduce potential loss to predation by older-aged Bull Trout. From 1999 to 2011, the trap was operated 4 d each week. Bull Trout captured on day 1 and day 2 were marked and released upstream and were recaptured on days 2, 3 and 4. In 2012 and 2013, the trap was operated 7 d a week, and Bull Trout were marked daily. Based on PIT tag recaptures in 2012 and 2013, 93% of recapture events occurred within 2 d of recycling. Catch was expanded for days when the trap was not operated and also for occasional short periods of inoperability due to debris or high water. Population estimates were calculated as described in Volkhardt et al. (Citation2007); variances were calculated via a bootstrap method with 1,000 iterations (Thedinga et al. Citation1994). Population estimates could not be calculated for 2002 (data missing) or 2009 (recapture data not collected). Basic assumptions in conducting trap-efficiency studies are that additional loss of released test fish to predation is negligible, and that marked, recycled test fish have the same susceptibility to capture at the trap as original migrants. As a check on the validity and consistency of these two assumptions, we compared annual calculated population estimates using trap efficiency with annual fish capture rates (individuals caught/d) during the 3-month spring monitoring period.
Expanding March–May estimates to annual estimates
Trap operation during the 9 months other than March–May varied, but each month was sampled in at least 1 year during the study. Downstream-migrant trapping during summer, fall, and winter, though infrequent, has shown that age 1 and older Bull Trout enter Lake Billy Chinook each month of the year. Recruitment estimates for the consistent March–May monitoring periods were expanded to estimates of total annual recruitment based on the proportionate catch during each of the other 9 months for the same years with available data. Mark–recapture estimates of recruitment during March through May were then multiplied by the inverse of that percentage to get rough estimates of total annual age 1 and older recruitment from 1999 through 2013 (except 2002 and 2009). The major assumptions inherent in these expansions are that capture rates in the hydrologically stable lower Metolius River are similar between months, and the percentages of the annual numbers of juvenile Bull Trout entering the lake each month are relatively consistent from year to year. To convert estimated annual recruitment to estimated natural stocking densities for Lake Billy Chinook (juvenile Bull Trout entering Lake Billy Chinook/surface ha), annual recruitment estimates were divided by the surface area of the Metolius River Arm (826 ha), and the surface area of the entire reservoir (1,619 ha). All young Bull Trout are produced in the Metolius River basin and must move through the 20.9-km Metolius River Arm before dispersing into the Deschutes and Crooked river arms of Lake Billy Chinook ().
Downstream-Migrant Capture by Age-Class
Age of spring emigration
For the 9 years after the B and B Fire (2005–2013), total capture of fish in each spring migration age-class was expanded by dividing by the percent of days sampled to a consistent 92-d sampling period. The proportion that each age-class composed of the total number of migrants captured each year during the 3-month sampling period was then calculated. We also determined percentage of age-1 and older Bull Trout captured each year that were ages 1, 2, 3 and older for the 9 years combined and compared the percentage of annual catch that was classified as age 1 to the percentage that was age 2 and older for each year.
Parent spawner abundance and juvenile capture by age-class
The timing and numbers for age-0 fry captured the following spring, and age-1 and age-2 progeny 1 and 2 years later, respectively, were compared between the high escapement (2004 brood) and the low escapement (2008 brood). We also examined variation in lower Metolius River flows during each of these six migration years to see if changes in daily mean flow within the March–May monitoring periods affected observed numbers of juvenile Bull Trout captured from these two broods. In addition, estimated annual spring recruitment by age-class into Lake Billy Chinook during the 3-month monitoring period for all years monitored was compared with parent spawner density to further illustrate the relationship between parent stock abundance and progeny recruitment. Trap efficiencies for fry were not determined, so population estimates and confidence limits could not be calculated. For fry, parent spawner abundance was compared with fry capture rate (individuals/d) the following spring. March–May mark–recapture population estimates and 95% confidence limits of age 1 and older were parsed out by proportionate length frequency to estimate total number of ages 1 and 2 recruited each year. We assumed that trap efficiency of each cohort was equal because recaptured fish were not measured.
RESULTS
Estimated Fry Recruited into Natal Habitats
Spawner densities
Bull Trout redd densities for the 27.1 km of Metolius basin spawning streams sampled from 1998 to 2013 steadily increased from 6.6 redds/km in 1998 to 38.6 redds/km in 2004, and exceeded 30 redds/km in 2006 (). Redd density declined to 14.1 redds/km in 2008, then increased to 15–22 redds/km in 2009–2013.
Egg deposition and swim-up fry abundance
We estimate 3.9 million eggs were deposited in redds counted in monitored stream reaches during the high-escapement brood year (2004), and 1.4 million in the low-escapement brood year (2008; ; ). An estimated total of 2.5 million swim-up fry emerged in late winter and spring 2005, and 1.0 million fry emerged in 2009 (). Mean estimated densities of emerging fry were highest in Roaring Creek at over 30 fry/m2 (SD, 9%) of wetted stream in both 2005 and 2009, and lowest in Jefferson Creek with estimates of 3.7 fry/m2 in 2005 and 0.9 fry/m2 (SD, 28%) in 2009. Jack Creek produced the most fry in 2005, whereas Roaring Creek produced more in 2009. Jefferson Creek produced the least fry of the monitored tributaries due to fewer spawners, and much lower estimated embryo survival (as measured by Bowerman et al. Citation2014). The hydrologically stable streams, Roaring and Jack creeks and Heising Spring, all had high estimated fry densities due to a combination of high spawner densities and high estimated rates of survival to emergence ().
TABLE 1. Estimated number and mean densities of Bull Trout fry emerging in the various spawning reaches of Metolius River basin streams from the 2004 high-escapement brood year and the 2008 low-escapement brood year (see ).
Recruitment of Age-1 and Older Juvenile Bull Trout
Estimated March–May recruitment
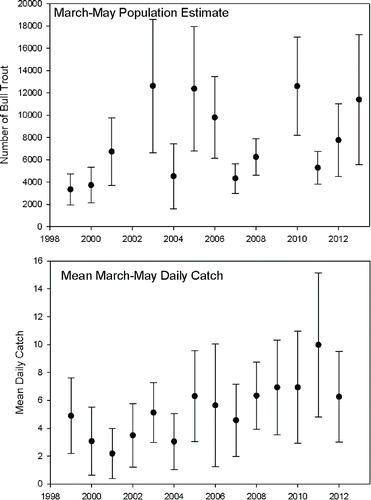
Estimates of the numbers of age-1 and older Bull Trout passing the trap site and entering Lake Billy Chiook during the spring monitoring period varied widely in the 13 years data were available (). Confidence intervals were also large most years due to the relatively low trap efficiency (4–11%). March–May recruitment estimates (±95% CI) from 2006 to 2013 (when juveniles reared in natal habitats modified by the B and B Fire) ranged from 4,322 (±1,334) in 2007 to 12,363 (±5,583) in 2010. The mean 2006–2013 estimated recruitment was 8,718 ± 2,696 95% CI. The mean (±95% CI) estimated recruitment for the three post-B and B Fire years with the highest trap efficiency (2007, 2008, and 2011) was 5,280 (±1,476).
FIGURE 3. Upper panel: estimated recruitment (±95% CI) of age-1 and older juvenile Bull Trout into Lake Billy Chinook during the March through May sampling periods from 1999 through 2013, except 2002 and 2009 when data are not available. Ranges represent 95% confidence intervals. Lower panel: annual mean daily capture rates (± SD) for age-1 and older juvenile Bull Trout during the March through May sampling period from 1999 through 2012.
Spring population estimates versus catch rates
Although spring population estimates were extremely variable, comparison with mean trap catch per day () indicates that variability in the population estimates came mainly from variability in the measured trap capture efficiency rather than variability in the catch rate. This indicates that the higher population estimates were possibly biased by violations in the basic assumption of equal capture rates of marked test fish compared with fish encountering the trap for the first time.
Expanding March–May estimates to annual estimates
About 36.2% of the pooled annual migration of age-1 and older Bull Trout from the Metolius River into Lake Billy Chinook was estimated to occur during March–May, and 63.8% occurred during the other 9 months (). Annual recruitment to the lake was estimated by expanding the March–May population estimates and confidence intervals () by 2.76 (the inverse of 36.2%). The mean (±95% CI) estimated annual recruitment for 2005–2013 was 24,083 (±7,440) age-1 and older Bull Trout into the lake. The mean of the annual expanded estimates using the three post B and B Fire years with the highest trap efficiency (2007, 2008, and 2011) was 14,586 (±4,074), which equates to an annual stocking densities of 13–23/ha into the Metolius River Arm and 7–12/ha into Lake Billy Chinook as a whole.
TABLE 2. Number of years trapping occurred at during specific months of the year (1999–2013), the capture rate of Age-1 and older Bull Trout catch data at the lower Metolius River downstream-migrant fish trap by month in comparison to the mean March through May catch rates for the same years.
Downstream Migrant Capture by Age-Class
Juvenile capture by age-class
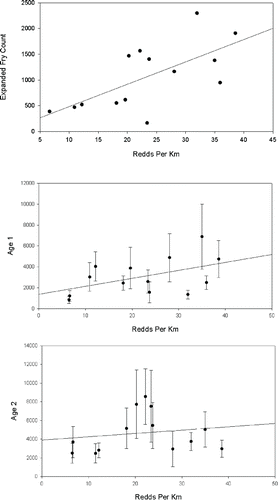
Maximum daily fry captures during late March and early April from the high-escapement (2004) brood were four times that of the low-escapement (2008) brood (). Daily captures of age-1 and age-2 juveniles were similar between the high and low escapements. However, most of the highest catch-days for age-1 juveniles were from the high 2004 escapement; whereas most of the highest catch days for age-2 juveniles were from the low 2008 escapement (). Parent spawner abundance and catch rate for age-0 fry had a positive linear relationship (R2 = 0.41, P = 0.01), whereas no significant relationships between estimated annual spring recruitment of age-1 and age-2 migrants and their corresponding parent densities were observed ().
FIGURE 4. Daily catch of age-0 fry and age-1 and age-2 juvenile Bull Trout progeny from the high-escapement 2004 brood year (BY) compared with low-escapement 2008 brood year at the lower Metolius River downsteam-migrant trap during cohorts' respective migration years. Also shown are the mean daily Metolius River flows at the trap site during the 3-month sampling periods for each of the six migration years.
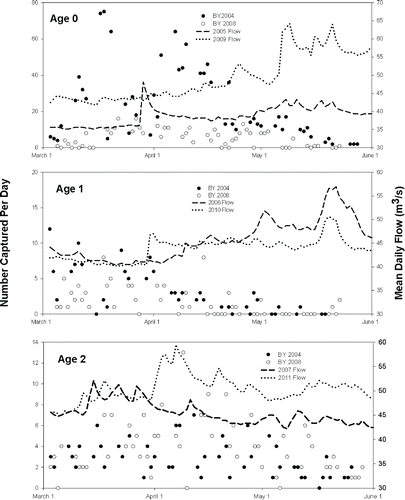
FIGURE 5. Comparison of adult Bull Trout redd densities (redds/km) and March–May expanded catch of Bull Trout fry (top panel) and age-1 and age-2 population estimates (middle and bottom panels). Lines are linear regression plots (fry: R2 = 0.44, P = 0.01; age 1: R2 = 0.24, P = 0.09; age 2: R2 = 0.03, P = 0.57. Error bars are 95% confidence intervals.
Age of spring downstream migration
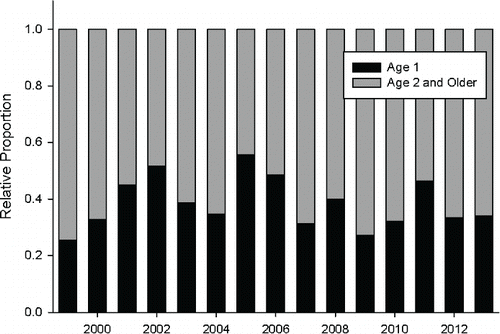
Proportionate age-class distribution during the March–May trapping periods from 2005 through 2013 was 68.1% age 0, 11.5% age 1, 18.9% age 2, and 1.5% age 3 or older. Of the age-1 and older emigrants captured, 34.0% were age 1, 60.8% were age 2, and 5.2% were age 3 and older. The percentage of age 1 and older juvenile Bull Trout that were age 1 varied from 25% to >50% between 1999 and 2013 ().
Timing of spring downstream migration
Daily capture of age-0 fry peaked from late March through April. The high-escapement 2004 year-class showed a much higher peak catch than the 2008 brood year (). Daily capture of age-1 juveniles was relatively high when sampling began in early March during both 2006 and 2010, but declined to low levels by late April. Capture numbers of age-2 juveniles were more evenly distributed through the March–May sampling periods than for age-0 and age-1 migrants. There was no indication that the relatively small fluctuations in Metolius River flows during the six migration years influenced migration timing ().
DISCUSSION
Estimated Fry Recruited into Natal Habitats
Adult spawner abundance
In many locations, the use of Bull Trout redd counts to monitor adult escapement is problematic due to observer error (Dunham et al. Citation2001; Muhlfeld et al. Citation2006), the presence of smaller adults with less defined redds (Al-Chokhachy et al. Citation2005), and changing environmental conditions that may change spawn timing or mask redds (Dunham et al. Citation2001). However, observer bias in redd counts can be reduced if experienced observers make the counts (Muhlfeld et al. Citation2006). Studies of an adfluvial population within an ecosystem similar to Metolius River–Lake Billy Chinook show that in stable systems with large adults, accurate counts can be obtained. During a 10-year study, adults ascending Smith-Dorrien Creek from Lower Kananaskis Lake, Alberta, were counted over a weir annually (Johnston et al. Citation2007). Redd abundance was found to be directly related to the number of females passed upstream each year and not significantly different from a 1:1 ratio, which was maintained despite a 20-fold increase in adults during their study period (Johnston et al. Citation2007). This indicates that adult females only created one redd, and that almost all redds were observed and counted in Smith-Dorrien Creek, even at high spawner densities. From these studies and earlier Metolius basin trapping versus redd count studies (Ratliff et al. Citation1996; see Methods), we conclude that annual redd counts made by experienced observers during our study were a relatively accurate estimator of annual adult spawner densities in the monitored streams.
Egg deposition and swim-up fry abundance
The cold groundwater-dominated spawning reaches in Roaring, Jack, and Heising Spring creeks had the highest densities of spawners and estimated numbers of emerging fry. Studies in Montana also showed high association of Bull Trout spawning with stream reaches experiencing hyporhheic and groundwater upwelling, which provide thermal moderation and maintain flows (Baxter and Hauer 2000; Bean et al. 2015). Baxter and Hauer (2000) found that Bull Trout spawning is often concentrated near geomorphic knick points that force groundwater to the surface, such as occurs in the Metolius River basin at Heising Spring and adjoining areas of Jack Creek and the main-stem Metolius River. Although Bowerman et al. (Citation2014) found that Jack Creek had a high percentage of fine sediments, they measured very high embryo survival due to the lack of sediment transport into redds that had been somewhat cleaned of fines during construction.
Fry production was the lowest in Jefferson Creek due to a combination of lower spawner numbers, and low estimated embryo survival. Bowerman et al. (Citation2014) attributed low survival to a combination of abundant fine sediments and variable high flows that transported sediments into some redds while scouring others. They observed that naturally spawning adult Bull Trout in Jefferson Creek often used sites just above obstacles where downwelling pressure was high, forcing water through natural redds, somewhat mitigating the effect of fine sediments. Bean et al. (Citation2015) also found that redd occurrence in the Flathead River basin, Montana, tended to be associated with concave-up bedforms (pool tailouts) with downwelling intragravel flows.
Juvenile Production
The methods we used to estimate fry emergence into natal habitats are uncertain due to multiple assumptions made in the calculations. However, all evidence supports the hypothesis that the numbers and densities are very high in many reaches. Spawning habitat quality or quantity is probably not limiting reproduction, even at the highest spawner densities observed. Excess fry above natal habitat capacity are displaced downstream. Fry capture rates (number/d) at the lower Metolius River downstream migrant trap from the high-escapement, 2004 brood year were up to four times that of the low-escapement 2008 brood year. Fry capture numbers during the annual spring monitoring periods were up to eight times the capture rates of older age-1 and age-2 Bull Trout and positively correlated with the abundance of parent spawners, even at the highest observed redd densities. Downs et al. (Citation2006) also observed large numbers of fry migrating into Lake Pend Oreille, Idaho, from Trestle Creek coinciding with high flows soon after emergence. They hypothesized that downstream movement of these small fish may have resulted from flushing flows, or that these fry were still seeking their own territories after being displaced by more dominant fry.
Mortality of fry is apparently very high in lacustrine environments. Growth patterns on scales of adult Bull Trout from Lake Pend Oreille (Downs et al. Citation2006) and Lake Kananaskis, Alberta (Stelfox and Egan Citation1995) revealed that none had emigrated at age 0. Pratt (Citation2000) examined scale samples from 304 subadult and adult Bull Trout sampled from Lake Billy Chinook in 1998, and similarly did not document any with a scale pattern indicating recruitment to the reservoir as fry. From these results we assume that few, if any, of the abundant Bull Trout fry moving downstream past the lower Metolius trap survive to maturity in Lake Billy Chinook.
Our analysis supports the hypothesis that production of age-1 and older juveniles from rearing habitats within the Metolius River basin is controlled by natal habitat rearing capacity. Johnston et al. (Citation2007) found that age-1 and age-2 Bull Trout rearing in Smith-Dorian Creek, Alberta, maintained similar late-summer densities over a 12-year study period, despite a 20-fold increase in adults spawning in the stream during that period. They attributed this stability in juvenile abundance to density-dependent mortality between the egg and age-1 stages and hypothesized that it took relatively few large, highly-fecund females to fully seed Smith-Dorian Creek. Our observations suggest that relatively low adult spawner abundance in 2007–2013 and high abundance in 2003–2006 produced similar annual recruitment of age-1 and older juveniles into Lake Billy Chinook.
Density-Dependent Mortality of Young Bull Trout
Bull Trout are allopatric within the cold tributary habitats of the Metolius River basin, and fry emerge in large numbers during late winter. Opportunistic cannibalism of newly emerged, naive Bull Trout fry by older, larger juveniles may be a significant population regulatory mechanism. Surviving fry may be forced into hiding during low-light periods, when older juveniles are actively feeding (Goetz Citation1997b) or forced downstream and out of the system (Downs et al. Citation2006; high fry capture rates, this study). Factors that may influence the numbers and densities of fry that can rear in high-quality, natal habitats and survive until age 1 include quality and quantity of low-velocity, nocturnal hiding cover enabling fry to avoid being cannibalized and competitive interactions between fry within these coverts. We encourage more intensive study of the interactions between young Bull Trout in relation to natal habitat characteristics that may regulate early Bull Trout survival, as well as the capacities of cold, densely-populated nursery streams to support and produce age-1 and older juvenile Bull Trout. Knowledge about the relationships between natal habitat characteristics and juvenile rearing capacity at a fine scale could aid in the design and justification of habitat enhancements to increase production capacity where natal habitat quantity is limiting and associated populations are in peril.
Density-dependent mortality in salmonid populations that rear for extended periods in small streams has been well described in the literature (Chapman Citation1966; Mortensen Citation1977; Grant and Kramer Citation1990; Elliott Citation1994). Many kinds of density-dependent processes can regulate fish populations, provided they do not act too intermittently, and are strong in relation to density-independent factors that may lead to population instability (Elliott Citation1994). Intraspecific, interference-type competition for feeding and cover locations among fry is the primary factor leading to density-related mortality for most salmonid species. The strongest, largest, and most aggressive individuals choose and defend feeding sites and territories while weaker, smaller, and timid individuals are displaced into less favorable locations or downstream (Mortensen Citation1977; Grant and Kramer Citation1990; Elliott Citation1990, Citation1994). However, with Bull Trout in coldwater habitats, energy-efficient exploitive competition may be a more important mechanism in density-dependent mortality than interference competition (Paul et al. Citation2000; Warnock and Rasmussen Citation2013).
Metolius Bull Trout juveniles normally rear for extended periods within coldwater natal habitats. In these habitats, successful fry must not only out-compete other fry, they must survive in close proximity to older, much larger, and potentially cannibalistic conspecifics. McPhail and Baxter (Citation1996) reported that in a small Skagit River tributary (St. Alice Creek), a 90-mm Bull Trout (likely age 1) contained a consumed 45-mm Rainbow Trout. Goetz (Citation1997a) documented cannibalism of Bull Trout fry by older juveniles rearing in cold Metolius tributary habitats and suggested that diurnal partitioning of near-bank, low-velocity habitats between fry and older year-classes may be a life history adaptation to avoid cannibalism (Goetz Citation1997b). He found that fry were easily observed during daylight, but hid in cover at night when older juveniles were out of cover and actively feeding. Our observations of juvenile Bull Trout in the Metolius River basin show they are opportunistically piscivorous and cannibalistic, even at age 1.
Natural, density-dependent mortality rates for young Bull Trout in the Metolius River basin appear to be very high. With estimated fry emergence of 1.0–2.5 million in study reaches and average annual juvenile recruits into Lake Billy Chinook of about 14,000, mortality from the swim-up fry stage to juvenile recruit stage exceeds 98%, not counting fry produced from unmonitored spawning reaches and the unknown fraction of fry that complete their life cycle in the Metolius River basin upstream from the lake. The vast majority of this mortality probably occurs within the first few weeks after emergence as fry vie for food, cover, and rearing space while trying to avoid older Bull Trout and other predators. Bowerman et al. (Citation2014) found peak fry emergence in the three study streams was from March 17 through April 4, 2010. In comparison, we observed peak daily catch rates of fry at the lower Metolius River downstream-migrant trap from the 2004, high-escapement brood year between March 15 and late April 2005.
Paul et al. (Citation2000), working with a long term data set for Eunice Creek, Alberta, hypothesized that exploitive competition for limited food resources was a likely cause for emigration of age-1 individuals at higher total Bull Trout densities because they observed almost no emigration of age-1 fish at low densities. Our observations support this hypothesis with the caveat that limited space and cover and food resources may prompt downstream migration. Daily catch numbers of age-1 migrants at the lower Metolius River trap were relatively high during March both years but declined to nearly zero by May 1. As more age-1 and age-2 juveniles vacated natal habitats in March and April and habitat availability increased, capture of age-1 Bull Trout from both brood years decreased dramatically, but not for age-2 individuals. Age-2 and age-3 Bull Trout in Eunice Creek also showed relatively high emigration rates regardless of densities (Paul et al. Citation2000).
Estimated Recruitment into Lake Billy Chinook
Conducting population estimates of juvenile Bull Trout in the lower Metolius River via mark–recapture methods (Volkhardt et al. Citation2007) is difficult due to our inability to control or even monitor cannibalism. Bull Trout routinely eat fusiform fishes up to 50% of their own body length, including other Bull Trout (Beauchamp and Van Tassell Citation2001). Studies in controlled environments require sorting and separation by size groups to reduce cannibalism (Mesa et al. 2013). Basic assumptions of insignificant predation mortality of test fish released above the trap, and equal recapture probability of test fish compared with first-time, natural migrants may not be met in some years due to cannibalism and potential modification of migration behavior after release (i.e., staying close to cover in the presence of predators). The lower Metolius River in the vicinity of the downstream-migrant trap provides excellent habitat for rearing subadult Bull Trout and supports previously adfluvial individuals moving upstream in search of prey during periods of low kokanee prey densities in Lake Billy Chinook. Adding support to this hypothesis, the high point estimates and wide confidence intervals in 2010 and 2013 coincide with weak maturing age-classes of kokanee during a period of high Bull Trout abundance in the lake. When these basic assumptions are not met, recapture of test fish is diminished, measured trap efficiency is possibly reduced, and the population estimates are unknowingly inflated. In our study, relatively stable annual spring capture rates after 2005 (post B and B Fire) versus calculated population estimates, which varied dramatically with measured trap efficiency, support the hypothesis of relatively stable annual recruitment based upon habitat capacity and higher trap efficiencies than were measured most years. In recognition of this disparity, we used the mean of the three lowest, most conservative population estimates (highest trap efficiencies) in recent years to estimate annual recruitment into Lake Billy Chinook.
Rieman and Myers (Citation1991) recommended a maximum juvenile stocking density for large salmonid predators of seven juveniles or less per hectare into kokanee waters in Idaho, unless there is no concern for the potential impacts to the kokanee population. High artificial and natural stocking densities of young Lake Trout S. namaycush have been linked to depression of kokanee populations in many lakes and reservoirs in western North America (Martinez et al. Citation2009). The estimated natural stocking densities of Bull Trout into Lake Billy Chinook appear to be especially high when considering they occur annually regardless of kokanee abundance and that current angling regulations protect Bull Trout from harvest until they are at least age 6. The three lowest, most conservative recruitment estimates during the postfire, March–May sampling periods, when expanded to annual estimates, predicted stocking densities of about 18 age-1 and older juveniles/ha into the Metolius River Arm and 9/ha into Lake Billy Chinook as a whole. Although uncertainties in our recruitment estimates are large, high natural stocking density estimates into the lake are corroborated by very abundant subadult Bull Trout in the reservoir after several years of higher kokanee densities.
Management Implications
Declining numbers of large adfluvial Bull Trout in the Metolius River-Lake Billy Chinook system in recent years should not be confused with population-level instability or weakness. Elliott (Citation1994) defines population stability as the ability of a population to return quickly to an equilibrium density following a transient perturbation. Under this definition, Bull Trout populations in the Metolius River basin are perennially stable, even though abundance of older age-classes within Lake Billy Chinook and adult spawner densities may vary significantly between years. The high quality and quantity of natal tributary habitats where Bull Trout are allopatric, and thus not subject to interspecific competition or displacement by other species, ensures continuing high juvenile production at a wide range of adult escapement levels.
Fluctuations in adult Bull Trout abundance in the lake are probably related to cyclic abundance of kokanee prey for subadults and adults. In peak cycles of kokanee production, juvenile Bull Trout have relatively high survival, and the population expands, providing high spawner densities and trophy harvest opportunity. Abundant Bull Trout prey on subsequent cohorts of kokanee, causing decline in abundance. During years of low kokanee abundance, mortality of age 1–3 juvenile Bull Trout recruiting into the lake is probably high, eventually resulting in low spawner densities for those cohorts.
Active management of Bull Trout juvenile recruitment and subadult numbers may result in better balance between the predator and prey populations. Beginning in 2011, a program was initiated of trapping Bull Trout in Lake Billy Chinook and the Metolius River and translocating them to reestablish Bull Trout in the Clackamas River (a lower Willamette River tributary in western Oregon) where they had been extirpated. This donor population group was selected based upon evaluation that it would not be adversely impacted by the removal. From 2011 to 2013, Barry et al. (Citation2014) reported that 1,182 juveniles, 158 subadults, and 61 adults were moved.
Future management options may include implementation of new angling regulations that allow harvest of some subadult Bull Trout. Harvest managers may want to model potential population responses from alternative Bull Trout harvest regulations designed to increase kokanee abundance and to promote more stability in both populations. Increased interannual density and stability of the kokanee population would also aid efforts to reestablish anadromous salmonid species, especially Sockeye Salmon, upstream of Round Butte Dam.
ACKNOWLEDGMENTS
We thank Walt Weber, Steve Marks, Brett Hodgson, Mike Harrington, Ted Wise, Erik Moberly, Mark Fritsch, Jennifer Graham, and others from ODFW and the CTWSRO, who coordinated and summarized annual Bull Trout redd counts. We also thank the dozens of fisheries personnel from many agencies, the Confederated Tribes, and countless volunteers that have assisted in these annual redd counts. Scott Lewis, Steve Thiesfeld, Gary Thiede, and Cory Quesada helped site and coordinate downstream-migrant trapping. We thank the numerous Portland General Electric Company fisheries technicians who diligently operated the lower Metolius River downstream-migrant trap during the past 15 years and the hydro maintenance crews that cheerfully deploy and pull this and other traps for us annually. We thank Lyman Jim and Jeff Hogle of the CTWSRO for information about Bull Trout distribution in Whitewater River and kokanee spawning escapement estimates. Tracy Bowerman from Utah State University generously shared results from studies measuring Bull Trout embryo survival in Metolius River tributaries with different flow and sediment conditions. Brad Wymore and Renny Schmidt of PGE assisted with data analysis and graphics. Terry Shrader, Chuck Huntington, Phil Howell, and an anonymous reviewer provided helpful comments on the manuscript.
REFERENCES
- Al-Chokhachy, R., P. Budy, and H. Schaller. 2005. Understanding the significance of redd counts: a comparison between two methods for estimating the abundance of and monitoring Bull Trout populations. North American Journal of Fisheries Management 25:1505–1512.
- ASRD (Alberta Sustainable Resource Development) and ACA (Alberta Conservation Association). 2009. Status of the Bull Trout (Salvelinus confluentus) in Alberta: update 2009. ASRD, Wildlife Status Report 39, Edmonton.
- Barry, P. M., J. M. Hudson, J. D. Williamson, M. L. Koski, and S. P. Clements. 2014. Clackamas River Bull Trout reintroduction project, 2013 annual report. Oregon Department of Fish and Wildlife, Salem and U.S. Fish and Wildlife Service, Oregon State Office, Portland, Oregon.
- Baxter, C. V., and F. R. Hauer. 2000. Geomorphology, hyporheic exchange, and selection of spawning habitat by Bull Trout (Salvelinus confluentus). Canadian Journal of Fisheries and Aquatic Sciences 57:1470–1481.
- Bean, J. R., A. C. Wilcox, W. W. Woessner, and C. C. Muhlfeld. 2015. Multiscale hydrogeomorphic influences on Bull Trout (Salvelinus confluentus) spawning habitat. Canadian Journal of Fisheries and Aquatic Sciences 72:514–526.
- Beauchamp, D. A., and J. J. Van Tassell. 2001. Modeling seasonal trophic interactions of adfluvial Bull Trout in Lake Billy Chinook, Oregon. Transactions of the American Fisheries Society 130:204–216.
- Bowerman, T., B. Neilson, and P. Budy. 2014. Effects of fine sediment, hyporheic flow, and spawning site selection on survival and development of Bull Trout embryos. Canadian Journal of Fisheries and Aquatic Sciences 71:1059–1071.
- Buchanan, D. V., M. L. Hanson, and R. M. Hooton. 1997. Status of Oregon's Bull Trout: distribution, life history, limiting factors, management considerations, and status. Technical Report to Bonneville Power Administration, Project 199505400, Portland, Oregon.
- Chapman, D. W. 1966. Food and space as regulators of salmonid populations in streams. American Naturalist 100:345–357.
- Corson, S. 2012. Fish passage: positive results from fish collection system at Round Butte Dam. Available: http://www.hydroworld.com/articles/hr/print/volume-31/issue-06/article/fish-passage-positive-results-from-fish-collection-system-at-round-butte-dam.html. (January 2015).
- DeHann, P., M. Diggs, and W. Ardren. 2008. Analysis of genetic variation in Metolius River basin Bull Trout populations. U.S. Fish and Wildlife Service, Abernathy Fish Technology Center Conservation Genetics Program, Longview, Washington.
- Downs, C. C., D. Horan, E. Morgan-Harris, and R. Jakubowski. 2006. Spawning demographics and juvenile dispersion of an adfluvial Bull Trout population in Trestle Creek, Idaho. North American Journal of Fisheries Management 26:190–200.
- Dunham, J., B. Rieman, and K. Davis. 2001. Sources and magnitude of sampling error in redd counts for Bull Trout. North American Journal of Fish Management 21:243–252.
- Elliott, J. M. 1990. Mechanisms responsible for population regulation in young migratory trout, Salmo trutta. Journal of Animal Ecology 59:803–818.
- Elliott, J. M. 1994. Quantitative ecology of the Brown Trout. Oxford University Press, Oxford, UK.
- Goetz, F. 1989. Biology of the Bull Trout Salvelinus confluentus, a literature review. Willamette National Forest, Eugene, Oregon.
- Goetz, F. A. 1997a. Habitat use of juvenile Bull Trout in Cascade Mountain streams of Oregon and Washington.. Pages 339–351 in W. C. Mackay, M. K. Brewin, and M. Monita, editors. Friends of the Bull Trout conference proceedings. Bull Trout Task Force, Trout Unlimited Canada, Calgary, Alberta.
- Goetz, F. A. 1997b. Diel behavior of juvenile Bull Trout and its influence on selection of appropriate sampling techniques. Pages 387–402 in W. C. Mackay, M. K. Brewin, and M. Monita, editors. Friends of the Bull Trout conference proceedings. Bull Trout Task Force, Trout Unlimited Canada, Calgary, Alberta.
- Grant, J. W. A., and D. L. Kramer. 1990. Territory size as a predictor of the upper limit to population density of juvenile salmonids in streams. Canadian Journal of Fisheries and Aquatic Sciences 47:1724–1737.
- Johnston, F. D., J. R. Post, C. J. Mushens, J. D. Stelfox, A. J. Paul, and B. Lajeunesse. 2007. The demography of recovery of an overexploited Bull Trout, Salvelinus confluentus, population. Canadian Journal of Fisheries and Aquatic Sciences 64:113–126.
- Martin, A. 1985. Reproduction of Bull Trout. Pages 88–101 in D. D. MacDonald, editor, Proceedings of the Flathead River Basin Bull Trout biology and populaiton dynamics modeling information exchange. British Columbia Ministry of Environment, Fisheries Branch, Cranbrook, British Columbia.
- Martinez, P. J., P. E. Bigelow, M. A. Deleray, W. A. Fredenberg, B. S. Hansen, N. J. Horner, S. K. Lehr, R. W. Schneidervin, S. A. Tolentino, and A. E. Viola. 2009. Western Lake Trout woes. Fisheries 34:424–442.
- McPhail, J. D., and J. S. Baxter. 1996. A review of Bull Trout (Salvelinus confluentus) life-history and habitat use in relation to compensation and improvement opportunities. University of British Columbia, Fisheries Management Report 104, Vancouver.
- Mesa, M. G., L. K. Weiland, H. E. Christiansen, S. T. Sauter, and D. A. Beauchamp. 2013. Development and evaluation of a bioenergetics model for Bull Trout. Transactions of the American Fisheries Society 142:41–49.
- Mortensen, E. 1977. Density-dependent mortality of trout fry (Salmon trutta L.) and its relationship to the management of small streams. Journal of Fish Biology 11:613–617.
- Muhlfeld, C. C., M. L. Taper, D. F. Staples, and B. B. Shepard. 2006. Observer error structure in Bull Trout redd counts in Montana streams: implications for inference on true redd numbers. Transactions of the American Fisheries Society 135:643–654.
- Paul, A. J., J. R. Post, G. L. Sterling, and C. Hunt. 2000. Density-dependent intercohort interactions and recruitment dynamics: models and a Bull Trout (Salvelinus confluentus) time series. Canadian Journal of Fisheries and Aquatic Sciences 57:1220–1231.
- Pratt, K. L. 1991. Bull Trout scale analysis, Metolius River basin. Deschutes National Forest, Final Report, Bend, Oregon.
- Pratt, K. L. 2000. Bull Trout Scale collections from early 1998 Metolius River/Lake Billy Chinook, Oregon. Prepared for Deschutes Basin Bull Trout Working Group by K. L. Pratt Independent Consultant, Boise, Idaho.
- Ratliff, D. 2012. Trophy Bull Trout prowl Lake Billy Chinook. Salmon Trout Steelheader Magazine (May):60–65.
- Ratliff, D. E. 1992. Bull Trout investigations in the Metolius River–Lake Billy Chinook system. Pages 37–44 in P. J. Howell and D. V. Buchanan, editors. Proceedings of the Gearhart Mountain Bull Trout workshop. American Fisheries Society, Oregon Chapter, Corvallis, Oregon.
- Ratliff, D. E., and P. J. Howell. 1992. The status of Bull Trout populations in Oregon. Pages 10–17 in P. J. Howell and D. V. Buchanan, editors. Proceedings of the Gearhart Mountain Bull Trout workshop. American Fisheries Society, Oregon Chapter, Corvallis, Oregon.
- Ratliff, D. E., S. L. Thiesfeld, W. G. Weber, A. M. Stuart, M. D. Riehle, and D. V. Buchanan. 1996. Distribution, life history, abundance, harvest, habitat, and limiting factors of Bull Trout in the Metolius River and Lake Billy Chinook, Oregon, 1983–1994. Oregon Department of Fish and Wildlife, Information Report 96-7, Portland.
- Riehle, M., W. Weber, A. M. Stuart, S. L. Thiesfeld, and D. E. Ratliff. 1997. Progress report of the multi-agency study of Bull Trout in the Metolius River system, Oregon. Pages 137–144 in W. C. Mackay, M. K. Brewin, and M. Monita, editors. Friends of the Bull Trout conference proceedings. Trout Unlimited Canada, Calgary, Alberta.
- Riehle, M. D. 1993. Metolius Basin water resources monitoring, 1988–1992 progress report. Deschutes National Forest, Sisters Ranger District, Sisters, Oregon.
- Rieman, B. E., and D. L. Myers. 1991. Kokanee population dynamics; cost, benefits and risks of salmonid predators in kokanee waters. Idaho Fish and Game, Project F-73-R-13, Job Completion Report, Boise.
- Stelfox, J. D., and K. L. Egan. 1995. Bull Trout investigations in the Smith-Dorrien Creek/lower Kananaskis Lake system. Alberta Environmental Protection, Natural Resources Service, Fisheries Management Division, Calgary.
- Stuart, A. M., S. L. Thiesfeld, S. E. Ratliff, T. T. Fies, and R. M. Hooton. 1997. Changes in management of Bull Trout in the Metolius River – from trash fish to trophy. Pages 59–66 in W. C. Mackay, M. K. Brewin, and M. Monita, editors. Friends of the Bull Trout conference proceedings. Trout Unlimited Canada, Calgary, Alberta.
- Thedinga, J. F., M. L. Murphy, S. W. Johnson, and J. D. Rodgers. 1994. Determination of salmonid smolt yield with rotary-screw traps in the Situk River, Alaska, to predict effects of glacial flooding. North American Journal of Fisheries Management 14:837–851.
- Thiede, G. P., J. C. Kern, M. K. Weldon, A. R. Dale, S. L Thiesfeld, and M. A. Buckman. 2002. Lake Billy Chinook Sockeye Salmon and kokanee research study 1996–2000. Oregon Department of Fish and Wildlife, High Desert Region, Bend.
- Thiesfeld, S. L., A. M. Stuart, D. A. Nolte, M. A. Buckman, and D. E. Ratliff. 1995. Angler surveys on Lake Billy Chinook, Oregon 1990–1993. Oregon Department of Fish and Wildlife, Information Report 95-1, Salem.
- Volkhardt, G. C., S. L. Johnson, B. A. Miller, T. E. Nickelson, and D. E. Seiler. 2007. Rotary screw traps and inclined plane screen traps. Pages 235–266 in D. H. Johnson, B. M. Shrier, J. S. O'Neal, J. A. Knutzen, X. Augerot, T. A. O'Neil, and T. N. Pearsons. Salmonid field protocols handbook: techniques for assessing status and trends in salmon and trout populations. American Fisheries Society, Bethesda, Maryland.
- Warnock, W. G., and J. B. Rasmussen. 2013. Assessing the effects of fish density, habitat complexity, and current velocity on interference competition between Bull Trout (Salvelinus confluentus) and Brook Trout (Salvelinus fontinalis) in an artificial stream. Canadian Journal of Zoology 91:619–625.