Abstract
Objective. The role of inhaled corticosteroids in the treatment of acute asthma exacerbations in children is controversial. This study compared the effect of inhaled budesonide and inhaled fluticasone in controlling acute asthma exacerbations in young children at home. Methods. In a quasi-randomized crossover design, children aged 5 months to 5 years with severe recurrent asthma episodes were treated either with inhaled budesonide 200 mcg or inhaled fluticasone 125 mcg delivered with a similar spacer. At the onset of asthma exacerbations, 2 puffs of inhaled terbutaline followed by inhaled budesonide or fluticasone was administered using one of the following treatment protocols: Citation 4-day protocol for a relatively mild exacerbation; Citation 8-day protocol for exacerbations that were more severe or uncontrolled by the 4-day protocol; and Citation 8-day protocol + azithromycin for exacerbations uncontrolled by the 8-day protocol or possibly associated with infection with atypical agents. Children were followed for 2 months after each exacerbation. Good response was defined as the absence of asthma symptoms for at least 2 weeks from completion of treatment. Results. One hundred children were recruited: 36 were treated with budesonide, 21 with fluticasone, and 44 with both on different occasions. The groups were similar for preliminary data. Good response was noted in 87% of the budesonide group, 85% of the fluticasone group, and 86% of the budesonide/fluticasone group. By protocol, rates of good response were 84%, 83%, and 94% for the 4-day, 8-day, and 8-day+azithromycin treatment protocols, respectively; corresponding symptom-free periods after treatment were 4.0, 4.9, and 4.3 weeks. None of the children received oral corticosteroids. Conclusion. Acute asthma exacerbations in young children can be effectively controlled at home with the use of high repetitive doses of inhaled budesonide or inhaled fluticasone, initially together with beta2-agonists, given at the beginning of the attack, for a period of 4–8 days.
Introduction
The potential benefits of inhaled corticosteroids for the treatment of acute asthma exacerbations include direct delivery to the airways and reduced systemic exposure. However, their effect in young children is still controversial. Several studies failed to note any positive effects of inhaled corticosteroids in the acute setting (1–4). The two studies of Schuh et al. (Citation[1], Citation[4]) included only children who presented to the emergency department with very severe asthma and forced expiratory volume in one second (FEV1) less than 60% Citation[1] or 50–79% of predicted Citation[4]. The other studies claimed that the acute use of inhaled corticosteroids provides only modest benefit in the control of asthma attack in children Citation[2] and that a combination of inhaled beta2-agonist and corticosteroids yields better results than inhaled corticosteroids alone Citation[3]. Two recent evidence-based reviews reported good results for repeated high doses given in the initial phase of the exacerbation (Citation[5], Citation[6]). It is apparently the high dose that is the key factor for clinical success, reaching up to 5 times the recommended amount Citation[7].
For the last 25 years, inhaled budesonide has been the only drug used in our pediatric asthma clinic for maintenance therapy as well as for treatment of episodic asthma exacerbations, however, budesonide became unavailable nationwide over a 3-month period, from December 2004 to March 2005, during which children in our clinic, including newly diagnosed ones, were treated with fluticasone. The aim of the present study was to compare the clinical efficacy of inhaled budesonide 200 mcg and inhaled fluticasone 125 mcg in controlling asthma exacerbations in young children at home.
Methods
The study group included 100 children aged 5 months to 5 years with recurrent asthma exacerbations who attended our clinic from December 2004 to February 2005 and were allocated for treatment of acute exacerbations at home with either inhaled budesonide 200 mcg or inhaled fluticasone 125 mcg. Only children with asthma symptoms during more than half the 3-month period preceding the study were included. A quasi-randomized crossover design was used.
The allocation for treatment was determined by external circumstance. Until 2004, only inhaled budesonide was used in our clinic for both maintenance therapy and control of asthma exacerbations. In December 2004, inhaled budesonide 200 mcg became commercially unavailable in our country, and all children who attended our clinic and required either maintenance treatment or treatment for acute asthma exacerbations were treated with inhaled fluticasone 125 mcg. Three months later, inhaled budesonide 200 mcg returned to the market, and the children treated with fluticasone switched to budesonide at the next acute asthma exacerbation. Thus, most of the children received both treatments in different episodes.
An asthma exacerbation is defined at our clinic as a sudden, progressive increase in asthma symptoms: shortness of breath, cough, wheezing, and chest tightness, alone or in combination, according to the revised Global Initiative for Asthma (GINA) guidelines 2006,8. We routinely invite all parents for an educational session on the detection, prevention, and treatment of asthma at their initial visit in our clinic, and instruct them to start treatment at the first signs of an exacerbation (usually cough or wheezing occurring during a respiratory viral infection). Before starting treatment in our clinic, all the children in the study had had at least 3 asthma episodes, and in all cases, nebulized beta2-agonists alone, oral; prednisolone, or sometimes nebulized budesonide 0.5 mg twice daily, failed to control the asthma attacks.
During the present study, a combination of inhaled terbutaline, 2 puffs of 0.25 mg/puff, and inhaled budesonide 200 mcg or fluticasone 125 mcg was administered at the beginning of all asthma attacks using one of the following 3 treatment protocols.
4-day protocol for relatively mild asthma episodes with mild cough, dyspnea, and wheezing that were recognized relatively early by the parents; treatment was started with beta2-agonists and than 1 puff of inhaled budesonide 200 mcg or 1 puff of inhaled fluticasone 125 mcg, 4 times daily for the first day followed soon thereafter by a gradual decrease in dose of the inhaled corticosteroids, as detailed in .
8-day protocol for more severe episodes with prolonged cough, dyspnea and wheezing and for episodes not controlled by the 4-day protocol; treatment was started with beta2-agonists and 2 puffs of inhaled budesonide 200 mcg or 2 puff of inhaled fluticasone 125 mcg, for the first 2 days, followed by a gradual decrease in dose of the inhaled corticosteroids, as described in .
8-day protocol + azithromycin for episodes not controlled by the 8-day protocol or for severe attacks with severe cough, dyspnea and wheezing, suspected to be associated with infection with atypical agents (such as Mycoplasma pneumoniae). In the first 2 or 4 days of the 4-or 8-day protocol, respectively, 2 puffs of inhaled terbutaline 0.25 mg/puff were administered prior to treatment with inhaled budesonide or fluticasone, as shown in . The dose of azithromycin (syrup) was 10 mg/kg once daily for 5 days.
Table 1 Treatment plan for the use of inhaled budesonide 200 mcg and inhaled fluticasone 125 mcg during asthma exacerbation or when starting preventive treatment.
The same treatment protocols were employed for both budesonide and fluticasone. Before starting the study, most of the participating children were treated with oral corticosteroids for asthma exacerbations that we believed met our criteria for the 4-day protocol. For all children, only the first asthma episode for which the treatment protocol was applied was analyzed in the present study. In the group of children who received both inhaled budesonide and fluticasone, only one treatment with each drug was evaluated. The maintenance doses of inhaled corticosteroids administered to the children participating in the study were 200 mcg budesonide twice daily or 125 mcg fluticasone twice daily. For drug delivery, all children used the NebuChamber (AstraSeneca) spacer, a nonelectrostatic, stainless-steel, valved holding chamber. The same NebuChamber has been used in our clinic for the last 12 years for the delivery of inhaled budesonide and inhaled terbutaline in children older than 3 months.
A good response to treatment was defined as complete absence of asthma symptoms at the end of the 4th or 8th day of the 4-and 8-day treatment protocols, respectively, and continued absence of asthma symptoms for at least 2 weeks thereafter. One physician (B.V.) provided the education sessions to the parents and examined all the children, and another physician (E.B.) collected the data according to a predesigned algorithm. The assessment of symptom improvement was based on parental reports provided during the routine clinical visit or by telephone interview 1–2 weeks after each acute asthma exacerbation.
Every child continued to be followed for at least 2 months after each asthma exacerbation. The study was approved by the Ethics Committee of Schneider Children's Medical Center of Israel. Student's t-test was used to analyze differences in continuous variables between children receiving budesonide or fluticasone (unpaired test for the single-drug groups and paired test for the children given both drugs) and chi-square test was used for categorical variables. p values < 0.05 were considered significant.
Results
Demographic Data
The study sample consisted of 42 boys and 58 girls, aged 22 ± 13 months. Fluticasone was given to all the children from December 2004 to February 2005, and budesonide, to all the children before and after this period; 44 children were treated with budesonide and fluticasone at different time points (26 started with budesonide, and 18 started with fluticasone). The total number of children ever treated with budesonide was 79, and the number ever treated with fluticasone was 65.
Before the study, the children used beta2-agonists and oral corticosteroids for acute asthma exacerbation, and inhaled budesonide (most of them) or montelukast (Singulair) as controller medications. The number of children using each of these drugs before the study was similar for all groups. The background characteristics of the sample are shown in . There were no differences among the groups in sex ratio, age at onset of asthma, and age at onset of treatment. Eighty-one percent of the children had repeated asthma symptoms on more than 75% of the 90 days preceding the study (mean, 86% of days), with no significant difference among the groups (). All 3 treatment groups contained children taking montelukast 4 mg (Singulair) as an additional preventive treatment. About 25% of the children were receiving montelukast at any time during the study, with no significant differences among the treatment groups.
Table 2 Preliminary data.
Response to Treatment
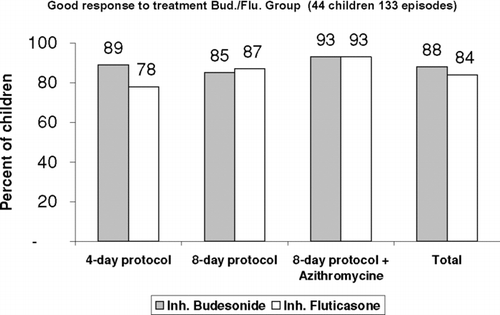
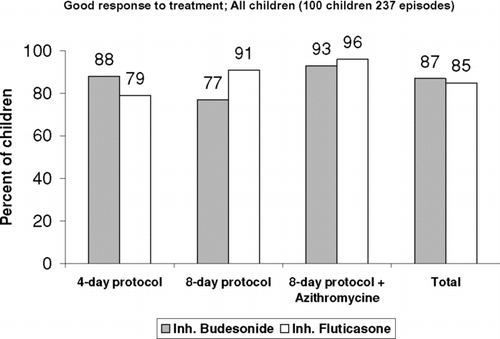
During the study period, the children had 237 asthma exacerbations: 138 treated with budesonide and 99 with fluticasone. The 4-day protocol was applied in 128 episodes, the 8-day protocol in 47, and the 8-day protocol + azithromycin in 62 (). Rates of good response to treatment were 87% for budesonide and 85% for fluticasone. The good response rate in the budesonide/fluticasone group was 86%, as was the overall rate for the whole sample (). Good response rates by protocol were 84% for the 4-day treatment, 83% for the 8-day treatment, and 94% for the 8-day + azithromycin treatment. The 8-day + azithromycin protocol was associated with the highest rate of good response, regardless of the drug used, even though the children who received this protocol had the most severe asthma exacerbations. No statistically significant difference in good response was observed among the different treatment protocols and in the same treatment protocol between budesonide and fluticasone.
Table 3 Number of children using inhaled budesonide or inhaled fluticasone in each treatment protocol.
Asthma Status after Treatment
For the whole sample, the mean duration of the symptom-free period after treatment in the good responders was 4.2 weeks for exacerbations treated with inhaled budesonide and 4.3 weeks for exacerbations treated with inhaled fluticasone. For the 4-day protocol, children treated with budesonide and fluticasone were symptom-free for the next 4.1 and 4.2 weeks, and for the 8-day protocol, the symptom-free periods by drug were 3.8 and 4.5 weeks, respectively. For the 8-day protocol + azithromycin, all 36 children (100%) treated with budesonide and 21 of the 22 children (95%) treated with fluticasone remained symptom-free for 3.8 and 4.5 weeks, respectively.
Budesonide treatment was associated with 0.6 asthma exacerbations during the first month after treatment, and fluticasone with 0.8. During the 2-month post-treatment period, 1.1 asthma exacerbations occurred in both groups.
The 44 children treated with both budesonide and fluticasone had a similar outcome to the cohort as a whole. None of the children was treated with oral corticosteroids during any of the 237 asthma episodes throughout the study. Despite the similar findings for the two drugs for all the objective clinical parameters, when the parents (one per child) of the children given both drugs were asked which one seemed to work better, 12 found no difference, 24 preferred budesonide, and 8 preferred fluticasone.
Discussion
This study indicates that the administration of high starting doses of inhaled corticosteroids, either budesonide or fluticasone, for a relatively short period of time (4–8 days) is very effective in controlling acute asthma exacerbations of varying severity in young children in an outpatient setting. The temporary unavailability of inhaled budesonide 200 mcg in Israel for the first time in 25 years gave us the unique opportunity to compare its effect with that of the substitute drug, inhaled fluticasone 125 mcg, in an acute setting. The findings obtained in this study are important because, after repeated requests over several years, the drug companies had refused us permission to perform a head-to-head comparison between these two drugs, and no other similar comparative study has been performed to date.
Efficacy of Inhaled Corticosteroids in the Acute Setting
Many pediatricians recommend inhaled corticosteroids for use in children in the acute setting.9 A Cochrane review suggested that early use of inhaled corticosteroids in acute asthma exacerbations reduces hospital admissions and improves pulmonary function compared with placebo Citation[10], McKean and Ducharme Citation[11], in an evidence-based review of the literature, suggested that episodic treatment with a high dose of inhaled corticosteroids is beneficial for mild, virally-induced wheezing in children. Several other randomized double-blind studies in the emergency setting yielded similar findings comparing oral prednisolone with various inhaled corticosteroids, such as budesonide (12–15), fluticasone Citation[16], flunisolide Citation[17], and dexamethasone Citation[18], administered with various devices, namely nebulizer (Citation[14], Citation[15], Citation[18], Citation[19]) turbohaler Citation[12] or metered dose inhaler and spacer (Citation[13], Citation[17]). Good results were also reported in more specific double-blind studies of children with wheezing due to respiratory infections (20–22). Volovitz et al. Citation[23] found that when using high starting dosages of budesonide in children aged 1 to 14 years, parents were able to control 1001 of the 1061 asthma exacerbations at home.
In the present study, the children were carefully selected to ensure homogenous comparative groups. All were younger than 5 years and had moderate to severe asthma. All the parents participated in the same asthma-education sessions. A comparable number of children were included in each treatment group, and a comparable number started with one or the other drug. The children were also evaluated during the same period of the year (the winter), by the same physician, and another physician collected the data from the electronic files. Due to the lack of objective evidence in the evaluation of the response, we cannot rule out that parents may overestimate the degree to which a their child's asthma is controlled.
The treatment protocols used in the present study have been used routinely in our outpatient clinic since 1983, and are described in our previous publications (Citation[12], Citation[23], Citation[24], Citation[25], Citation[26]). However; this is the first time, that we were able to compared inhaled fluticasone 125 mcg to inhaled budesonide 200 mcg in the same setting. The similar efficacy of the 2 drugs in all 3 protocols indicates that inhaled corticosteroids, as a group, are effective in the control of acute asthma episodes in children.
Efficacy of High-Dose Corticosteroids in Children
Studies have shown that simply doubling the usual maintenance dose of inhaled corticosteroids may be not sufficient for controlling asthma exacerbations Citation[27] and that doses at least 4–5 times the maintenance dose may be necessary (12–16,18). The drugs/doses/inhalers investigated in these studies included budesonide 2400 μ g via nebulizer Citation[14], budesonide 2000 μ g via nebulizer Citation[15], budesonide 1600 μ g dry powder inhaledCitation[12], dexamethasone 1.5 mg/kg via nebulizer Citation[18], budesonide 1200 μ g via metered dose inhaler with spacer Citation[13], and fluticasone 1000 μ g via nebulizer Citation[16], In only a few studies did the inhaled route prove to be less effective than the oral route (Citation[1], Citation[2], Citation[4]).
Volovitz et al. Citation[12] in a study of 22 older children (aged 6–16 years) who presented to the emergency department with moderately severe asthma attacks, found that tapering dry powder inhaled budesonide conferred similar benefits to tapering oral corticosteroid therapy in terms of spirometry, pulmonary indices, wheezing, accessory muscle use, and oxygen saturation. Symptom scores improved more quickly with inhaled budesonide than with oral prednisolone during the first week after discharge. Devidayal et al. Citation[14], in a study of 80 children aged 2–12 years, found that the rate of full recovery and subsequent discharge from the emergency department was threefold faster when a single oral dose of prednisolone 2 mg/kg was replaced with 3 doses of budesonide 800 mcg given at 30-minute intervals via nebulizer. These findings were supported by 2 recent evidence-based reviews which reported a good response to high-dose inhaled corticosteroids given repeatedly during the initial phase of an acute asthma exacerbation (Citation[5], Citation[6]). Apparently, the high dose of inhaled corticosteroids combined with the high frequency of administration provided a rapid, additive effect to regimens and were the key factor for success.
In the present study, we used a 4-day protocol with relatively moderate doses of inhaled corticosteroids (budesonide 800–400 mcg/day and fluticasone 500–250 mcg/day) or an 8-day-protocol with high doses of inhaled corticosteroids (budesonide 1600–800 mcg/day and fluticasone 1000–500 mcg/day). Although the dose of inhaled corticosteroids was just doubled (over the maintenance dose) in the 4-day protocol and quadrupled in the 8-day protocol, both were successful. These good clinical results, which contrast with the finding of Garrett et al. Citation[27] may be explained by the good compliance to treatment of our patients, which we attributed to our extensive asthma education program, and our instruction of the parents to start treatment immediately at the early signs of asthma exacerbation.
Additive Effect of Azithromycin in Acute Asthma
Macrolide antibiotics have been considered beneficial for patients with asthma since the1950s Citation[28], thanks to their anti-inflammatory effects on the chronically inflamed airways in addition to their anti-infective action Citation[29]. There is growing evidence of a key role of atypical respiratory pathogens, such as Chlamydia pneumoniae and Mycoplasma pneumoniae, in the pathogenesis of asthma exacerbations. Infection with Mycoplasma pneumoniae was found to be significantly associated with hospitalization for acute exacerbation of asthma Citation[30]. Furthermore, Chlamydia pneumoniae infections may account for the symptoms of asthma that are poorly controlled by steroids Citation[31]. One study demonstrated a positive effect of macrolides on reducing the number of eosinophils and markers of eosinophilic inflammation in patients with asthma Citation[32].
The present study indicates that the addition of azithromycin to treatment with high-dose corticosteroids is effective in children who do not respond to inhaled corticosteroids alone. Our rates of improvement with this protocol were 93% for inhaled budesonide and 96% for inhaled fluticasone. It is important to emphasize that these children had the most severe asthma attacks. Before azithromycin became available, we used doxycycline, with similar results Citation[33].
Advantage of Inhaled Corticosteroids
Oral corticosteroids are associated with adverse effects in young children even when administered in short intermittent courses (Citation[12], Citation[34], Citation[35], Citation[36], Citation[37]). This is not true for inhaled corticosteroids Citation[38], probably because they are delivered directly to the site of action Citation[39]. The two most harmful effects of oral corticosteroids are impeded height growth and depressed serum cortisol concentration Citation[40]. In addition, oral corticosteroids often cause a significant state of restlessness in the child, especially when methylprednisolone is used. Oral prednisolone is associated with fewer adverse effects, but it is prescribed much less often for young children because it is not soluble in water and has a very bad taste which children find unacceptable Citation[41].
In previous studies by our group using similar protocols (Citation[12], Citation[42]), we showed that unlike oral corticosteroids, high-dose inhaled budesonide was not associated with a decrease in cortisol plasma concentration (either after fasting at 8 am or 1 hour after ACTH stimulation test). Moreover, 5 years of treatment with inhaled corticosteroids in young children did not impair growth Citation[24]. De Benedictis et al. Citation[43] reported that 10 days' administration of nebulized budesonide 500 mcg or fluticasone 250 mcg was not associated with hypophysial pituitary axis suppression. Using a similar protocol, we showed that after discharge from hospital, treatment with inhaled corticosteroids led to a faster alleviation of asthma symptoms than oral corticosteroids Citation[12].
It is recognized that many children are able to tolerate a few days of oral corticosteroid treatment without clinically significant problems. However, all the children in our study were younger than 5 years and were unable to swallow tablets of prednisolone. Furthermore, most of the mother preferred not to give the children methylprednisolone because of their previous bad experience with this drug. The Expert Panel Report 3 (EPR3)44 Guidelines for the Diagnosis and Management of Asthma August 2007 (Section 5: Managing exacerbation of asthma) recommends that for home management of asthma exacerbations, treatment should be initiated with oral systemic corticosteroids. However, the panel also notes that preliminary evidence indicates that quadrupling the dose of ICS starting with the appearance of worsening symptoms may prevent exacerbations warranting treatment with oral systemic corticosteroids.
Comparisons Between Budesonide and Fluticasone
Ours is the first study to compare inhaled budesonide to inhaled fluticasone for acute asthma using metered dose inhaler + spacer in an outpatient setting. De Benedictis et al. Citation[40] found that a short course of nebulized fluticasone had the same effects as a double dose of nebulized budesonide when either drug was added to bronchodilator therapy in children with acute asthma. Accordingly, the present study indicated that multiple doses of inhaled budesonide 200 mcg and inhaled fluticasone 125 mcg are similarly effective in all ranges of asthma severity. The preference of the parents for inhaled budesonide may be explained by their awareness that budesonide was the main drug used by our clinic for many years and that fluticasone was a relatively new drug, serving as a substitute.
Inhaled corticosteroids, both budesonide and fluticasone, can be used in children who are already receiving preventive treatment with inhaled corticosteroids or children who will be starting preventive treatment immediately after an asthma attack Citation[45]. They can also be given to younger children who cannot tolerate the available oral corticosteroid preparations. Perhaps efforts are need to develop new preparations of prednisolone with a better taste so that they may be more amenable to treatment in young children for short periods as an alternative to inhaled corticosteroids.
Conclusions
On the basis of the present findings and studies reported in the literature (Citation[5], Citation[6]), we suggest that high repetitive doses of inhaled corticosteroids, either budesonide or fluticasone, administered with gradual decease in dosage, according to a 4–8-day protocol, may be recommended for the effective control of acute asthma exacerbations in young children at home.
References
- Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, Alothman G, Tennis O, Canny G. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. N Engl J Med 2000; 343: 689–694
- Gibson P G, Powell H. Initial corticosteroid therapy for asthma. Curr Opin Pulm Med 2006; 12(1)48–53
- Hendeles L, Sherman J. Are inhaled corticosteroids effective for acute exacerbations of asthma in children?. J Pediatr 2003; 142: S26–S33
- Schuh S, Dick P T, Stephens D, Hartley M, Khaikin S, Rodrigues L, Coates A L. High-dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics 2006; 118(2)644–650
- Volovitz B. Inhaled budesonide in the management of acute worsenings and exacerbations of asthma: A review of the evidence. Respir Med. 2007; 101(4)685–695, Epub 2006, Nov 27
- Rodrigo G J. Rapid effects of inhaled corticosteroids in acute asthma: an evidence-based evaluation. Chest 2006; 130(5)1301–1311
- Volovitz B, Nussinovitch M. Management of children with severe asthma exacerbation in the emergency department. Paediatr Drugs 2002; 4: 141–148
- GINA. Pocket guide for asthma management and prevention. The Global Initiative for Asthma (GINA). 2006; 18
- Garrett J, Williams S, Wong C, Holdaway D. Application of asthma action plans to childhood asthma: a national survey. NZ J Med. 1997; 110: 308–310
- Edmonds M L, Camargo C A, Jr., Pollack C V, Rowe B H. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma (Cochrane review). The Cochrane Library, Version 4. Update Software, Oxford 2003
- McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood (Cochrane review). The Cochrane Library. Update Software, Oxford 2000, Issue 4
- Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch M, Varsano I. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: a controlled comparative study with oral prednisolone. J Allergy Clin Immunol 1998; 102: 605–609
- Singhi S, Banerjee S, Nanjundaswamy H. Inhaled budesonide in acute asthma. J Paediatr Child Health 1999; 35: 483–487
- Devidayal Singhi S, Kumar L, Jayshree M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatr 1999; 88: 835–840
- Matthews E E, Curtis P D, McLain B I, Morris L S, Turbitt M L. Nebulized budesonide versus oral steroid in severe exacerbations of childhood asthma. Acta Paediatr 1999; 88: 841–843
- Manjra A I, Price J, Lenney W, Hughes S, Barnacle H. Efficacy of nebulized fluticasone propionate compared with oral prednisolone in children with an acute exacerbation of asthma. Respir Med 2000; 94: 1206–1214
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. Am J Respir Crit Care Med 1998; 157: 698–703
- Scarfone R J, Loiselle J M, Wiley J F, 2nd, Decker J M, Henretig F M, Joffe M D. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med 1995; 26: 480–486
- Sung L, Osmond M H, Klassen T P. Randomized, controlled trial of inhaled budesonide as an adjunct to oral prednisone in acute asthma. Acad Emerg Med 1998; 5: 209–213
- Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Arch Dis Child 1993; 68: 85–87
- Svedmyr J, Nyberg E, Thunqvist P, Asbrink-Nilsson E, Hedlin G. Intermittent treatment with inhaled steroids for deterioration of asthma due to upper respiratory tract infections. Acta Paediatr 1995; 84: 884–888
- Svedmyr J, Nyberg E, Asbrink-Nilsson E, Hedlin G. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatr 1999; 88: 42–47
- Volovitz B, Nussinovitch M, Finkelstein Y, Harel L, Varsano I. Effectiveness of inhaled corticosteroids in controlling acute asthma exacerbations in children at home. Clin Pediatr 2001; 40: 79–86
- Volovitz B, Amir J, Malik H, Kaushansky A, Varsano I. Growth and pituitary-adrenal function in children with severe asthma treated with inhaled budesonide. N Engl J Med 1993; 329: 1703–1738
- Varsano I, Volovitz B, Malik H, Amir Y. Safety of one year treatment with budesonide in young children with asthma. J Allergy Clin Immunol 1990; 5: 914–920
- Volovitz B, Soferman R, Blau H, Nussinovitch M, Varsano I. Rapid induction of clinical response with a short-term high-dose starting-schedule of budesonide nebulizing suspension in young children with recurrent wheezing episodes. J Allergy Clin Immunol 1998; 101: 464–469
- Garrett J, Williams S, Wong C, Holdaway D. Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid. Arch Dis Child 1998; 79: 12–17
- Kaplan M A, Goldin M. The use of triacetyloleandomycin in chronic infectious asthma. Antibiotics Manual, 1958–1959, H Welch, F Marti-Ibanez. Interscience, New York 1959; 273–276
- Ferrara G, Losi M, Franco F, Corbetta L, Fabbri M, Richeldi L. Macrolides in the treatment of asthma and cystic fibrosis. Respir Med Jan, 2005; 99(1)1–10
- Lieberman D, Lieberman D, Printz S, Ben-Yaakov M, Lazarovich Z, Ohana B, Friedman M G, Dvoskin B, Leinonen M, Boldur I. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 2003; 167: 406–410
- Hahn D L, Bukstein D, Luskin A, Zeitz H. Evidence for Chlamydia pneumoniae infection in steroid-dependent asthma. Ann Allergy Asthma Immunol Jan, 1998; 80(1)45–49
- Amsden G W. Anti-inflammatory effects of macrolides–an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions?. J Antimicrob Chemother Jan, 2005; 55(1)10–21
- Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M. Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila.) 2007; 46(2)121–126
- Hodsman A B, Toogood J H, Jennings B, Fraher L J, Baskerville J C. Differential effects of inhaled budesonide and oral prednisolone on serum osteocalcin. J Clin Endocrinol Metab 1991; 72: 530–540
- Wolthers O D, Riis B J, Pedersen S. Bone turnover in asthmatic children treated with oral prednisolone or inhaled budesonide. Pediatr Pulmonol 1993; 16: 341–346
- Hedlin G, Svedmyr J, Ryden A-C. Systemic effects of a short course of betamethasone compared with high-dose inhaled budesonide in early childhood asthma. Acta Paediatr 1999; 88: 48–51
- Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther 1972; 13: 694–702
- Pedersen S. Clinical Safety of inhaled corticosteroids for asthma in children: An update of long-term trials. Drug Saf 2006; 29(7)599–612
- Barnes P J. Inhaled glucocorticoids for asthma. N Engl J Med 1995; 332: 868–875
- Zora J A, Zimmerman D, Carey T L. Hypothalamic-pituitary-adrenal axis suppression after short-term, high-dose glucocorticoid therapy in children with asthma. J Allergy Clin Immunol 1986; 77: 9–13
- Hendeles L. Selecting a systemic corticosteroid for acute asthma in young children. J Pediatr 2003; 142(2 Suppl)S40–S44
- Volovitz B, Kauschansky A, Nussinovitch M, Harel I, Varsano I. Normal diurnal variation in serum cortisol concentration in asthmatic children treated with inhaled budesonide. J Allergy Clin Immunol 1995; 96: 874–878
- De Benedictis F M, Del Giudice M M, Vetrella M, Tressanti F, Tronci A, Testi R, Dasic G; Flic12 Study Group. Nebulized fluticasone propionate vs. budesonide as adjunctive treatment in children with asthma exacerbation. J Asthma 2005; 42(5)331–336
- Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. NHLBI produced publications. August 28, 2007
- FitzGerald J M, Shragge D, Haddon J, Jennings B, Lee J, Bai T, Pare P, Kassen D, Grunfeld A. A randomized, controlled trial of high dose, inhaled budesonide versus oral prednisone in patients discharged from the emergency department following an acute asthma exacerbation. Can Respir J 2000; 7(1)61–67