Abstract
Introduction: Bronchial asthma (BA) and obstructive sleep apnea syndrome (OSAS) are common causes of respiratory disturbance. Many cases of patients with both conditions have been reported, and BA and OSAS may exacerbate each other, but information remains sparse.
Methods:We retrospectively evaluated 60 patients under treatment for BA in our department between April 2016 and March 2018 who also underwent portable polysomnography (PSG) for suspected OSAS to assess potential association between PSG results and asthma treatment or respiratory function. BA was diagnosed and treated according to the Asthma Prevention and Management Guideline 2015.
Results: We found that BA treatment intensity step was significantly higher for patients with BA who had concurrent moderate or severe OSAS (p = 0.0016). However, neither respiratory function, fraction of exhaled nitric oxide (FeNO), nor forced oscillation technique (FOT) differed significantly between patients with and without OSAS, and apnea hypopnea index was not significantly correlated with respiratory function, FeNO or FOT parameters.
Conclusion:We conclude that even though BA patients with OSAS had good respiratory function, their BA was more severe than that of patients without OSAS, suggesting that OSAS may exacerbate BA. Background factors and asthma parameters were not predictive of PSG results, and patients with suspected OSAS should be evaluated proactively by using PSG.
Introduction
Bronchial asthma (BA) and obstructive sleep apnea syndrome (OSAS) are common in Japan, both having a prevalence of around 5% (Citation1,Citation2), and they both can cause nocturnal respiratory failure. A high proportion of patients have both BA and OSAS, which are known to exacerbate each other (Citation2,Citation3). The nocturnal upper airway stenosis and nocturnal hypoxia of OSAS have the potential to aggravate BA symptoms directly. In addition, TNF-α, interleukin-6, vascular endothelial growth factor, leptin, and other inflammatory mediators and adipokines produced during OSAS are believed to induce airway changes and sensitization, indirectly aggravating BA and making it intractable to treatment (Citation2,Citation4,Citation5). Several epidemiologic studies have shown that BA patients are more likely than the public as a whole to have OSAS (Citation6–8), thus suggesting an interaction between BA and OSAS. In OSAS patients who develop BA, the lower airway stenosis due to BA increases the treatment intractability of OSAS, and BA-associated allergic rhinitis and steroid treatment may further exacerbate OSAS (Citation2,Citation4). Other studies, however, have failed to identify a direct interaction between OSAS and BA (Citation9), and a clear consensus regarding the effects of OSAS and BA on each other has yet to be reached.
Although some studies have shown that treating OSAS in patients with both BA and OSAS enables reduction of BA treatment (Citation10,Citation11), others indicate that this improvement is restricted to the nocturnal symptoms of OSAS and unrelated to respiratory function or BA symptoms (Citation9,Citation12). Therefore, the association between OSAS and BA remains unclear. In daily asthma medical care, useful tests for asthmatic patients are increasing, such as FeNO (Th2 inflammatory marker) or forced oscillation test (FOT: airway resistance and lung compliance). If some methods detect SAS in asthmatic patients more easily, it is useful in clinical situation of asthma treatment.
In this study, we hypothesized that some factors detect SAS in asthmatic patients and we investigated the association between BA and OSAS by calculating the apnea hypopnea index (AHI), an index of OSAS severity, in BA patients who were evaluated for suspected OSAS by using simple polysomnography (PSG). We compared the PSG results with patients’ scores for several BA assessment parameters, including severity, respiratory function, forced oscillation technique (FOT) parameters, fraction of exhaled nitric oxide (FeNO), and BA treatment intensity step.
Patients and methods
We retrospectively compared 60 patients with BA who were treated in the Division of Respiratory Medicine of St. Marianna University School of Medicine Yokoyama City Seibu Hospital and who were investigated between 1 April 2016 and 31 March 2018 for suspected OSAS for reasons including nocturnal snoring, dyspnea, laryngopharngeal reflux, or obesity. BA was diagnosed according to Asthma Prevention and Management Guideline 2015 (Citation1), and treatment was adjusted as required by the attending physician in accordance with this guideline. Rhinitis and laryngopharngeal reflux was diagnosed through interview. Patients who did not undergo simple PSG, those with thoracic deformity, those known to have another respiratory disorder, pregnant and lactating women, and patients hospitalized for lower respiratory tract infection were excluded. OSAS was assessed by portable PSG (SAS-2100, Nihon Kohden, Tokyo, Japan) and the AHI was calculated. BA was assessed by FeNO measurement (NIOX VERO, Chest M.I., Tokyo, Japan) carried out during PSG, spirometry (AC8800, Chest M.I., Tokyo, Japan), and FOT by a MostGraph-01 (Chest M.I., Tokyo, Japan). The tests were conducted by experienced technicians in the Clinical Laboratory of St. Marianna University School of Medicine Yokoyama City Seibu Hospital.
Statistical analysis was completed by using SPSS version 20 (IBM, New York, NY). All parameters were expressed as means and standard deviations. Groups were compared by using a χ2 test or a Mann–Whitney U test, with p < 0.05 regarded as significant. Correlations between parameters were investigated by using Pearson's correlation coefficient, with p < 0.05 regarded as significant. In addition, Mantel–Haenzel test were performed to exclude confounding factors for chi-square test. Parameters were investigated by using this test coefficient, with p < 0.05 and HR > 1 (95% CI) regarded as significant. Sleep study level is 3 as using portable PSG.
Because FeNO was not conducted during the study period in seven cases and MostGraph testing was not done in 3, those patients were excluded from the individual analyses of these parameters.
This study was approved by the Clinical Study Group of St. Marianna University Medical School (University No. 4105) and Seibu Hospital (Seibu No. 726) on August 21, 2018.
Results
Patient attributes are shown in . The study subjects comprised 60 patients (12 men and 48 women) who were 65.00 ± 13.38 years (mean ± 1 SD) of age and had a body mass index (BMI) of 26.54 ± 4.78. The treatment step as defined according to Asthma Prevention and Management Guideline 2015 (Citation1) was Step 3 for 25 subjects and Step 4 for the remaining 35. AHI was 0.4–73.1 (19.33 ± 16.88), percentage forced vital capacity in 1 s was 50–160% (104.05 ± 21.89%), and FeNO was 5–129 ppb (31.64 ± 28.53 ppb). Most graph parameters were: R5, 1.73–13.2 cmH20/L/S (4.34 ± 2.36 cmH20/L/S); X5, –9.57 to 0.83 cmH20/L/S (–1.31 ± 2.02 cmH20/L/S); and resonant frequency, 4–27.4 (10.09 ± 5.07).
Table 1. Patient demographics.
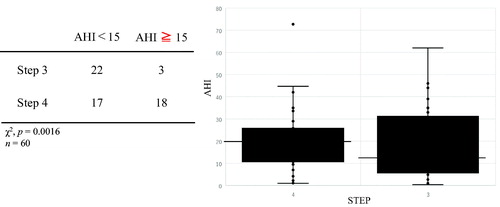
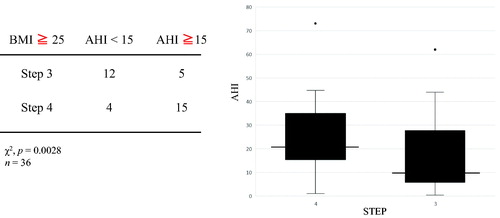
We investigated the association between BA assessment parameters and the OSAS parameters of AHI and minimum SpO2 but found no clear correlation in any case (). When we divided patients into those with mild OSAS (AHI < 15) and moderate or severe OSAS (AHI ≥ 15) and used a χ2 test to investigate the effect of BA treatment step on OSAS, significantly more of the patients with moderate or severe OSAS were undergoing Step 4 treatment (p = 0.0016) (), but there were no significant differences between the two groups in terms of respiratory function, FeNO, or any of the FOT parameters (). In addition, rhinitis had no significant effect on AHI, such that we considered that rhinitis and AHI were not associated (). We divided patients into an overweight group (BMI ≥ 25) and a nonoverweight group (BMI < 25), and compared the AHI values and treatment steps of the two groups to investigate the effect of obesity. In the overweight group, patients with higher AHI were significantly more likely to be at treatment step 4 (p = 0.0028), but there was no clear association between AHI score and treatment step in the nonoverweight group (). To eliminate the confounding factor of obesity, Mantel–Haenzel test was performed. It shows that patients with higher AHI were significantly more likely to be at treatment step 4 with or without obesity (p = 0.003). The relative risk of higher AHI (AHI ≧ 15) to the frequency of STEP4 is 10.667 (95% CI: 2.283–49.838). There was a significant correlation with or without obesity. As for gender differences, there were few men and could not do significant statistics. The effects of GERD and medication were not clear.
Table 2. Correlation between various parameters and AHI or lowest SpO2 at PSG.
Table 3. Comparison of patient characteristics between low AHI and high AHI groups.
Table 4. Relationship between rhinitis and AHI.
Discussion
We found that patients with more severe OSAS were significantly more likely to be at BA treatment step 4, and this tendency was more pronounced in patients with a high BMI. However, neither AHI nor minimum SpO2 were correlated with any BA parameter. In addition, we did not find factors to detect SAS efficiently by the measurement of spirometry, FOT or FENO.
We propose two potential explanations for the fact that patients with serious OSAS were more likely to be at BA Treatment Step 4 irrespective of respiratory function: (Citation1) obesity itself, which is a common risk factor for both BA and OSAS, led to the aggravation of BA; and (Citation2) the direct and indirect adverse effects of OSAS on BA increased its severity.
Regarding the first possible explanation, obesity is an important factor contributing to the severity of BA (explanation 1), and epidemiologic studies have shown that this association is particularly strong in women (Citation13,Citation14). In addition, obesity is a known risk factor for OSAS (Citation15) and may have both worsened BA and OSAS. In this study, AHI had a strong effect on BA treatment intensity step in patients with a higher BMI. Even if the effect of obesity is excluded by the Mantel–Haenzel test, the severity of asthma was related to the severity of SAS.
In terms of possible direct and indirect adverse effects of OSAS on BA (explanation 2), we did not observe any direct effects in this study, given the lack of correlation between AHI or minimum SpO2 and either BA treatment step or respiratory function. Moreover, because we did not evaluate the levels of any inflammatory mediators, we were also unable to assess potential indirect effects. Prolonged evaluation of patients treated for OSAS is required to address this point. A literature search yielded several reports in which patients with both OSAS and BA who received continuous positive airway pressure (CPAP) treatment for OSAS also demonstrated decreased BA (Citation10–12,Citation16,Citation17). In some of these cases, the reports also noted decreased airway sensitivity (Citation16) or decreased inflammatory mediator release in obese persons (Citation10), suggesting that the presence of SAS may have a negative effect on BA control. However, other reports indicate that although CPAP ameliorated SAS, this therapy had no effect on BA (Citation9,Citation17). Consequently, no consensus has been achieved, and additional studies are warranted.
We also found little correlation between patient attributes or BA assessment parameters and portable PSG results, with no factor predictive of a high AHI. Previous reports have suggested that concomitant rhinitis may worsen BA (Citation18), but this association was not significant in our current study. Because the nocturnal symptoms of BA and SAS do not overlap, evaluation by PSG is required.
This study had several limitations. This was a cross-sectional retrospective study, and because the subjects had undergone testing for clinical purposes, it may have been affected by the clinical attitudes of the attending physicians concerned. The circumstances under which clinical intervention occurred were unclear, and although BA assessment was conducted during the same time period as PSG, the tests were not done simultaneously. In addition, tests were conducted during the day, meaning that differences in respiratory function between sitting and lying down or between day and night were not considered. Prospective all-case studies and the collection of additional case studies are required to ascertain the frequency with which OSAS and BA occur together and to identify those patients who would benefit from therapeutic intervention.
Conclusions
We found that patients with high AHI tended to require treatment for serious BA despite having good respiratory function. This association suggests that OSAS should be suspected in BA patients with unstable symptoms and that this potential comorbidity should actively be investigated through PSG.
Declaration of interest
The authors have no conflict of interest.
Acknowledgements
We thank Dr. Yoshiaki Ono and Dr. Junichiro Hisata of the Yokohama Respiratory Clinic Sleep Breathing Disorder Center for their cooperation with this study and their detailed investigation and treatment of OSAS.
Additional information
Funding
References
- Ichinose M, Sugiura H, Nagase H, Yamaguchi M, Inoue H, Sagara H, Tamaoki J, Tohda Y, Munakata M, Yamauchi K, et al. Japanese guidelines for adult asthma 2017. Allergol Int 2017;66:163–189. doi: 10.1016/j.alit.2016.12.005.
- Razak A, Chirakalwasan N. Obstructive sleep apnea and asthma. Asian Pac J Allergy Immunol 2016;34:265–271. doi: 10.12932/AP0360.32.2.2013.
- Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med 2009;5:71–78.
- Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med 2014;62:665–675. doi: 10.2310/JIM.0000000000000065.
- Sundbom F, Janson C, Malinovschi A, Lindberg E. Effects of coexisting asthma and obstructive sleep apnea on sleep architecture, oxygen saturation, and systemic inflammation in women. J Clin Sleep Med 2018;14:253–259. doi: 10.5664/jcsm.6946.
- Teodorescu M, Barnet J, Hagen E, Palta M, Young T, Peppard P. Association between asthma and risk of developing obstructive sleep apnea. JAMA 2015;313:156–164. doi: 10.1001/jama.2014.17822.
- Kong D-L, Qin Z, Shen H, Jin H-Y, Wang W, Wang Z-F. Association of obstructive sleep apnea with asthma: a meta-analysis. Sci Rep 2017;7:4088.doi: 10.1038/s41598-017-04446-6.
- Lu H, Fu C, Li W, Jiang H, Wu X, Li S. Screening for obstructive sleep apnea syndrome in asthma patients: a prospective study based on Berlin and STOP-Bang questionnaires. J Thorac Dis 2017;9:1945–1958.
- Ng SSS, Chan T-O, To K-W, Chan KKP, Ngai J, Yip W-H, Lo RLP, Ko FWS, Hui DSC. Continuous positive airway pressure for obstructive sleep apnoea does not improve asthma control. Respirology 2018;23:1055–1062. doi: 10.1111/resp.13363.
- To Y, Koshino T, Kubo M, Muramatsu H, Kudo K, Kabe J. A case of refractory bronchial asthma improving with treatments of sleep apnea syndrome (in Japanese). Arerugi 1998;47:34–40.
- Serrano-Pariente J, Plaza V, Soriano JB, Mayos M, López-Viña A, Picado C, Vigil L, CPASMA Trial Group. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy 2017;72:802–812. doi: 10.1111/all.13070.
- Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med 2005;99:529–534. doi: 10.1016/j.rmed.2004.10.011.
- Wang L, Wang K, Gao X, Paul TK, Cai J, Wang Y. Sex difference in the association between obesity and asthma in U.S. adults: Findings from a national study. Respir Med 2015;109:955–962. doi: 10.1016/j.rmed.2015.06.001.
- Fukutomi Y, Taniguchi M, Tsuburai T, Tanimoto H, Oshikata C, Ono E, Sekiya K, Higashi N, Mori A, Hasegawa M, et al. Obesity and aspirin intolerance are risk factors for difficult-to-treat asthma in Japanese non-atopic women. Clin Exp Allergy 2012;42:738–746. doi: 10.1111/j.1365-2222.2011.03880.x.
- Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, Fiander PM, Grunstein RR, Gurubhagavatula I, Kapur VK, et al. The role of weight management in the treatment of adult obstructive sleep apnea. Am J Respir Crit Care Med 2018;198:e70–e87. doi: 10.1164/rccm.201807-1326ST.
- Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J 2007;29:307–311. doi: 10.1183/09031936.00059706.
- Kauppi P, Bachour P, Maasilta P, Bachour A. Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep Breath 2016;20:1217–1224.
- Chirakalwasan N, Ruxrungtham K. The linkage of allergic rhinitisand obstructive sleep apnea. Asian Pacif J Allergy Immunol 2014;32:276–286.