Abstract
Objective
Patients with severe asthma require high-dose inhaled corticosteroids, with or without add-on treatments, to maintain asthma control. Because symptom control remains unsatisfactory in some patients despite these therapies, maintenance therapy with oral corticosteroids (OCS) remains considered a treatment option by physicians. Besides physician-diagnosed exacerbations, many patients intermittently self-medicate with OCS during episodes of worsening symptoms or as a prevention of such episodes. However, long-term OCS use is associated with several comorbidities that may decrease health-related quality of life, worsen prognosis, and should ideally require monitoring and management. In this review, we discuss the adverse effects of OCS use, the OCS-sparing effect of biologics in severe asthma, and the need for optimal referral pathways to ensure the best outcomes for those at-risk asthma patients.
Data sources
PubMed.
Study selection
Studies with results on the OCS-sparing effect of biologics in adult severe asthma were selected.
Results
Chronic and intermittent OCS use in asthma is associated with considerable adverse effects in asthma. Omalizumab, mepolizumab, benralizumab, and dupilumab reduce the need for OCS in severe asthma, while also reducing the exacerbation rate and improving several patient-related outcomes.
Conclusion
Targeted biologic therapies have revolutionized the treatment of uncontrolled severe asthma by reducing or even eliminating the need for OCS and improving other major outcomes. Novel agents are now rapidly increasing the therapeutic armamentarium, but additional efforts are needed to optimize referral pathways in order to ensure sustainable access to these therapies.
Introduction
Severe asthma is defined in the European Respiratory Society and American Thoracic Society (ERS/ATS) guidelines as asthma which requires treatment with high-dosage inhaled corticosteroids (ICS) plus a second controller (and/or systemic corticosteroids) to prevent it from becoming uncontrolled, or asthma which remains uncontrolled despite this therapy () (Citation1). The distinction between uncontrolled asthma and severe asthma is important; not all uncontrolled asthma is severe asthma and vice versa.
Table 1. ERS/ATS definition of severe asthma for patients aged ≥6 years.
Patients with severe asthma are estimated to comprise approximately 10% of the total asthma population (Citation2,Citation3), with approximately 40% of severe asthma remaining uncontrolled (Citation2,Citation4). While severe asthma is present in a minority of asthma patients, its contribution to asthma morbidity and economic burden is considerable, especially if it remains uncontrolled (Citation5–7). Uncontrolled asthma leads to more days off work (Citation8), a limitation in daily activities, a decreased health-related quality of life and more frequent emergency department visits, and hospitalizations compared to controlled asthma (Citation9). In addition, patients with uncontrolled asthma require more medication, including rescue inhaler use and oral corticosteroids (OCS) (Citation9).
ICS and ICS-long-acting beta2 agonist (LABA) combination remain the mainstay therapies for asthma. If these therapies are not sufficient to achieve asthma control, confounding factors, such as poor treatment adherence, poor inhaler technique, comorbidities, and exposure to modifiable risk factors, should first be ruled out before increasing therapy dosage or resorting to add-on treatments (Citation10). If these confounding factors are not adequately addressed, the asthma is referred to as difficult-to-control asthma. Only when patients require high-dosage ICS-LABA therapy despite addressing all confounding factors, the asthma is referred to as (refractory) severe asthma (Citation11). Based on this definition, a study in the Netherlands indicated that only 20.5% of patients with difficult-to-control asthma met the definition of refractory severe asthma, corresponding to 3.6% of the Dutch asthma population (Citation12). When including patients who require treatment with a high-dosage ICS-LABA to prevent their asthma from becoming uncontrolled after ICS-LABA tapering, this becomes 4.5% (Citation12).
For patients with uncontrolled severe asthma, short-term or maintenance OCS add-on therapy is still widely used. It has been estimated that for 30% of adult patients with severe asthma, OCS therapy is used in addition to ICS to maintain an acceptable level of asthma control (Citation1). In a patient sample from a national United Kingdom registry, 42% of patients with refractory severe asthma were prescribed maintenance OCS at baseline and 57% at a median follow-up of 3.1 years (Citation13). In the Netherlands, approximately 20% of the asthma patients are prescribed OCS escalation therapy one or more times a year on top of ICS therapy (Citation14), and in Belgium, 24% of severe asthmatics were treated with OCS on a daily basis (Citation15).
Long-term use of OCS is associated with many comorbidities, as detailed later in this review. While, historically, there were no real alternatives, various OCS-sparing therapies now allow reducing or stopping maintenance OCS therapy. In particular, some immunosuppressants and add-on biologic therapies are effective in reducing OCS exposure (Citation16).
In this article, we review the burden of OCS in severe asthma, and we provide an overview of the OCS-sparing effect of various biologic therapies that are currently available. Finally, ensuring that OCS exposure is minimized and that the right patient receives the right treatment requires a timely confirmation of the diagnosis of uncontrolled severe asthma and a thorough patient characterization, which both depend on optimal referral pathways. In the discussion, we provide an expert opinion on this later topic.
Burden of oral corticosteroids in severe asthma
Frequent or regular exposure to OCS is a common cause of adverse events in various groups of patients, with some of the most frequently reported morbidities being osteoporosis, dyspeptic disorders, sleep disturbance, hypertension, diabetes, bone fractures, and cataract (Citation17‒Citation19). In addition, withdrawal from maintenance OCS therapy following long-term use may result in prolonged adrenal insufficiency that requires appropriate substitution and preventive measures (Citation20,Citation21). For patients with severe asthma, the burden of OCS use is high, and OCS-related adverse events affect the majority. In a British cross-sectional study, 93% of patients with severe asthma were found to have at least one condition linked to systemic corticosteroid exposure (Citation22). The most important types of OCS-related morbidity are illustrated in .
Figure 1. Burden of OCS in severe asthma.
OR, odds ratio; OCS, oral corticosteroids; GI, gastrointestinal.
Severe vs mild/moderate asthma data: Sweeney et al. (Citation22); data for ≥4 OCS prescriptions: Sullivan et al. (Citation23).
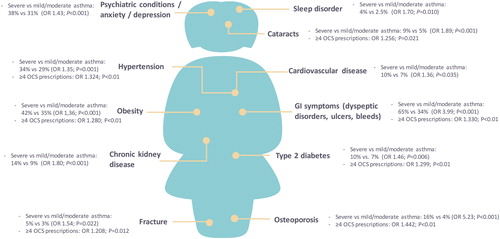
It is difficult to distinguish the corticosteroid burden that is due to ICS therapy (especially with high dosages), acute OCS use, and/or maintenance OCS in patients with severe asthma. Also, the burden of topical corticosteroids and OCS bursts for concomitant sinonasal disease may add to the overall burden. Moreover, randomized controlled trials (RCTs) that generally run over a limited period are not designed to study long-term adverse effects of OCS use. Findings from a retrospective cohort study in the United States suggest that each prescription for an OCS results in a cumulative burden on current and future health, regardless of dosage and duration. The incidence of adverse events appears to increase with each year of exposure, particularly for patients with four or more prescriptions of OCS per year (even in case of short-term bursts of OCS use), and results in a greater risk of an adverse effect during the current year (odds ratio [OR] range: 1.21–1.44 depending on the adverse effect) (Citation23). These data strongly argue that even repeated short courses of OCS might considerably impact patients’ health-related quality of life. A recent systematic review of systemic corticosteroid use for asthma management further establishes that OCS use is prevalent in asthma management and that risks of acute and chronic complications increase with cumulative OCS dosage (Citation24).
Methods
Data sources: PubMed. Methodology: An initial search was performed in Aug 2018, with a final update in Oct 2019, with the following search string: ((((“oral corticosteroid”[Title/Abstract] OR “systemic corticosteroid”[Title/Abstract]) OR “oral glucocorticoid”[Title/Abstract]) OR “systemic glucocorticoid”[Title/Abstract]) AND (“antibody name”[MeSH Terms] OR “antibody name”[All Fields])) AND (“2008/01/01”[PDAT]: “3000”[PDAT]). Only original studies were selected (i.e., comments, reviews, and meta-analysis, … were excluded). Only studies with results on the OCS-sparing effect of the biologics were selected. Studies in children were excluded. Only articles in English were included. Quality assessment (including use and details of appropriate methods, baseline comparability of groups, reporting of relevant outcomes, …) was performed by the authors and disagreements were resolved through consensus. Out of 109 identified articles, 29 original studies met the criteria for inclusion. This article is written as a narrative review.
Results
OCS-sparing effect of biologic therapies
Several biologic therapies for the treatment of uncontrolled severe asthma are currently available. Omalizumab targets immunoglobulin E (IgE) and benefits patients with allergic asthma. Other biologics target interleukin (IL)-5 (mepolizumab, reslizumab), IL-5 receptor alpha (IL-5Rα) (benralizumab), and IL-4 receptor alpha (IL-4Rα) (dupilumab). The OCS-sparing effect of these therapies, except for reslizumab, has been shown in RCTs (). This is not surprising considering that OCS are acting mainly on type 2 inflammation, and the current biologic therapies target specific mediators of this pathway (i.e., IgE, IL-5, IL-4, IL-13).
Table 2. Results from OCS-sparing studies.
The OCS-sparing effect of omalizumab has been evaluated in two RCTs of which the results were heterogenous. In the double-blind, placebo-controlled 011 trial, there was no treatment benefit with omalizumab (Citation52), possibly due to a poorly optimized OCS dosage at baseline. In the randomized, open-label EXALT study, patients on omalizumab reduced or stopped OCS around twice as often as those on optimized asthma therapy alone (Citation25). In the double-blind, placebo-controlled SIRIUS trial, mepolizumab resulted in a median OCS dosage reduction of 50% versus placebo (OR: 2.39), 6% more patients able to discontinue OCS versus placebo (OR: 1.67), and 36% of patients not able to reduce OCS dosage (Citation47). Benralizumab every 8 weeks resulted in a median OCS dosage reduction of 50% versus placebo (OR: 4.12), 33% more eligible patients able to discontinue OCS versus placebo (OR: 4.19), and 21% of patients not able to reduce OCS dosage in the double-blind, placebo-controlled ZONDA trial (Citation50). The OCS-sparing effect of both mepolizumab and benralizumab was maintained in extension trials (Citation53,Citation54). Finally, dupilumab resulted in a median OCS dosage reduction of 50% versus placebo, 23% more patients able to discontinue OCS versus placebo (OR: 2.74), and 14% of patients not able to reduce OCS dosage in the double-blind, placebo-controlled VENTURE trial (Citation51).
Real-world observational studies confirm the OCS-sparing effects of biologics in severe asthma. This is the case for omalizumab, which has been available the longest, but now also for mepolizumab and benralizumab (). As the outcomes of both RCTs and observational studies can be greatly impacted by the study design (e.g., baseline characteristics of included patients, OCS dosage at baseline), direct comparisons between biologics cannot be made.
Discussion
Optimization of the patient journey before initiation of a biologic – expert opinion
Currently, severe asthma patients often do not routinely receive the optimal care in a timely manner, leading to possibly preventable OCS use and its associated risks. To address this, an important challenge lies in the optimization of referral pathways, as patients are sometimes confined in primary care or are consulting several physicians before a clear care pathway could be proposed.
First, it is important to create awareness at the general practitioner (GP) level around timely referral of patients who could have severe asthma. A niche consists of at-risk patients who frequently take courses of OCS or use OCS as a regular therapy could potentially benefit from treatment with a biologic. Initiatives around referral are best developed at a local level, since clinical practice and organization of health care can vary between different countries and regions. In Belgium, the authors of this review agreed on a referral signal to identify patients who should be assessed for referral to a pulmonologist to confirm the diagnosis and optimize therapy, including possible initiation of a biologic therapy. Patients should be considered for referral when they meet the following criteria: the use of medium- to high-dosage ICS-LABA with at least one OCS prescription for a respiratory indication. Referral to a list of ICS dose equivalences may be useful (). Additionally, while repeated antibiotic use is not in itself indicative of severe asthma, the use of medium- to high-dosage ICS-LABA with two or more antibiotics prescriptions for a respiratory indication should also be considered as a potential referral signal. Antibiotics tend to be overused in the acute setting by GPs as add-on to OCS or to avoid OCS use (Citation56). These criteria may be adapted based on local experience to accommodate differences in practice. Alternatively, a more general referral signal to a pulmonologist could be used, such as the use of OCS for more than two weeks cumulative per year for a respiratory indication.
Table 3. Low, medium, and high daily doses of inhaled corticosteroids (mcg).
To optimize referral and interplay between GPs and pulmonologists specialized in the management of severe asthma, better collaboration and communication could be put in place. For example, communication should be standardized in a way that meets the GP’s requirements and biologics could be prescribed by the pulmonologist and injected by the GP.
Pharmacists can also play a role in identifying those patients that should be seen by a pulmonologist. This could be the case for asthma patients who repeatedly receive OCS prescriptions for a respiratory indication or have excessive use of short-acting beta agonists. In Belgium, pharmacists are recommended to have counseling interviews with asthma patients: one information interview and one follow-up discussion (“Begeleidingsgesprek Goed Gebruik Geneesmiddelen” https://upb-avb.be/nl/dossiers/begeleidingsgesprekken-nieuwe-medicatie-bnm/). Another possibility could be the integration of pharmacy data with hospital patient files, as is currently already the case in several countries. In countries where prescription data are centralized, a feedback system allowing GPs to be aware of the total amount of OCS delivered to their patients could be useful and at least prompt discussions about current therapy.
Repeated measurements of blood eosinophils, as well as total and specific serum IgE, should be standard along the patient journey because of their importance for asthma characterization and choosing between biologic therapies. When possible, blood samples should be taken before the administration of systemic corticosteroids because these drastically and rapidly decrease circulating eosinophils. When an increased count of circulating eosinophils is observed in an uncontrolled asthma patient by the GP or at the emergency department, this should be flagged because a count greater than 300 eosinophils per µL blood strongly supports a diagnosis of eosinophilic asthma (Citation57,Citation58). Of note, 300 eosinophils per µL blood lies within the “normal” range (in a healthy population, 90% has an eosinophil level between 0.5 and 400 per µL blood (Citation59)). In addition, investigations are underway to establish new biomarkers or sets of biomarkers that could be more reliable (Citation60).
In several countries including Belgium, the reimbursement criteria for anti-IL-5 and anti-IL-5Rα therapies currently include ≥300/µL blood eosinophils during the last year and at initiation. This might be problematic for patients on maintenance OCS, or for patients with repeated serious asthma attacks who are given systemic corticosteroids, as both settings lead to depletion of blood eosinophils. Consequently, some pulmonologists ask for an exception for reimbursement. Others will try to temporarily lower the patient’s OCS dosage allowing their eosinophil levels to recover, although this entails a risk of worsening asthma or exacerbations. Finally, some pulmonologists may not even initiate a biologic at all. Hence, there is an argument to be made to lower the eosinophil threshold for reimbursement for patients on maintenance OCS until a better biomarker becomes available.
When a patient is hospitalized or admitted to the emergency department for an asthma attack, a pulmonologist should be consulted, and a diagnosis of severe asthma should be considered. In the absence of clinical evidence for the efficacy of targeted biologic therapy in the acute setting, systemic administration of corticosteroids remains the standard of care. Of note, early-phase studies exploring the use of biologics in the acute setting have been performed and warrant further investigation (Citation61).
Patients with uncontrolled severe asthma are often eligible for multiple biologic therapies. In the IDEAL study, about one-third of patients eligible for mepolizumab were also eligible for omalizumab. Of those patients eligible for omalizumab, eligibility for mepolizumab varied considerably depending on the eligibility criteria used, ranging from 35% to 73% (Citation62). Responses to omalizumab and mepolizumab in combined allergic and eosinophilic severe asthma will be compared in the PREDICTUMAB study (Citation63). The importance of selecting the right biologic therapy for the right patient is further exemplified by recent cost-effectiveness analyses, advocating adjusting pricing structures and directing biologic therapy to responders (Citation64,Citation65). An updated algorithmic approach to identifying patients who can be considered candidates for biologics has recently been published by the Global Initiative for Asthma. When choosing between biologic therapies, local reimbursement criteria, predictors of asthma response, cost, dosing frequency, delivery route and patient preference should be considered (https://ginasthma.org/severeasthma/)
Conclusion
OCS have long been the only option for uncontrolled severe asthma patients, especially for patients non-allergic severe asthma. However, OCS use has a great patient and societal burden, especially in case of long-term use. Therefore, OCS should no longer be considered as a first-line add-on treatment in the long term, and repeated intermittent OCS use should be avoided since novel biologics offer a safer alternative that targets the same biological processes. OCS should be tapered to a minimal dosage at which asthma control is maintained. To ensure that OCS exposure is minimized and that the right patient receives the right sustainable therapy, it is critical to optimize the patient’s journey, to determine the asthma endotype of patients via existing biomarkers (serum IgE, blood and/or sputum eosinophils, exhaled NO) and to develop new biomarkers and predictors of (non)response to biologic therapies. Finally, it is of utmost importance to correctly diagnose severe asthma before resorting to any add-on therapy. Patients whose asthma remains difficult to control with ICS should first be assessed for therapy adherence, correct use of inhaler devices, comorbidities, and exposure to modifiable risk factors.
Trademarks
Xolair is a registered trademark of NOVARTIS AG. Nucala is a registered trademark of the GSK group of companies. Cinqaero/Cinqair is a registered trademark of Teva Pharmaceutical Industries Ltd. Fasenra is a registered trademark of AstraZeneca. Dupixent is a registered trademark of Sanofi Biotechnology.
Acknowledgments
The authors wish to thank Joke Vandewalle (Modis) for writing assistance and coordination of manuscript development, and Roy Heusschen (AstraZeneca) for writing assistance. This assistance was funded by AstraZeneca.
Declaration of interest
Dr. Louis reports grants and personal fees from GSK, grants and personal fees from AZ, grants and personal fees from Novartis, grants from Chiesi, personal fees from Sanofi, outside the submitted work. Dr. Michils has nothing to disclose. Dr. Ninane reports personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from Astra Zeneca, personal fees from BMS, personal fees from Novartis, outside the submitted work. Dr. Peche reports personal fees from ASTRA ZENECA, during the conduct of the study. Dr. Pilette reports grants from AstraZeneca, grants from TEVA, personal fees from Novartis, personal fees from GSK group of companies, personal fees from Chiesi, personal fees from ALK-Abello, outside the submitted work. Dr. Florence has nothing to disclose. Dr. Hanon reports personal fees from AstraZeneca, during the conduct of the study; personal fees and other from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Novartis, outside the submitted work. Dr. Cataldo reports personal fees from AstraZeneca, during the conduct of the study; personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Chiesi, personal fees from Sanofi, personal fees from GSK Group of Companies, other from Aquilon Pharmaceuticals, outside the submitted work.
Additional information
Funding
References
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Stone B, Davis JR, Trudo F, Schiffman B, Alzola C, Brown D, Fox KM. Characterizing patients with asthma who received Global Initiative for Asthma steps 4-5 therapy and managed in a specialty care setting. Allergy Asthma Proc 2018;39(1):27–35. doi:10.2500/aap.2018.39.4094.
- Lang DM. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc 2015;36(6):418–424. doi:10.2500/aap.2015.36.3908.
- Fernandes AG, Souza-Machado C, Coelho RC, Franco PA, Esquivel RM, Souza-Machado A, Cruz AA. Risk factors for death in patients with severe asthma. J Bras Pneumol 2014;40(4):364–372. doi:10.1590/S1806-37132014000400003.
- World Allergy Organization (WAO). Economic Analysis of the Cost of Treatments for Severe Asthma. Available from: http://www.worldallergy.org/educational_programs/world_allergy_forum/anaheim2005/blaiss.php [last accessed 15 March 2019].
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC Pulm Med 2009;9(1):24. doi:10.1186/1471-2466-9-24.
- Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002;19(1):61–67. doi:10.1183/09031936.02.00232001.
- Dean BB, Calimlim BM, Kindermann SL, Khandker RK, Tinkelman D. The impact of uncontrolled asthma on absenteeism and health-related quality of life. J Asthma 2009;46(9):861–866. doi:10.3109/02770900903184237.
- Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med 2014;24(14009).
- Blaiss MS, Castro M, Chipps BE, Zitt M, Panettieri RA, Jr., Foggs MB. Guiding principles for use of newer biologics and bronchial thermoplasty for patients with severe asthma. Ann Allergy Asthma Immunol 2017;119(6):533–540. doi:10.1016/j.anai.2017.09.058.
- Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, Wagener AH, Wagers SS, Sterk PJ, Compton CH, on behalf of the members of the Unbiased Biomarkers for the Prediction of Respiratory Disease Outcome (U-BIOPRED) Consortium, Consensus Generation. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI). Thorax 2011;66(10):910–917. doi:10.1136/thx.2010.153643.
- Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042.
- Sweeney J, Brightling CE, Menzies-Gow A, Niven R, Patterson CC, Heaney LG, on behalf of the British Thoracic Society Difficult Asthma Network. Clinical management and outcome of refractory asthma in the UK from the British Thoracic Society Difficult Asthma Registry. Thorax 2012;67(8):754–756. doi:10.1136/thoraxjnl-2012-201869.
- Ferns M. Real world OCS use in asthma in The Netherlands. In: Week van de Longen; 2018. Available from: https://www.weekvandelongen.nl/en/home
- Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, Peche R, Manise M, Joos G. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med 2014;108(12):1723–1732. doi:10.1016/j.rmed.2014.10.007.
- Nguyen VQ, Ulrik CS. Measures to reduce maintenance therapy with oral corticosteroid in adults with severe asthma. Allergy Asthma Proc 2016;37(6):125–139. doi:10.2500/aap.2016.37.4004.
- Fardet L, Kassar A, Cabane J, Flahault A. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf 2007;30(10):861–881. doi:10.2165/00002018-200730100-00005.
- Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther 2011;33(10):1413–1432. doi:10.1016/j.clinthera.2011.09.009.
- Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103(7):975–994. doi:10.1016/j.rmed.2009.01.003.
- Broersen LH, Pereira AM, Jorgensen JO, Dekkers OM. Adrenal Insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab 2015;100(6):2171–2180. doi:10.1210/jc.2015-1218.
- Al Nofal A, Bancos I, Benkhadra K, Ospina NM, Javed A, Kapoor E, Muthusamy K, Brito JP, Turcu AF, Wang Z, et al. Glucocorticoid replacement regimens in chronic adrenal insufficiency: a systematic review and meta-analysis. Endocr Pract 2017;23(1):17–31. doi:10.4158/EP161428.OR.
- Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, Chaudhuri R, Price D, Brightling CE, Heaney LG. British Thoracic Society Difficult Asthma Network. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016;71(4):339–346. doi:10.1136/thoraxjnl-2015-207630.
- Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol 2018;141(1):110–116.e117. doi:10.1016/j.jaci.2017.04.009.
- Bleecker ER, Menzies-Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, Alacqua M, Tran TN. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. [Epub ahead of print]. doi:10.1164/rccm.201904-0903SO.
- Siergiejko Z, Świebocka E, Smith N, Peckitt C, Leo J, Peachey G, Maykut R. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr Med Res Opin 2011;27(11):2223–2228. doi:10.1185/03007995.2011.620950.
- Molimard M, de Blay F, Didier A, Le Gros V. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med 2008;102(1):71–76. doi:10.1016/j.rmed.2007.08.006.
- Molimard M, Buhl R, Niven R, Le Gros V, Thielen A, Thirlwell J, Maykut R, Peachey G. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med 2010;104(9):1381–1385. doi:10.1016/j.rmed.2010.06.001.
- Pelaia G, Gallelli L, Romeo P, Renda T, Busceti MT, Proietto A, Grembiale RD, Marsico SA, Maselli R, Vatrella A. Omalizumab decreases exacerbation frequency, oral intake of corticosteroids and peripheral blood eosinophils in atopic patients with uncontrolled asthma. CP 2011;49(12):713–721. doi:10.5414/CP201586.
- Domingo C, Moreno A, Jose Amengual M, Monton C, Suarez D, Pomares X. Omalizumab in the management of oral corticosteroid-dependent IGE-mediated asthma patients. Curr Med Res Opin 2011;27(1):45–53. doi:10.1185/03007995.2010.536208.
- Costello RW, Long DA, Gaine S, Mc Donnell T, Gilmartin JJ, Lane SJ. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci 2011;180(3):637–641. doi:10.1007/s11845-011-0716-2.
- Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma 2012;49(1):78–82. doi:10.3109/02770903.2011.637598.
- Lafeuille MH, Dean J, Zhang J, Duh MS, Gorsh B, Lefebvre P. Impact of omalizumab on emergency-department visits, hospitalizations, and corticosteroid use among patients with uncontrolled asthma. Ann Allergy Asthma Immunol 2012;109(1):59–64. doi:10.1016/j.anai.2012.04.015.
- Subramaniam A, Al-Alawi M, Hamad S, O'Callaghan J, Lane SJ. A study into efficacy of omalizumab therapy in patients with severe persistent allergic asthma at a tertiary referral centre for asthma in Ireland. QJM 2013;106(7):631–634. doi:10.1093/qjmed/hct072.
- Braunstahl GJ, Chlumsky J, Peachey G, Chen CW. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol 2013;9(1):47. doi:10.1186/1710-1492-9-47.
- Barnes N, Menzies-Gow A, Mansur AH, Spencer D, Percival F, Radwan A, Niven R. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma 2013;50(5):529–536. doi:10.3109/02770903.2013.790419.
- Gouder C, West LM, Montefort S. The real-life clinical effects of 52 weeks of omalizumab therapy for severe persistent allergic asthma. Int J Clin Pharm 2015;37(1):36–43. doi:10.1007/s11096-014-0034-7.
- Sousa AS, Pereira AM, Fonseca JA, Azevedo LF, Abreu C, Arrobas A, Calvo T, Silvestre MJ, Cunha L, Falcao H, Drummond M, et al. Severe Asthma Specialist N. Asthma control and exacerbations in patients with severe asthma treated with omalizumab in Portugal. Rev Port Pneumol 2006;2015. doi:10.1016/j.rppnen.2015.03.002.
- Gibson PG, Reddel H, McDonald VM, Marks G, Jenkins C, Gillman A, Upham J, Sutherland M, Rimmer J, Thien F, et al. Effectiveness and response predictors of omalizumab in a severe allergic asthma population with a high prevalence of comorbidities: the Australian Xolair Registry. Intern Med J 2016;46(9):1054–1062. doi:10.1111/imj.13166.
- Niven RM, Saralaya D, Chaudhuri R, Masoli M, Clifton I, Mansur AH, Hacking V, McLain-Smith S, Menzies-Gow A. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open 2016;6(8):e011857. doi:10.1136/bmjopen-2016-011857.
- Bhutani M, Yang WH, Hebert J, de Takacsy F, Stril JL. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX Observational study. PLoS One 2017;12(8):e0183869. doi:10.1371/journal.pone.0183869.
- Celebi Sozener Z, Aydin O, Misirligil Z, Mungan D, Demirel YS, Celik GE, Sin BA, Bavbek S. Omalizumab in non-allergic asthma: a report of 13 cases. J Asthma 2018; 55:756–763. doi:10.1080/02770903.2017.1362427.
- Lee JH, Lee HY, Jung CG, Ban GY, Shin YS, Ye YM, Nahm DH, Park HS. Therapeutic effect of omalizumab in severe asthma: a real-world study in Korea. Allergy Asthma Immunol Res 2018;10(2):121–130. doi:10.4168/aair.2018.10.2.121.
- Pilon D, Kavati A, Ortiz B, Paknis B, Vegesna A, Schiffman B, Zhdanava M, Lefebvre P, Stone B. Asthma control, lung function, symptoms, and corticosteroid sparing after omalizumab initiation in patients with allergic asthma. Allergy Asthma Proc 2018;39(2):127–135. doi:10.2500/aap.2018.39.4111.
- Tarraf HN, Masoud HH, Zidan M, Wahba B. Effectiveness and safety of omalizumab in severe, persistent IgE-mediated asthma in pediatric and adult patients: a real-world observational study in Egyptian population. J Asthma 2018;1–7. doi:10.1080/02770903.2018.1553051.
- Hutyrová B, Bystroň J, Collaborators CA-IR. Czech Anti-IgE Registry collaborators. The effect of omalizumab treatment on severe allergic asthma and allergic comorbidities: real-life experience from the Czech Anti-IgE Registry. PDIA 2018;35(5):510–515. doi:10.5114/ada.2018.77243.
- Pelaia C, Calabrese C, Barbuto S, Busceti MT, Preianò M, Gallelli L, Savino R, Vatrella A, Pelaia G. Omalizumab lowers asthma exacerbations, oral corticosteroid intake and blood eosinophils: results of a 5-YEAR single-centre observational study. Pulm Pharmacol Ther 2019;54:25–30. doi:10.1016/j.pupt.2018.11.002.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID, Investigators S. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Kurosawa M, Sutoh E. Prospective open-label study of 48-week subcutaneous administration of mepolizumab in Japanese patients with severe eosinophilic asthma. J Investig Allergol Clin Immunol 2019;29(1):40–45. doi:10.18176/jiaci.0285.
- Kurosawa M, Ogawa K, Dorwal P. Favorable clinical efficacy of mepolizumab on the upper and lower airways in severe eosinophilic asthma: a 48-week pilot study. Eur Ann Allergy Clin Immunol 2019;51(05):213–221. doi:10.23822/EurAnnACI.1764-1489.94.
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501.
- Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093.
- FDA (2/2007, updated 7/2007). Alert: information for healthcare professionals: Omalizumab (marketed as Xolair). Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126456.htm [last accessed 15 October 2009]
- Fitzgerald JM, Bleecker ER, Bourdin A, Busse WW, Ferguson GT, Brooks L, Barker P, Martin U. Two-year integrated efficacy and safety analysis of benralizumab SIROCCO, CALIMA, ZONDA, and BORA trials in severe asthma. Presented at the American Thoracic Society (ATS) International Conference; 2019 May 17–22; Dallas, Texas. doi:10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2676.
- Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, Kato M, Albers FC, Bradford ES, Gilson MJ, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther 2019; 41(10):2041–2056.e5. doi:10.1016/j.clinthera.2019.07.007.
- GINA. 2018. Pocket Guide for Asthma Management and Prevention. Available from: https://ginasthma.org/2018-pocket-guide-for-asthma-management-and-prevention/ [last accessed 6 August 2018].
- Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med 2016;176(11):1637–1638. doi:10.1001/jamainternmed.2016.6046.
- Coumou H, Bel EH. Improving the diagnosis of eosinophilic asthma. Expert Rev Respir Med 2016;10(10):1093–1103. doi:10.1080/17476348.2017.1236688.
- Demarche SF, Schleich FN, Paulus VA, Henket MA, Van Hees TJ, Louis RE. Is it possible to claim or refute sputum eosinophils >/= 3% in asthmatics with sufficient accuracy using biomarkers? Respir Res 2017;18(1):133. doi:10.1186/s12931-017-0615-9.
- Colak Y, Afzal S, Nordestgaard BG, Marott JL, Lange P. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen General Population Study. Eur Respir J 2018;52:1800616. doi:10.1183/13993003.00616-2018.
- Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015;46(5):1308–1321. doi:10.1183/13993003.00779-2015.
- Kirkland SW, Cross E, Campbell S, Villa-Roel C, Rowe BH. Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma. Cochrane Database Syst Rev 2018;6:CD012629. doi:10.1002/14651858.CD012629.pub2.
- Albers FC, Mullerova H, Gunsoy NB, Shin JY, Nelsen LM, Bradford ES, Cockle SM, Suruki RY. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55(2):152–160. doi:10.1080/02770903.2017.1322611.
- Study of Magnitude and Prediction of Response to Omalizumab and Mepolizumab in Adult Severe Asthma. (PREDICTUMAB). Available from: https://clinicaltrials.gov/ct2/show/NCT03476109 [last accessed 15 March 2019].
- Anderson WC, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic?. Ann Allergy Asthma Immunol 2019;122(4):367–372. doi:10.1016/j.anai.2019.01.018.
- Tice JA, Campbell JD, Synnott PG, Walsh JME, Kumar VM, Whittington M, Adair E, Rind D, Pearson SD. The effectiveness and value of biologic therapies for the treatment of uncontrolled asthma. JMCP 2019;25(5):510–514. doi:10.18553/jmcp.2019.25.5.510.
