Abstract
Objective
To investigate the current prevalence of physician-diagnosed obstructive airway diseases by respiratory symptoms and by sex in Sweden and Finland.
Method
In 2016, a postal questionnaire was answered by 34,072 randomly selected adults in four study areas: Västra Götaland and Norrbotten in Sweden, and Seinäjoki-Vaasa and Helsinki in Finland.
Results
The prevalence of asthma symptoms was higher in Norrbotten (13.2%), Seinäjoki-Vaasa (14.8%) and Helsinki (14.4%) than in Västra Götaland (10.7%), and physician-diagnosed asthma was highest in Norrbotten (13.0%) and least in Västra Götaland (10.1%). Chronic productive cough was most common in the Finnish areas (7.7–8.2% versus 6.3–6.7%) while the prevalence of physician-diagnosed chronic bronchitis (CB) or chronic obstructive pulmonary disease (COPD) varied between 1.7 and 2.7% in the four areas. Among individuals with respiratory symptoms, the prevalence of asthma was most common in Norrbotten, while a diagnosis of COPD or CB was most common in Västra Götaland and Seinäjoki-Vaasa. More women than men with respiratory symptoms reported a diagnosis of asthma in Sweden and Seinäjoki-Vaasa but there were no sex differences in Helsinki. In Sweden, more women than men with symptoms of cough or phlegm reported a diagnosis of CB or COPD, while in Finland the opposite was found.
Conclusion
The prevalence of respiratory symptoms and corresponding diagnoses varied between and within the countries. The proportion reporting a diagnosis of obstructive airway disease among individuals with respiratory symptoms varied, indicating differences in diagnostic patterns both between areas and by sex.
Introduction
Increased awareness of asthma and possible differences in diagnostic practices between different countries contribute to the difficulties in making accurate epidemiological estimates of prevalence (Citation1). It is unclear whether the increase in asthma prevalence observed during the last decades is due to a real increase or an increased awareness of respiratory symptoms and obstructive airway diseases, both in society and among healthcare providers (Citation2,Citation3). Whilst there appears to be no global decline in the prevalence of asthma (Citation4), there are large geographical variations (Citation5,Citation6). The global prevalence of asthma has been estimated to be around 4% (Citation5), while in Northern Europe, the prevalence of asthma is approximately 10% (Citation7–10). In Sweden, both an increase and a leveling of prevalence of physician-diagnosed asthma have been reported during the last decades (Citation7,Citation8,Citation10). In Helsinki in Finland, the prevalence of physician-diagnosed asthma increased until the 2000s (Citation9) but leveled during the last decade (Citation11). This increase in prevalence in Finland may be associated with an increased awareness of asthma in primary health care attributed to the implementation of a specific national asthma program (Citation12).
Distinguishing between asthma and chronic obstructive pulmonary disease (COPD) is difficult partly due to the development of irreversible bronchial obstruction in persons with asthma, thereby fulfilling the criteria of COPD. Both asthma and COPD can also occur in the same person, which may complicate the diagnostic procedure (Citation13,Citation14). Further, there has been a misdiagnosis of obstructive airway diagnoses due to lack of knowledge and lack in use of spirometry (Citation15–17). Several studies including post-bronchodilator spirometry have shown that the under-diagnosis of COPD is extensive, around 50–80% (Citation17–20). In addition, misclassification is common, and a recent study reported that more than 50% had an incorrect COPD diagnosis, as they were non-obstructed after post-bronchodilator spirometry (Citation21). However, there are studies showing improvements in the diagnostic procedures: both in Norway and Finland there has been an improved diagnostic accuracy of COPD in primary health care, which may be ascribed to improved adherence to guidelines (Citation22) and the Finnish national COPD program (Citation23). The global prevalence of COPD is estimated to 10.7% (Citation24) and in Sweden and Finland, the prevalence of COPD is estimated to 5.9–8.5% when based on spirometry in general population samples (Citation25,Citation26).
Although respiratory symptoms are common in the general population (Citation27–30), some studies suggest a decreasing or leveling trend of respiratory symptoms, chronic bronchitis (CB) and obstructive lung diseases during the last decades (Citation7,Citation8,Citation10,Citation11,Citation25,Citation31,Citation32). This decrease is partly a result of a decrease in tobacco smoking (Citation7,Citation8,Citation31). Most studies have reported higher prevalence of asthma among women (Citation7,Citation9,Citation10), and higher prevalence of COPD among men (Citation33,Citation34). However, the sex differences in COPD prevalence tend to diminish (Citation14,Citation25,Citation33) as a consequence of equalization in smoking habits in men and women (Citation7,Citation35).
A large population-based multicenter study performed in the mid-1990s suggested that different diagnostic practices regarding obstructive airway diseases existed between Finland, Estonia and Sweden (Citation36). Thus, the aim was to investigate the current prevalence of physician-diagnosed obstructive airway diseases by respiratory symptoms and by sex in Sweden and Finland
Methods
This study presents results from the Nordic EpiLung project, which is a collaboration between large epidemiological research programs in Sweden, Finland and Norway. The Nordic EpiLung project stems from previously established research collaboration between Finland, Estonia and Sweden – the FinEsS-study (Citation26,Citation27,Citation36–39). The current study consists of random samples of adults in ages between 20 and 69 years drawn from the National Population registries in Sweden and Finland. In 2016, postal questionnaires were sent to the selected samples in two Swedish areas, Norrbotten and Västra Götaland and in two Finnish areas, Seinäjoki-Vaasa and Helsinki.
Participants in the questionnaire surveys
In Sweden, the postal questionnaire study was conducted within the Obstructive Lung Disease in Northern Sweden (OLIN) in Norrbotten, the northernmost county in Sweden, and within the West Sweden Asthma Study (WSAS) in Västra Götaland (Citation10), a county in southwest of Sweden. The response rate in Norrbotten was 53% (n = 5,466) (Citation7) and in Västra Götaland 50% (n = 20 435).
In Finland, the study was performed in Helsinki, the capital of Finland located in the southern part of the country, where 50.3% (n = 3,998) responded (Citation11) and in Seinäjoki-Vaasa, an area in western Finland, where 52.5% (n = 4,173) responded (Citation40).
Thus, in total, 34 072 randomly selected individuals in ages between 20 and 69 years from four study areas constitute the study population.
Questionnaires
The postal questionnaires included identical questions in all four areas. The questionnaires were based on the OLIN questionnaire (Citation41) and were provided in Swedish and Finnish. In Helsinki, an English version of the questionnaire was additionally provided. The questionnaire, which has been used in several cross-sectional studies (Citation7,Citation9,Citation10,Citation27,Citation36–39), includes questions about respiratory symptoms, physician diagnoses of asthma, CB or COPD, and potential risk factors such as smoking habits.
Definitions
The following definitions were used:
Physician-diagnosed asthma: Have you been diagnosed as having asthma by a doctor?
Physician-diagnosed CB or COPD: Have you been diagnosed as having chronic bronchitis (CB), chronic obstructive pulmonary disease (COPD) or emphysema by a doctor?
Asthma symptoms: Have you had asthma symptoms during the last 12 months? (Have you, during the last 12 months, had intermittent attacks or periodic breathlessness, with or without cough or wheezing/whistling in your chest?)?
Recurrent wheeze: Do you usually have wheezing, whistling or a noisy sound in your chest when breathing?
Any wheeze: Have you had wheezing or whistling in your chest at any time during the last 12 months?
Wheezing with breathlessness: Have you been at all breathless when the wheezing or whistling was present during the last 12 months?
Wheezing without a cold: Have you had wheezing or whistling when you did not have a cold during the last 12 months?
Asthmatic wheeze: Any wheeze with breathlessness without having a cold during the last 12 months.
Sputum production: Do you usually have phlegm when coughing, or do you have phlegm in your chest, which is difficult to bring up?
Chronic productive cough: Have you had periods of phlegm when coughing, or phlegm, which is difficult to bring up at least three months during at least two successive years?
Woken up by tightness in chest: Have you woken up with tightness in your chest at any time during the last 12 months?
Dyspnea: Do you have to walk slower than other people of the same age on level ground because of breathlessness (corresponding to mMRC grade 2)? (Citation42)
Analyses
The analyses were conducted using IBM SPSS Statistics version 25 (Citation43) and the Chi-square test calculator (Citation44). Descriptive statistics, i.e. frequencies and percentages, were used to describe the study populations and chi-square test to calculate p values for comparisons between men and women and between study areas. A p value of <0.05 was regarded as statistically significant. Unadjusted and adjusted logistic regressions were used to investigate the relation of different respiratory symptoms to physician-diagnosed asthma and physician-diagnosed CB or COPD, respectively. The analyses were adjusted for age, sex, smoking habits, heredity of asthma, CB or COPD, and expressed as odds ratios and 95% confidence intervals (OR 95% CI). Due to legislation (Citation45), it was impossible to transpose data across national borders to pool data into a common dataset. Therefore, comparisons between countries regarding prevalence estimates were conducted using the Chi-square test calculator (Citation44).
Ethical considerations
The regional ethical review boards in Gothenburg and Umeå approved the studies in Sweden. The studies in Finland were approved by the Coordinating Ethics Committee of Helsinki and Uusimaa Hospital District.
Results
The prevalence of current smoking was similar in the Swedish areas, 12.7% in Norrbotten and 13.0% in Västra Götaland, but higher in the Finnish areas, 20.1% in Seinäjoki-Vaasa and 24.7% in Helsinki. Additional characteristics of the study populations are presented in online supplementary file Table 1.
Table 1. Prevalence (%) of respiratory symptoms and physician diagnoses of asthma and chronic bronchitis (CB) or chronic obstructive pulmonary disease (COPD).
Prevalence of respiratory symptoms and diagnoses
Asthma symptoms were more common in Norrbotten (13.2%), Seinäjoki-Vaasa (14.8%) and Helsinki (14.4%) than in Västra Götaland (10.7%) (p < 0.001). The prevalence of recurrent wheeze was higher in Norrbotten (11.6%) than in the other study areas (p < 0.001). Sputum production was more common in Helsinki (22.5%) and in Seinäjoki-Vaasa (19.2%) than in Norrbotten (16%) and Västra Götaland (14.7%) (p < 0.001). There was no difference in prevalence of any wheeze last 12 months between the four study areas ().
Physician-diagnosed asthma was more common in Norrbotten (13.0%) than in Västra Götaland (10.1%), and in Finland the prevalence was somewhere between the Swedish areas, i.e. 11.2% in Seinäjoki-Vaasa and 10.9% in Helsinki (p < 0.001). Physician-diagnosed CB or COPD was low in all areas but somewhat higher in Västra Götaland (2.7%) and in Seinäjoki-Vaasa (2.6%) than in Helsinki (2.0%) and Norrbotten (1.7%) (p < 0.001), ().
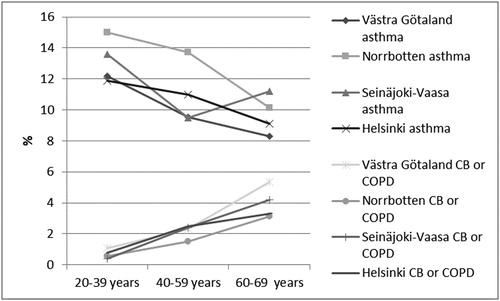
The prevalence of physician-diagnosed asthma decreased by increasing age in three of the study areas, Norrbotten, Västra Götaland, Helsinki, but in Seinäjoki-Vaasa the decrease was followed by an increase among the eldest. The prevalence of physician-diagnosed CB or COPD increased by increasing age in all study areas ().
Figure 1. Prevalence (%) of physician-diagnosed asthma and physician-diagnosed chronic bronchitis (CB) or chronic obstructive pulmonary disease (COPD), respectively, by age groups and study area; in Västra Götaland in southwest Sweden, in Norrbotten in northern Sweden, in Seinäjoki-Vaasa in western Finland and in Helsinki the capital of Finland.
Prevalence of physician-diagnosis of asthma and CB or COPD among individuals with respiratory symptoms
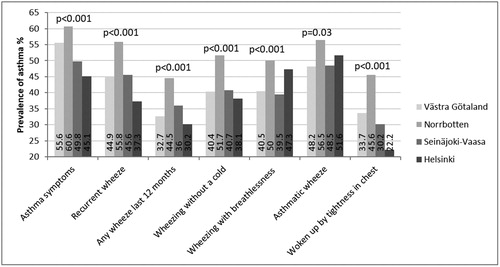
In general, among individuals reporting different respiratory symptoms, the prevalence of physician-diagnosed asthma was highest in Norrbotten and lowest in Helsinki. However, among individuals in Helsinki reporting wheeze with breathlessness and asthmatic wheeze, the prevalence of physician-diagnosed asthma was almost as high as in Norrbotten ().
Figure 2. Prevalence (%) of physician-diagnosed asthma among individuals with different respiratory symptoms in Västra Götaland in southwest Sweden, in Norrbotten in northern Sweden, in Seinäjoki-Vaasa in western Finland and in Helsinki the capital of Finland. Chi-square test was used for comparisons between the study areas.
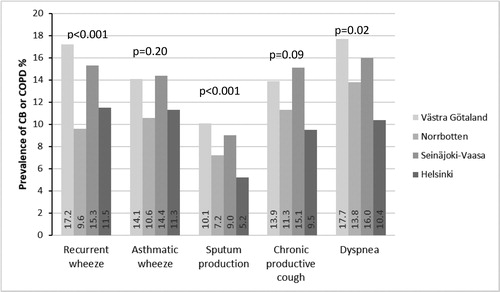
Among individuals reporting recurrent wheeze, sputum production and dyspnea, it was more common to have physician-diagnosed CB or COPD in Västra Götaland and Seinäjoki-Vaasa than in the other study areas ().
Figure 3. Prevalence (%) of physician-diagnosed chronic bronchitis (CB) or chronic obstructive pulmonary disease (COPD) among individuals with different respiratory symptoms in Västra Götaland in southwest Sweden, in Norrbotten in northern Sweden, in Seinäjoki-Vaasa in western Finland and in Helsinki the capital of Finland. Chi-square test was used for comparisons between the study areas.
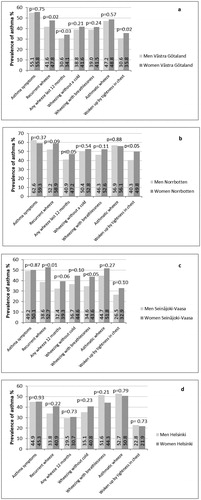
The prevalence of physician-diagnosed asthma in men and women with different respiratory symptoms are presented in . Differences between men and women were found in Västra Götaland, where it was more common to report physician-diagnosed asthma among women with recurrent wheeze (p = 0.02), any wheeze last 12 months (p = 0.03) and having woken up by tightness in the chest (p = 0.02) than among men having these symptoms. In Norrbotten, the prevalence of physician-diagnosed asthma was higher among women reporting any wheeze last 12 months (p < 0.05) and having woken up by tightness in the chest (p < 0.05) than among men. In Seinäjoki-Vaasa, the prevalence of physician-diagnosed asthma was higher among women reporting recurrent wheeze (p = 0.01) and wheezing with breathlessness (p < 0.05) than among men having these symptoms. In Helsinki, no differences between men and women were found.
Figure 4. Comparing prevalence (%) of physician-diagnosed asthma by sex by different respiratory symptoms in a) in Västra Götaland in southwest Sweden, b) in Norrbotten in northern Sweden, c) in Seinäjoki-Vaasa in western Finland and d) in Helsinki the capital of Finland. Chi-square test was used for comparisons between men and women.
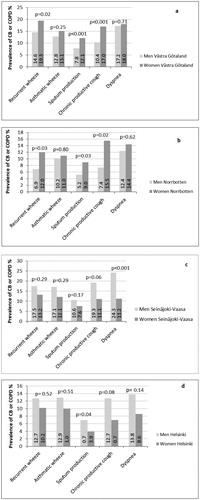
The prevalence of physician-diagnosed CB or COPD in men and women who had different respiratory symptoms are shown in . In both Swedish areas, it was more common to have a diagnosis among women who reported recurrent wheeze (p = 0.02 in Västra Götaland and p = 0.03 in Norrbotten), sputum production (p < 0.001 in Västra Götaland and p = 0.03 in Norrbotten) and chronic productive cough (p < 0.001 in Västra Götaland and p = 0.02 in Norrbotten) than among men who reported these symptoms. In Finland, it was more common to have a diagnosis of CB or COPD among men than among women who reported respiratory symptoms, significantly for dyspnea (p = 0.001) in Seinäjoki-Vaasa, and sputum production (p = 0.04) in Helsinki.
Figure 5. Comparing prevalence (%) of physician-diagnosed chronic bronchitis (CB) or chronic obstructive pulmonary disease (COPD) among men and women with different respiratory symptoms in a) in Västra Götaland in southwest Sweden, b) in Norrbotten in northern Sweden, c) in Seinäjoki-Vaasa in western Finland and d) in Helsinki the capital of Finland. Chi-square test was used for comparisons between men and women.
Associations between respiratory symptoms and physician-diagnoses of asthma and CB or COPD
Among the respiratory symptoms studied, the association between asthma symptoms and physician-diagnosed asthma was the strongest in all areas. The associations between respiratory symptoms and physician-diagnosed asthma tended to be strongest in Norrbotten (). For the associations between the respiratory symptoms and physician-diagnosed CB or COPD, the strongest associations were found for recurrent wheeze and dyspnea, and the associations being particularly strong in Seinäjoki-Vaasa and Norrbotten (). Unadjusted models showed almost identical results as the adjusted (data not shown).
Table 2. AdjustedTable Footnote# logistic regression analyses showing respiratory symptoms (analyzed one by one) in relation to physician-diagnosed asthma.
Table 3. AdjustedTable Footnote# logistic regression analyses showing respiratory symptoms (analyzed one by one) in relation to physician-diagnosed chronic bronchitis or chronic obstructive pulmonary disease.
Discussion
This population-based multicentre study including four areas in Sweden and Finland, using identical methods in all centers, showed that the prevalence of respiratory symptoms and diagnoses of obstructive airway diseases varied both within and between the countries. The highest prevalence of physician-diagnosed asthma was found in northern Sweden and the associations between respiratory symptoms and asthma diagnosis were strongest here as well. Both current smoking and chronic productive cough, corresponding to the clinical definition of chronic bronchitis, were most common in Finland, however, a physician diagnosis of CB or COPD was likewise low in Finland as in Sweden. Furthermore, more women than men with respiratory symptoms were diagnosed with asthma in both areas in Sweden and in Seinäjoki-Vaasa in Finland while no sex difference was found in Helsinki. In Sweden, more women than men with sputum production and chronic productive cough had a diagnosis of CB or COPD while the opposite was found in Finland where more men reported a diagnosis. This indicates differences in diagnostic patterns between the study areas as well as between men and women.
In the current study, the prevalence of physician-diagnosed asthma was higher in both Sweden and Finland than in the 1990s when the prevalence was 7.8% in Sweden and 5.9% in Finland (Citation7,Citation36). However, in Northern Sweden an ongoing increase was reported from 1996 to 2006 and further to 2016 (Citation7), while in Helsinki an increase in asthma diagnosis was found until 2006 (9), but leveling until 2016 (Citation11). The introduction of the Finnish asthma program (Citation12) and the implementation of asthma/COPD clinics and national guidelines in Sweden (Citation46) may have contributed to better recognition of asthma in primary healthcare, which might be reflected in our findings. A previous multicenter study from the 1990s showed that 27.2% of individuals reporting any wheeze in Sweden and 17.7% in Finland were diagnosed with asthma (Citation36) while in the current study between 30.2 and 44.5% of individuals with any wheeze had an asthma diagnosis. Furthermore, between 45.1% and 60.6% of the individuals with asthma symptoms reported an asthma diagnosis in the current study, which may indicate an improvement in diagnostic patterns since the 1990s when only 30 to 40.5% of the individuals with asthma symptoms had an asthma diagnosis (Citation36). Despite that more symptomatic individuals were diagnosed with asthma both in Finland and Sweden in the present study, there are still differences between the countries. The health care structure is similar in Sweden and Finland. Both are tax-funded, which means that all citizens have equal access to healthcare services. The primary health care take care of most patient groups and diseases, including asthma and COPD, while unusual and complicated diseases are treated within the specialist healthcare at hospitals. However, the differences may be due to variations in awareness of respiratory symptoms and obstructive airway diseases, both in society and among healthcare providers, but also to differences in diagnostic guidelines. In Finland, the diagnosis of asthma must be confirmed by demonstrating reversible obstruction in spirometry, a PEF follow-up for 2 weeks, or by bronchial provocation tests, and much attention is paid on confirming the asthma diagnosis because otherwise the patient does not get a special higher reimbursement for the medication. Consequently, asthma medication is much more expensive for patients in Finland without a diagnosis. In Sweden, the national guidelines also stipulate that spirometry with reversibility test is to be performed but a normal result from a spirometry cannot exclude asthma and more diagnostics are not mandatory. Thus, the more strict diagnostic criteria in Finland with mandatory verified bronchial variability probably explain the lower prevalence of physician-diagnosed asthma in relation to reported asthma symptoms in Finland compared with Sweden.
However, the current study suggests differences in diagnostic patterns also within the countries. In Norrbotten, the large population-based studies about obstructive airway diseases and allergy performed within the OLIN since 1985 may further have increased the awareness of these conditions, and thus contributed to the national differences in Sweden. The prevalence of asthma decreased by age in three of the four study areas but in Seinäjoki-Vaasa the decrease was followed by an increase in the oldest age group. This finding was surprising because this pattern, which probably was related to misclassification of asthma among elderly, was seen in Sweden (Citation7) and areas in Finland (Citation36) during the 1990s but not in later decades (Citation7). The explanation for this difference within Finland is unclear despite late-onset asthma has been reported to be somewhat more common in Seinäjoki-Vaasa than in Helsinki (Citation40).
Generally, the prevalence of asthma among adults is higher among women (Citation7,Citation47,Citation48). The reason for this sex difference is not clear. However, in the current study we found that a higher proportion of women than men with respiratory symptoms were diagnosed with asthma in the two Swedish areas and in one of the Finnish, Seinäjoki-Vaasa, while no corresponding differences were found in Helsinki. These results indicate that it was more likely for women to be diagnosed when having asthma-like symptoms, and this may thus contribute to the higher prevalence of asthma among women. One explanation for the current results may be that women tend to be more observant of asthma symptoms (Citation49) and experience them as more severe than men do, which can be reflected in more frequent healthcare seeking among women (Citation48).
The prevalence of physician-diagnosed CB or COPD was still low and seemed to have decreased further, from 3.1% in Sweden and 2.9% in Finland in 1996 (Citation36) to the current 1.7–2.5% in Sweden and 2.0–2.6% in Finland. This could be related to decreasing prevalence of current smoking since the 1990s both in Sweden and Finland (Citation7,Citation36). However, smoking is still more prevalent in Finland and may explain why typically smoke-related symptoms such as sputum production and chronic productive cough were more frequent in Finland than in Sweden. In the current study, 9.5–15.1% of the individuals with chronic productive cough reported physician-diagnosed CB or COPD. This result can be seen in relation to the study conducted by Pallasaho et al. (Citation36) showing that during the 1990s between 9.2 and 9.5% of the individuals with chronic productive cough reported a diagnosis of CB or COPD. In light of that, and the remaining low prevalence of reported physician diagnosed CB or COPD in our study populations, the current study indicates a continued under-diagnosis of COPD. An extensive under-diagnosis of COPD has been described in several studies based on spirometry (Citation17–20,Citation50). Notably, difference in prevalence of COPD especially in elderly could be related to use of FEV1/FVC fixed cutoff or LLN. In Norrbotten, the prevalence of COPD in 2009 was 6.3–8.5% depending on spirometric definition of COPD (Citation25), and in Finland the corresponding prevalence around the millennium shift was 5.9–6.8% (Citation26) and 5.4–9.4% (Citation20). Altogether, this highlight the need for improvement in the diagnostic practices of COPD.
Importantly, the current study showed differences in diagnostic patterns between men and women regarding physician-diagnosed CB or COPD. In Sweden, more women than men with respiratory symptoms reported a diagnosis while in Finland the opposite was found. This was an unexpected finding and we cannot explain this difference between the countries. However, it has been argued that there are differences in clinical presentation between men and women with COPD, where for instance women present with more dyspnea but less sputum production than men (Citation51,Citation52). The prevalence of sputum production was similar among men and women in all the four study areas, while the prevalence of dyspnea was higher among women than among men. In the two Swedish study areas, the prevalence of physician-diagnosed CB or COPD was higher among women both with chronic productive cough and sputum production. These results are contrasting the Finnish study areas, where it was more likely to have a diagnosis among men than among women with sputum production.
A strength with the current study is that each study area contributed with a large random general population study sample of adults. Another strength is that the results are based on postal questionnaires with identical questions enabling comparisons between the study areas, and the questionnaires used have recently been validated (Citation53). A limitation is that the physician-diagnoses are based on self-reports and not clinically verified. Due to legislation (Citation45), it was not possible to transport data across national borders to pool data into a joint dataset, which both complicated and limited the analyses. Another possible weakness is the increasing nonparticipation in population-based studies during the last decades (Citation7,Citation54), which potentially could introduce bias and influence the estimates. However, the response rate was similar in all four areas and a previous non-response study conducted within the WSAS study showed no differences in the prevalence of symptoms between responders and non-responders (Citation55).
Conclusion
There are still differences in diagnostic patterns of obstructive airway diseases between Sweden and Finland, between the study areas in each country, and between men and women. Although some improvements were seen since the 1990s, the current study suggests that increased knowledge and awareness of obstructive lung diseases including diagnostic practices are needed both in society and among healthcare providers in order to act on the under-diagnosis and misclassification of obstructive airway diseases. Furthermore, our study supports the large under-diagnosis of COPD reported from studies based on spirometry.
Supplemental Material
Download MS Word (25.4 KB)Acknowledgment
Zandra Lundgren, Bo Selinder and Tessa Pohjanen are acknowledged for questionnaire management and compilation of data in Norrbotten, Sweden. Antti Sepponen, technician, and Aino Sepponen, RN, are acknowledged for their input with western Finland FinEsS sample. All the research nurses working within the West Sweden Asthma Study are acknowledged for greatly assisting the research.
Declaration of interest
Malin Axelsson, Helena Backman, Linda Ekerljung, Linnea Hedman, Arnulf Langhammer, Ari Lindqvist, Bright I. Nwaru, Paula Pallasaho, Anssi Sovijärvi, Iida Vähätalo, Päivi Piirilä, Eva Rönmark report no conflict of interest.
Pinja Ilmarinen reports personal fees from Astra Zeneca, Novartis, Mundipharma, and from GlaxoSmithKline, outside the submitted work.
Anne Lindberg reports personal fees from Boehringer-Ingelheim, AstraZeneca, Novartis, and from GlaxoSmithKline, outside the submitted work.
Hanna Hisinger-Mölkänen reports other from GlaxoSmithKline, outside the submitted work.
Hannu Kankaanranta reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer-Ingelheim, Orion Pharma, SanofiGenzyme, personal fees from Chiesi Pharma AB, Novartis, Mundipharma and from GlaxoSmithKline, outside the submitted work.
Funding
Financial support for the Nordic Epilung project was received from Nordforsk.
Financial support for the study in Norrbotten was received mainly from The Swedish Heart & Lung Foundation, ALF – a regional agreement between Umeå University and Norrbotten County Council, Norrbotten County Council, The Swedish Asthma-Allergy Foundation, and Visare Norr.
Financial support for the study in Västra Götaland was received from VBG Group’s Herman Krefting Foundation for asthma and allergy research.
Financial support for the study in Seinäjoki-Vaasa was received from the Tampere Tuberculosis Foundation (Tampere, Finland), the Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (Tampere, Finland) and the Medical Research Fund of Seinäjoki Central Hospital (Seinäjoki, Finland).
Financial support for the study in Helsinki was received from Nummela Sanatorium Foundation (AS), Ida Montin Foundation (H H-M) and from Helsinki University Central Hospital (Project TYH 2013354).
None of the sponsors had any involvement in the planning, execution, drafting or write-up of this study.
Data availability
The data are not publicly available due to legislation.
References
- Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–1422. doi:10.1056/NEJMoa022363.
- Barraclough R, Devereux G, Hendrick DJ, Stenton SC. Apparent but not real increase in asthma prevalence during the 1990s. Eur Respir J. 2002;20(4):826–833. doi:10.1183/09031936.02.00822002.
- Lundbäck B, Rönmark E, Jonsson E, Larsson K, Sandström T. Incidence of physician-diagnosed asthma in adults-a real incidence or a result of increased awareness? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2001;95(8):685–692. doi:10.1053/rmed.2001.1126.
- Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65(2):152–167. doi:10.1111/j.1398-9995.2009.02244.x.
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet L-P. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi:10.1186/1471-2458-12-204.
- Burney P, Chinn S, Jarvis D, Luczynska C, Lai E. Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J. 1996;9(4):687–695.
- Backman H, Räisänen P, Hedman L, Stridsman C, Andersson M, Lindberg A, Lundbäck B, Rönmark E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy. 2017;47(11):1426–1435. doi:10.1111/cea.12963.
- Ekerljung L, Andersson A, Sundblad B-M, Rönmark E, Larsson K, Ahlstedt S, Dahlén S-E, Lundbäck B. Has the increase in the prevalence of asthma and respiratory symptoms reached a plateau in Stockholm, Sweden? Int J Tuberc Lung Dis. 2010;14(6):764–771.
- Kainu A, Pallasaho P, Piirilä P, Lindqvist A, Sovijärvi A, Pietinalho A. Increase in prevalence of physician-diagnosed asthma in Helsinki during the Finnish Asthma Programme: improved recognition of asthma in primary care? A cross-sectional cohort study. Prim Care Respir J. 2013;22(1):64–71. doi:10.4104/pcrj.2013.00002.
- Lötvall J, Ekerljung L, Rönmark EP, Wennergren G, Lindén A, Rönmark E, Torén K, Lundbäck B. West Sweden Asthma Study: prevalence trends over the last 18 years argues no recent increase in asthma. Respir Res. 2009;10:94. doi:10.1186/1465-9921-10-94.
- Hisinger-Mölkänen H, Pallasaho P, Haahtela T, Lindqvist A, Sovijärvi A, Piirilä P. The increase of asthma prevalence has levelled off and symptoms decreased in adults during 20 years from 1996 to 2016 in Helsinki, Finland. Respir Med. 2019;155:121–126. doi:10.1016/j.rmed.2019.07.014.
- Haahtela T, Lehtimäki L, Ahonen E, Harju T, Jartti T, Kankaanranta H, Korhonen K, Mäkelä M, Puurunen M, Sovijärvi A, et al. Update on current care guidelines: asthma. Duodecim. 2013;129(9):994–995.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Available from: http://www.ginasthma.org.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2019. Available from: http://www.goldcopd.org.
- Heffler E, Crimi C, Mancuso S, Campisi R, Puggioni F, Brussino L, Crimi N. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med. 2018;142:48–52. doi:10.1016/j.rmed.2018.07.015.
- José B. P d S, Camargos PAM, Cruz Filho Á. A S d, Corrêa R. d A. Diagnostic accuracy of respiratory diseases in primary health units. Rev Assoc Med Bras (1992)). 2014;60(6):599–612. doi:10.1590/1806-9282.60.06.021.
- Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43(1):75–80. doi:10.1080/02770900500448738.
- Lamprecht B, Soriano JB, Studnicka M, Kaiser B, Vanfleteren LE, Gnatiuc L, Burney P, Miravitlles M, García-Rio F, Akbari K, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971–985. doi:10.1378/chest.14-2535.
- Lindberg A, Bjerg A, Bjerg-Bäcklund A, Rönmark E, Larsson L-G, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100(2):264–272. doi:10.1016/j.rmed.2005.04.029.
- Kotaniemi J-T, Sovijärvi A, Lundbäck B. Chronic obstructive pulmonary disease in Finland: prevalence and risk factors. COPD. 2005;2(3):331–339. doi:10.1080/15412550500218122.
- Sator L, Horner A, Studnicka M, Lamprecht B, Kaiser B, McBurnie MA, Buist AS, Gnatiuc L, Mannino DM, Janson C, et al. Overdiagnosis of COPD in subjects with unobstructed spirometry: a BOLD analysis. Chest. 2019;156(2):277–288. doi:10.1016/j.chest.2019.01.015.
- Melbye H, Drivenes E, Dalbak LG, Leinan T, Høegh-Henrichsen S, Østrem A. Asthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or more. Int J Chron Obstruct Pulmon Dis. 2011;6:597–603.
- Vasankari T, Pietinalho A, Lertola K, Junnila SY, Liippo K. Use of spirometry and recording of smoking habits of COPD patients increased in primary health care during national COPD programme. BMC Fam Pract. 2011;12(1):97. doi:10.1186/1471-2296-12-97.
- Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi:10.7189/jogh.05-020415.
- Backman H, Eriksson B, Rönmark E, Hedman L, Stridsman C, Jansson S-A, Lindberg A, Lundbäck B. Decreased prevalence of moderate to severe COPD over 15 years in northern Sweden. Respir Med. 2016;114:103–110. doi:10.1016/j.rmed.2016.03.013.
- Kainu A, Pallasaho P, Pietinalho A. No change in prevalence of symptoms of COPD between 1996 and 2006 in Finnish adults - a report from the FinEsS Helsinki Study. Eur Clin Respir J. 2016;3(1):31780. doi:10.3402/ecrj.v3.31780.
- Axelsson M, Lindberg A, Kainu A, Rönmark E, Jansson SA. Respiratory symptoms increase health care consumption and affect everyday life - a cross-sectional population-based study from Finland, Estonia, and Sweden. Eur Clin Respir J. 2016;3:31024. doi:10.3402/ecrj.v3.31024.
- Grønseth R, Vollmer WM, Hardie JA, Ólafsdóttir IS, Lamprecht B, Buist AS, Gnatiuc L, Gulsvik A, Johannessen A, Enright P, et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J. 2014;43(6):1610–1620. doi:10.1183/09031936.00036813.
- Maio S, Baldacci S, Carrozzi L, Pistelli F, Angino A, Simoni M, Sarno G, Cerrai S, Martini F, Fresta M, et al. Respiratory symptoms/diseases prevalence is still increasing: a 25-yr population study. Respir Med. 2016;110:58–65. doi:10.1016/j.rmed.2015.11.006.
- Wheaton AG, Ford ES, Thompson WW, Greenlund KJ, Presley-Cantrell LR, Croft JB. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States–National Health and Nutrition Examination Survey 2007–2010. BMC Public Health. 2013;13(1):854. doi:10.1186/1471-2458-13-854.
- Axelsson M, Ekerljung L, Eriksson J, Hagstad S, Rönmark E, Lötvall J, Lundbäck B. Chronic bronchitis in West Sweden - a matter of smoking and social class. Eur Clin Respir J. 2016;3:30319doi:10.3402/ecrj.v3.30319.
- Soriano JB, Ancochea J, Miravitlles M, Garcia-Rio F, Duran-Tauleria E, Munoz L, Jimenez-Ruiz CA, Masa JF, Viejo JL, Villasante C, et al. Recent trends in COPD prevalence in Spain: a repeated cross-sectional survey 1997–2007. Eur Respir J. 2010;36(4):758–765. doi:10.1183/09031936.00138409.
- Bhatta L, Leivseth L, Mai X-M, Chen Y, Henriksen AH, Langhammer A, Brumpton BM. Prevalence and trend of COPD from 1995-1997 to 2006-2008: the HUNT study, Norway. Respir Med. 2018;138:50–56. doi:10.1016/j.rmed.2018.03.020.
- Landis SH, Muellerova H, Mannino DM, Menezes AM, Han MK, van der Molen T, Ichinose M, Aisanov Z, Oh Y-M, Davis KJ, et al. Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis. 2014;9:597–611. doi:10.2147/COPD.S61854.
- Vasankari TM, Impivaara O, Heliövaara M, Heistaro S, Liippo K, Puukka P, Saarelainen S, Kanervisto M, Jousilahti P. No increase in the prevalence of COPD in two decades. Eur Respir J. 2010;36(4):766–773. doi:10.1183/09031936.00178109.
- Pallasaho P, Meren M, Raukas-Kivioja A, Rönmark E. Different labelling of obstructive airway diseases in Estonia, Finland, and Sweden. Eur J Epidemiol. 2005;20(12):975–983. doi:10.1007/s10654-005-4117-6.
- Jannus-Pruljan L, Meren M, Polluste J, Loit HM, Kiviloog J, Baburin A, et al. Postal survey on asthma, chronic bronchitis and respiratory symptoms among adult Estonians and non-Estonians (FinEsS-study). Eur J Public Health. 2004;14(2):114–119. doi:10.1093/eurpub/14.2.114.
- Meren M, Raukas-Kivioja A, Jannus-Pruljan L, Loit HM, Rönmark E, Lundbäck B. Low prevalence of asthma in westernizing countries-myth or reality? Prevalence of asthma in Estonia-a report from the "FinEsS" study“”. J Asthma. 2005;42(5):357–365. doi:10.1081/JAS-62985.
- Pallasaho P, Lundbäck B, Meren M, Kiviloog J, Loit HM, Larsson K, Laitinen LA. Prevalence and risk factors for asthma and chronic bronchitis in the capitals Helsinki, Stockholm, and Tallinn. Respir Med. 2002;96(10):759–769. doi:10.1053/rmed.2002.1308.
- Honkamäki J, Hisinger-Mölkänen H, Ilmarinen P, Piirilä P, Tuomisto LE, Andersén H, Huhtala H, Sovijärvi A, Backman H, Lundbäck B, et al. Age- and gender-specific incidence of new asthma diagnosis from childhood to late adulthood. Respir Med. 2019;154:56–62. doi:10.1016/j.rmed.2019.06.003.
- Lundbäck B, Nyström L, Rosenhall L, Stjernberg N. Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J. 1991;4(3):257–266.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581.
- SPSS IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
- Chi-Square Test Calculator. 2018. Avaliable from: https://www.socscistatistics.com/tests/chisquare2/Default2.aspx.
- Council of the European Union, European Parliament. General Data Protection Regulation. The Regulation (EU) 2016/679.
- National guidelines for treatment of asthma and COPD. The National Board of Health and Welfare. 2015. https://www.socialstyrelsen.se/publikationer2018/2018-1-36.
- Kankaanranta H, Tuomisto LE, Ilmarinen P. Age-specific incidence of new asthma diagnoses in Finland. J Allergy Clin Immunol Pract. 2017;5(1):189–191.e3. doi:10.1016/j.jaip.2016.08.015.
- Pignataro FS, Bonini M, Forgione A, Melandri S, Usmani OS. Asthma and gender: The female lung. Pharmacol Res. 2017;119:384–390. doi:10.1016/j.phrs.2017.02.017.
- McCallister JW, Mastronarde JG. Sex differences in asthma. J Asthma. 2008;45(10):853–861. doi:10.1080/02770900802444187.
- Annesi-Maesano I, Lundbäck B, Viegi G (editors). ERS Monograph. Respiratory Epidemiology. 2014. doi:10.1183/2312508X.10008514.
- Aryal S, Diaz-Guzman E, Mannino DM. COPD and gender differences: an update. Transl Res. 2013;162(4):208–218. doi:10.1016/j.trsl.2013.04.003.
- Sawalha S, Hedman L, Rönmark E, Lundbäck B, Lindberg A. Pre- and post-bronchodilator airway obstruction are associated with similar clinical characteristics but different prognosis - report from a population-based study. COPD. 2017;12:1269–1277. doi:10.2147/COPD.S127923.
- Ekerljung L, Rönmark E, Lötvall J, Wennergren G, Torén K, Lundbäck B. Questionnaire layout and wording influence prevalence and risk estimates of respiratory symptoms in a population cohort. Clin Respir J. 2013;7(1):53–63. doi:10.1111/j.1752-699X.2012.00281.x.
- Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–653. doi:10.1016/j.annepidem.2007.03.013.
- Rönmark EP, Ekerljung L, Lötvall J, Toren K, Rönmark E, Lundbäck B. Large scale questionnaire survey on respiratory health in Sweden: effects of late- and non-response. Respir Med. 2009;103(12):1807–1815. doi:10.1016/j.rmed.2009.07.014.
