Abstract
Objective
To evaluate the cost-effectiveness of mepolizumab added to standard of care (SOC) compared with SOC alone among patients with severe uncontrolled eosinophilic asthma in the Singapore setting.
Methods
A Markov model with three health states (asthma on mepolizumab and SOC, asthma on SOC alone, and death) was developed from a healthcare system perspective over a lifetime horizon. During each 4-week cycle, patients in the non-death health states could experience asthma exacerbations requiring oral corticosteroid burst, emergency department visit, or hospitalization. Asthma-related mortality following an exacerbation or all-cause mortality could also occur at each cycle. The model was populated using local costs while utilities were derived from international literature. Transition probabilities were obtained from a mixture of Singapore-specific and internationally published data.
Results
The base-case analysis comparing mepolizumab plus SOC with SOC alone resulted in an incremental cost-effectiveness ratio (ICER) of SGD335 486 (USD238 195) per quality-adjusted life-year (QALY) gained. Sensitivity analysis demonstrated that the ICER was most sensitive to the price of mepolizumab, followed by the proportion of exacerbations which required hospital intensive care. Despite restricting mepolizumab use to patients with a higher baseline exacerbation rate (3 in the past year) in a scenario analysis, the ICER remained high at SGD238 876 (USD 169 602) per QALY gained.
Conclusion
At its current price, mepolizumab is not considered a cost-effective use of healthcare resources in Singapore. Substantial price reductions for mepolizumab are required to improve its cost-effectiveness to an acceptable range. These results will be useful to inform national funding decisions.
Introduction
The estimated prevalence of current asthma among Singapore residents aged 18 to 69 years is 3.9% (Citation1). Although severe asthma only affects 5–10% of all asthmatics (Citation2), it is associated with a high disease burden and accounts for a disproportionately high level of healthcare utilization compared to non-severe asthma (Citation3). Furthermore, Singapore has a high asthma mortality rate at 16 per 100 000, three times that of other developed nations such as the US and New Zealand (Citation4,Citation5). A retrospective medical records review of four restructured hospitals in Singapore also reported 461 severe life-threatening asthma exacerbation events requiring intensive care admissions between 2011–2015 (Citation6).
Severe asthma is defined as asthma which requires treatment with high-dose inhaled corticosteroids plus a second controller and/or systemic corticosteroids to prevent it from becoming uncontrolled or which remains uncontrolled despite this therapy (Citation2). It is a heterogeneous disease and current clinical practice guidelines (Citation2,Citation7) recommend phenotyping patients with severe asthma to guide further treatment if they remain uncontrolled despite high doses of inhaled controller medications. In severe uncontrolled eosinophilic asthma, the use of biologics such as mepolizumab, an anti-IL-5 monoclonal antibody, in addition to standard of care (SOC) has been shown to improve asthma outcomes such as reducing clinically significant exacerbation rates, improving symptoms and health-related quality of life, and reducing the need for maintenance oral corticosteroids (Citation8–11). While there is a need for add-on therapy for patients with severe uncontrolled asthma (Citation12,Citation13), biologics such as mepolizumab are expensive (∼SGD1690 [USD1200] every 4 weeks), which raises the question of whether they are cost effective on a healthcare system level.
The healthcare financing system in Singapore is based on a philosophy of shared responsibility and comprises a combination of government subsidies, compulsory individual health care savings accounts, risk-pooling via voluntary private and mandatory government health insurance plans, and out-of-pocket contributions from patients (Citation14). Government subsidies cover 50–75% of drug costs and play a significant role in ensuring patient access to effective drugs. The decision by the government to subsidize a health technology is informed by multiple factors, such as unmet need, clinical effectiveness, safety, cost-effectiveness and budget impact (Citation15). Although there is no explicit cost-effectiveness threshold in Singapore, incremental cost-effectiveness ratios (ICERs) for drugs used to treat chronic diseases (such as diabetes, chronic obstructive pulmonary disease and atrial fibrillation) which have been previously recommended for subsidy in Singapore generally ranged from dominance to < SGD45 000 (USD31 950) per QALY gained (Citation16–18).
Several cost-effectiveness analyses of mepolizumab have been performed in the UK, Canada and US (Citation7,Citation19–22), but none were conducted in our local setting. This study aims to evaluate the cost-effectiveness of mepolizumab plus SOC compared with SOC alone among patients with severe eosinophilic asthma in Singapore, to inform local drug subsidy decisions.
Methods
Clinical effectiveness
A systematic literature search was conducted to identify all relevant randomized controlled trials (RCTs) which compared mepolizumab 100 mg subcutaneous (SC) injection or 75 mg intravenous (IV) injection every 4 weeks added to SOC with SOC alone for the treatment of severe eosinophilic asthma. Although not licensed in Singapore, mepolizumab 75 mg IV injection has been accepted by overseas regulatory agencies to have comparable bioequivalence and efficacy to mepolizumab 100 mg SC (Citation23). SOC was defined as high dose inhaled corticosteroid (ICS) plus long-acting beta2-agonist (LABA) or an additional controller, with or without maintenance oral corticosteroids (mOCS).
Four relevant studies were identified. MENSA (Citation10) (ClinicalTrials.gov number NCT01691521), MUSCA (Citation9) (NCT02281318), and DREAM (Citation11) (NCT01000506) studied mepolizumab as add-on therapy to high-dose ICS plus additional controllers among patients with severe eosinophilic asthma and at least two exacerbations in the previous 12 months. Baseline characteristics of patients included in these trials (age, number of exacerbations in the past 12 months, oral corticosteroid doses) matched closely to that of the local population. Across the three trials, mepolizumab plus SOC reduced clinically significant exacerbations by 48–58% compared with SOC alone. SIRIUS (Citation8) (NCT01691508) was designed as a steroid-sparing trial and showed a 30% median reduction in the average OCS dose after 24 weeks of mepolizumab treatment. These results were considered generalizable to the local population and were used in the model.
Model structure and study cohort
A Markov state-transition model was developed using TreeAge Pro Version: 2019 software (TreeAge Software, Williamstown, MA; available at http://www.treeage.com) to compare mepolizumab plus SOC versus SOC alone. The model’s starting cohort, in line with the population included in the pivotal trials of mepolizumab, comprised patients aged 12 years and older with severe asthma and high blood eosinophil counts (≥150/µL at screening or ≥300/µL in the previous year). In order to capture the benefits related to the OCS-sparing effect of mepolizumab, patients were divided into two groups: those who were receiving mOCS and those who were not receiving mOCS. Transition probabilities, utilities and costs associated with treatment and adverse event management applied in the model differed for the two groups of patients. Patients not receiving mOCS had at least two asthma exacerbations in the past year.
Patients could transition between three mutually exclusive health states, namely, “asthma on mepolizumab and SOC”, “asthma on SOC alone”, and “death” (). During each cycle, patients in the non-death health states could experience any one of three levels of asthma exacerbations, characterized by the required intensity of their clinical management: OCS burst, emergency department (ED) visit or hospitalization. Asthma-related mortality following an exacerbation or all-cause mortality could also occur. Patients in the “asthma on mepolizumab and SOC” health state could discontinue mepolizumab and move to the “asthma on SOC alone” health state.
Figure 1. Markov model structure. OCS: oral corticosteroids; SOC: standard of care; ED: emergency department.
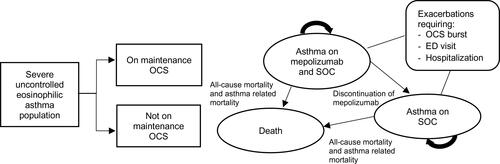
The model was developed from the Singapore healthcare system perspective, and included all direct medical costs borne by the patient, insurance providers and government subsidies. A lifetime horizon and a cycle length of 4 weeks were used. Half-cycle correction and an annual discounting rate of 3% were applied to both costs and benefits in the base case.
Interventions
The intervention was 100 mg of mepolizumab administered subcutaneously every 4 weeks by a healthcare professional. The local treatment mix for SOC drugs, including ICS plus LABA, montelukast and tiotropium, was based on expert opinion from local clinicians (Table S1). The daily dose of prednisolone used in the base case analysis followed the SIRIUS trial (median: 12.5 mg), and was consistent with clinical experts’ estimates of the average dose required in the local setting (10 to 12.5 mg).
Table 1. Key model inputs (base case).
Model inputs
Clinically significant exacerbations
Key model inputs are presented in . The rates of clinically significant exacerbations and the rate ratio reduction for patients who were not receiving mOCS were obtained from MENSA, MUSCA and DREAM trials while the rates for patients on mOCS were obtained from the SIRIUS trial.
The proportion of exacerbations requiring an ED visit or hospitalization was substantially higher in the local setting compared to that in the trials. To obtain cost-effectiveness estimates most reflective of the local setting, data from local hospitals was used to inform the distribution of exacerbation types in the model. Approximately 10% of all exacerbations requiring hospitalization were treated in the intensive care unit (ICU).
Mortality and discontinuation
Asthma-related mortality rates were 1.32% and 13.72% following an exacerbation that requires general ward hospitalization and ICU admission, respectively (Ministry of Health (MOH) Singapore Casemix data 2013 to 2017). A similar mortality rate (12.5%) was reported in a local study among patients who experienced a life-threatening asthma event requiring ICU or high-dependency unit care (Citation6). Mortality from exacerbations requiring ED visits was 0.06% (Citation24). Mortality rates following exacerbations requiring OCS burst were assumed to be similar to rates for exacerbations requiring an ED visit, due to a lack of local data. The risk of mortality from other causes was estimated using age- and gender-specific Singapore life tables for all-cause mortality over 5 years (2014 to 2018) (Citation25). Asthma-related deaths were not specifically removed from all-cause mortality rates due to the lack of data, but this was considered unlikely to impact results significantly as asthma-related deaths accounted for a very small proportion of all deaths.
An annual discontinuation rate of 10% for mepolizumab treatment was considered appropriate by local clinicians and assumed in the base case. Discontinuation rates of 5% to 15% were tested in one-way sensitivity analysis.
Maintenance OCS use and associated adverse events
Based on data from two local respiratory specialist centers, 16.67% of patients in the study cohort received mOCS. Using results from the SIRIUS trial, patients in the intervention arm had a 30% median dose reduction after 24 weeks of treatment, while no dose reduction occurred in the control arm. Patients who had a reduction in mOCS dose while on mepolizumab treatment were assumed to return to the baseline OCS dose upon discontinuation of mepolizumab.
As long-term OCS use is associated with an increased incidence of adverse events, additional costs and disutilities were applied to patients receiving mOCS in the model. Incidence of adverse events and their associated disutilities were estimated from internationally published sources in the absence of local estimates (Citation7,Citation27). Local costs were used (Appendix S1) (Citation28,Citation29).
Utility values
Treatment-specific utilities were assigned to all health states in the model, consistent with clinical expert opinion that mepolizumab improved asthma symptom control and health-related quality of life (HRQoL) in the day-to-day non-exacerbation state. Utility weights based on direct elicitation from patients using a multi-attribute utility instrument (e.g. EQ-5D) were preferred and hence utilities reported by the DREAM study were used to inform health state utilities of patients who were not receiving mOCS in the base case. Utility weights for patients receiving mOCS were derived by mapping the St George’s Respiratory Questionnaire (SGRQ) total scores reported by the SIRIUS study to the EQ-5D instrument using the mapping algorithm by Starkie et al. 2011, as utility weights directly elicited from patients using EQ-5D were not available (Citation30).
Disutilities from exacerbations were estimated using a published study conducted in the UK (Citation26), and the duration of exacerbations was estimated by local clinicians. Quality adjusted life year (QALY) gains in the mepolizumab arm were therefore assumed to be as a result of both treatment-specific effects on symptom control and HRQoL, as well as a reduction in disutility from exacerbations.
Costs
Costs of drugs (2018), consultation visits (2019) and monitoring tests (2019) were locally sourced (, Tables S1–S3). Among patients who experienced an exacerbation requiring OCS burst, the distribution of patients who would visit a GP (∼40%) or a respiratory specialist (∼15%) was estimated by local clinicians. Remaining exacerbation events requiring OCS burst (∼55%) were assumed to only incur the cost of prednisolone. Costs of ED visits and hospitalizations were obtained from public healthcare institutions in 2019 and MOH Singapore Casemix & Subvention data (2013–2017), respectively.
Sensitivity analyses
Deterministic one-way sensitivity analyses (OWSA) were conducted to explore the impact of uncertain model parameters on the ICER. Each parameter was varied individually by the lower and upper range of the 95% confidence interval. An arbitrary reduction in the cost of mepolizumab was tested (Table S4).
A probabilistic sensitivity analysis (PSA) was performed using second-order Monte Carlo simulation of 10 000 iterations and represented on a scatterplot. Uncertainty in each parameter was represented by an appropriate probability distribution that corresponded with the nature of the variable (Table S4). As there is no explicit cost-effectiveness threshold in Singapore, a cost-effectiveness acceptability curve (CEAC) was generated to display the probability of the treatment arms being cost-effective across a range of willingness-to-pay (WTP) thresholds.
Scenario analyses
Two additional scenario analyses were performed to examine how certain changes to the base case assumptions may influence the ICER. In scenario A, mepolizumab was restricted to patients with a higher baseline exacerbation rate (3 exacerbations in the past year), which was assessed by local clinicians to be reasonable and implementable. To be conservative, the same rate ratios reported for the general population in trials (at least 2 exacerbations in previous year) were applied. A range of hypothetical price reductions was also tested in this scenario.
In scenario B, alternative utility values obtained from mapped SGRQ results of the MENSA trial were used to inform the health state utility value of patients who were not receiving OCS. Of note, utility values were reported by both Whittington et al. (Citation21) and NICE TA431 (Citation7), with slight differences between them, possibly due to the use of tariffs from different countries. Both sets of values were tested ().
Table 2. Summary of mapped SGRQ utility weights by treatment strategies used in scenario B.
Results
Base-case results
In the base-case over a lifetime time horizon, treatment with mepolizumab plus SOC accrued more QALYs and incurred higher costs relative to SOC alone (). This resulted in an ICER of SGD335 486 (USD238 195) per QALY gained, whilst the incremental cost per LY gained was SGD208 234 (USD147 846). With mepolizumab treatment, an average of five exacerbations were avoided per patient (three were exacerbations requiring OCS burst, one requiring ED visit and one requiring hospitalization) over a lifetime time horizon.
Table 3. Quality adjusted life-years and life years per patient.
Sensitivity analyses
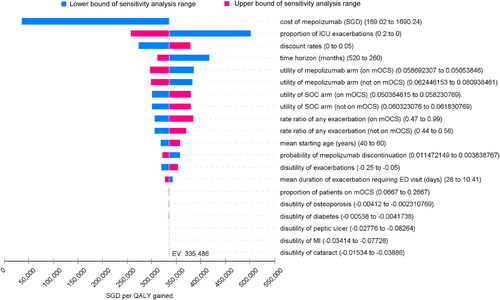
OWSA showed that, apart from the price of mepolizumab, the ICER was most sensitive to the proportion of exacerbations that required ICU stay (). The model was also sensitive to the time horizon, discount rates and utility values of the non-exacerbation health states. Variation in values of parameters such as disutility from adverse events associated with mOCS use had minimal influence on the ICER. Overall, the ICERs were found to be consistently above SGD250 000 (USD177 500) per QALY gained when parameters other than the price of mepolizumab were varied over their uncertainty range.
Figure 2. Tornado diagram for one-way sensitivity analysis. mOCS: maintenance oral corticosteroids; SOC: standard of care; ED: emergency department; ICU: intensive care unit; MI: myocardial infarction; SGD: Singapore dollars.
The PSA result was congruent with the base-case analysis with a mean ICER of SGD336 088 (USD238 622) per QALY gained. Mepolizumab as add-on therapy to SOC consistently produced higher QALYs at an increased cost compared with SOC alone, across 100% of the 10 000 PSA iterations (Figure S1). The CEAC demonstrated that across a hypothetical WTP threshold range of 0 to SGD330 000 (USD234 300) per QALY, SOC was more cost-effective than mepolizumab plus SOC (Figure S2).
Scenario analysis
Results of the two scenario analyses conducted are provided in Table S5. In scenario A, restricting the use of mepolizumab to patients with a higher baseline exacerbation rate (three per year) resulted in an ICER of SGD238 876 (USD169 602) per QALY gained. Ranging the hypothetical mepolizumab price reductions between 20% and 80% reduced the ICER to between SGD190 995 (USD135 606) and SGD47 354 (USD33 621) per QALY gained. In scenario B, using utility weights mapped from SGRQ results gave a larger gain in utility with the use of mepolizumab. The resulting ICER from utility weights reported by Whittington et al. and NICE TA431 were SGD206 265 (USD146 448) and SGD211 328 (USD150 043) per QALY gained, respectively. In the absence of any price reduction, ICERs from both scenarios remained high relative to ICERs for previous chronic disease treatments that were considered cost effective in Singapore’s context (Citation16–18).
Discussion
The base case ICER of SGD335 486 (USD238 195) per QALY gained was exceedingly high relative to ICERs of drugs used for the treatment of chronic diseases which previously received positive subsidy recommendations in Singapore (generally ranging from dominance to < SGD45 000/QALY [USD 31 950]) (Citation31). Thus, while there is no fixed WTP threshold in Singapore, mepolizumab is unlikely to represent a cost-effective treatment option at its current price in the Singapore setting. The base case ICER was driven mainly by the high cost of mepolizumab. The proportion of patients who required ICU treatment among those who were hospitalized was also a major driver, but the ICER remained high (>SGD250 000 [USD177 500]/QALY gained) when the proportion of ICU admissions was varied across its uncertainty range, giving confidence in the cost-effectiveness conclusion.
Published cost effectiveness evaluations (CEAs) corroborate the results of our analysis. High ICERs were demonstrated in CEAs by Whittington et al. (Citation21) (USD385 546/QALY gained) and CADTH (Citation19) (CAD521 000 [USD391 026]/QALY gained). However, the model from the manufacturer’s submission to NICE (TA431 report) had a much lower ICER of £29 163 (USD38 043)/QALY gained as it differed from our model in a number of ways.
First, the population in the NICE model was restricted to patients with more severe disease: patients had a blood eosinophil count of ≥300/µL in the previous year and either had four or more exacerbations in the previous year or were on maintenance corticosteroids. Our model, similar to the model by CADTH and Whittington et al. included a broader population in line with the general population included in the mepolizumab pivotal trials (blood eosinophil count of ≥150/µL at screening or ≥300/µL in the previous year and either two or more exacerbations in the previous year or receiving maintenance corticosteroids).
Restriction of the starting cohort to patients with a baseline exacerbation rate of three per year was conducted in a scenario analysis in our model. A further restriction to a subgroup with a baseline exacerbation rate higher than three in the past year was not tested as a very low number of patients in Singapore would be eligible for mepolizumab under this criterion. Using the same rate ratio for exacerbation reduction reported for the general population (patients not receiving mOCS: 0.50, patients receiving mOCS: 0.68), the resultant ICER was reduced to SGD238 845 (USD169 580) per QALY gained in this scenario analysis. However, this is likely to be conservative as results from one of the three pivotal studies (DREAM) demonstrated a lower exacerbation rate ratio (0.43, 95%CI 0.29 to 0.65) among patients with a baseline exacerbation rate of three. The lower rate ratio was not incorporated into the model as it was from a subgroup analysis in only one study (DREAM) and results should be interpreted with caution.
Second, the NICE model included a continuation criterion of a 50% reduction in exacerbations in the first year of treatment (Citation7,Citation20). This was not incorporated in our model due to a lack of published efficacy data for responders. Furthermore, such criteria would pose significant implementation challenges due to the additional administrative burden on the local healthcare system. Instead, an annual discontinuation rate of 10% for mepolizumab was assumed, which may account for some patients who choose to discontinue mepolizumab due to poor efficacy. A reduction in the ICER is expected, if patients were assumed to continue receiving mepolizumab only if a target response is achieved.
A confidential patient access scheme is also available for mepolizumab in the UK which reduces the effective price of mepolizumab below the list price of £840 (USD1096) per dose. As the price of mepolizumab is a major driver of the model, further price discounts may have contributed to the significantly lower ICER.
Our study also differs from other mepolizumab CEAs in that the distribution of the three levels of clinically significant exacerbations (OCS burst, ED visit, hospitalization) used in the model were informed by local hospital data, instead of data from pivotal trials. The proportion of exacerbations requiring an ED visit or hospitalization combined (47.2–47.9% across treatment arms) was similar to that reported for the control arm of a local CEA on bronchial thermoplasty (47.9%) (Citation32), but substantially higher than that reported in the mepolizumab pivotal trials (∼7–9% requiring hospitalization, 3–7% requiring ED visit). This disparity is unsurprising as ED attendances and hospitalization rates for asthma in Singapore have been observed to be higher than other developed countries. The reasons are multifactorial, including easy accessibility of EDs resulting in inappropriate visits (Citation33), socio-economic factors (Citation34,Citation35), a lower level of confidence or competency in self-management among patients (Citation36), and poor adherence to and/or lack of standard protocol in the management of acute asthma in EDs (Citation37–39).
Even though the steroid sparing effect of mepolizumab was incorporated in our model, there was little impact on the ICER. This may be attributed to the low proportion of patients who receive mOCS in the local setting (16.67%) and the low incidence rate of adverse events associated with mOCS derived from the Clinical Practice Research Datalink (CPRD) database (Appendix S1). Similarly, a high ICER was also demonstrated in the CEA by Whittington et al. (Citation21) which incorporated the costs and disutilities associated with adverse events from mOCS use. The manufacturer’s submission to CADTH (Citation19) had also included a scenario analysis which modeled the benefits of reduced mOCS use; however, the ICER did not differ appreciably from the base case analysis.
Nonetheless, we acknowledge that the steroid sparing beneficial effect of mepolizumab could be under-estimated in our model. It is well established that long-term systemic OCS use is associated with significant morbidity, and greater steroid exposure results in a higher risk of adverse events and increased healthcare resource use (Citation40–47). Although costs and disutilities of five major adverse events (cataract, diabetes, myocardial infarction, osteoporosis, peptic ulcer) associated with mOCS use were incorporated in our model to reflect the steroid-sparing effect of mepolizumab, other corollary adverse events such as obesity, renal impairment and infections were not included due to the lack of suitable data to populate our model. Similarly, treatment of exacerbations using OCS bursts was assumed not to result in any increase in OCS-related adverse events even though we acknowledge that there is increasing evidence that cumulative doses of short-term OCS bursts contribute to corticosteroid morbidity as well (Citation48,Citation49). In addition, as longer-term RCT data was not available, the reduction in mOCS dose with long-term use of mepolizumab was capped at 30%, in line with results from the 24-week SIRIUS study. Nevertheless, data inputs relating to adverse events from mOCS were not found to be drivers of our model and are likely to have little impact on the ICER.
Whilst utility weights directly elicited from patients using the EQ-5D are preferred to mapped data and were accepted by NICE, the high baseline EQ-5D results from the DREAM study may have led to a small incremental QALY gain in the intervention arm. Thus, SGRQ-mapped utility weights which showed a larger difference between treatment arms were tested in a scenario analysis. The ICER was substantially reduced from SGD335 486 (USD238 195)/QALY gained in the base case to SGD206 265 (USD146 448) and SGD211 328 (USD150 043)/QALY gained with the use of mapped SGRQ data from two different sources, but nonetheless, it remained beyond an acceptable cost-effectiveness range.
There are other limitations to our study. EQ-5D and SGRQ values were derived largely from Western trial participants and may not reflect the health status of our local Asian population of severe asthmatics, hence this may either over- or under-estimate the actual utility values. Also, the number of exacerbations requiring OCS burst in the local setting was captured based on patient reporting during clinician visits, thus the proportion of exacerbations requiring OCS burst in our study could be underestimated due to recall bias. Thirdly, the response to mepolizumab which was observed in clinical trials may lack external validity due to stringent trial criteria. However, several studies have reported similar outcomes in the real-world setting, suggesting that the results are consistent and likely to be generalizable (Citation50–52). Lastly, some of the model inputs such as the local mortality rate from asthma exacerbations at the ED (Citation24) and utility decrements due to asthma exacerbations (Citation26) were informed by data older than 5 years. Nonetheless, these inputs are likely to still be valid as they were verified by local clinical experts, and similar utility decrements were used in the CEAs conducted by Whittington et al. (Citation21) and NICE TA431 (2017).
In conclusion, although mepolizumab plus SOC is more effective than SOC alone for the treatment of severe eosinophilic asthma, it is also costlier. Mepolizumab is not considered cost-effective at its current price in Singapore. We are cognizant that there may be limitations to our model, however, these are unlikely to affect the cost-effectiveness conclusion.
Supplemental Material
Download MS Word (710.3 KB)Declaration of interest
MSK has received speaker fees and fees for serving on the advisory boards of GlaxoSmithKline, AstraZeneca, and Sanofi, all paid to her hospital, Singapore General Hospital.
References
- National Health Survey 2010: Epidemiology & Disease Control Division, Ministry of Health, Singapore. Available from: https://www.moh.gov.sg/resources-statistics/reports/national-health-survey-2010.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:https://doi.org/10.1183/09031936.00202013.
- Tay TR, Wong HS, Ihsan R, Toh HP, Choo X, Tee AK. Comparison of the proportion and healthcare utilisation of adult patients with uncontrolled severe asthma versus non-severe asthma seen in a Southeast Asian hospital-based respiratory specialist clinic. Ann Acad Med Singapore. 2017;46(6):217–228.
- Koh MS, Yii AC, Ong YY. Asthma in Singapore: past, present and future. Ann Acad Med Singap. 2017;46(3):81–83.
- Chai H, Koh M. Zero tolerance towards asthma deaths in Singapore: role of the family doctor. SFP. 2019;44(4):10–13. doi:https://doi.org/10.33591/sfp.44.4.u2.
- Yii ACA, Tay TR, Puah SH, Lim HF, Li A, Lau P, Tan R, Neo LP, Chung KF, Koh MS. Blood eosinophil count correlates with severity of respiratory failure in life-threatening asthma and predicts risk of subsequent exacerbations. Clin Exp Allergy. 2019;49(12):1578–1586. doi:https://doi.org/10.1111/cea.13465.
- Mepolizumab for treating severe refractory eosinophilic asthma, Technology appraisal guidance [TA431]: National Institute for Health and Care Excellence. Available from: https://www.nice.org.uk/guidance/ta431.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID, SIRIUS Investigators Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:https://doi.org/10.1056/NEJMoa1403291.
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, ten Brinke A. Ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:https://doi.org/10.1016/s2213-2600(17)30125-x.
- Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:https://doi.org/10.1056/NEJMoa1403290.
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:https://doi.org/10.1016/s0140-6736(12)60988-x.
- McDonald VM, Maltby S, Reddel HK, King GG, Wark PA, Smith L, Upham JW, James AL, Marks GB, Gibson PG. Severe asthma: Current management, targeted therapies and future directions-A roundtable report. Respirology. 2017;22(1):53–60. doi:https://doi.org/10.1111/resp.12957.
- Beasley R. Unmet need in inadequately controlled asthma. Respirology. 2007;12(s3):S18–S21. doi:https://doi.org/10.1111/j.1440-1843.2007.01046.x.
- Pearce F, Lin L, Teo E, Ng K, Khoo D. Health technology assessment and its use in drug policies: Singapore. Value Health Reg Issues. 2019;18:176–183. doi:https://doi.org/10.1016/j.vhri.2018.03.007.
- Drug Evaluation Methods and Process Guide v2.0 Agency for Care Effectiveness, Ministry of Health, Singapore. Available from: https://www.ace-hta.gov.sg [last accessed 12 August 2020].
- Novel oral anticoagulants (NOACs) for the prevention of stroke and systemic embolism in non-valvular atrial fibrillation: agency for Care Effectiveness, Ministry of Health, Singapore. Available from: https://www.ace-hta.gov.sg/our-guidance/novel-oral-anticoagulants-noacs-for-the-prevention-of-stroke-and-systemic-embolism-in-non-valvular-atrial-fibrillation-rivaroxaban-dabigatran-and-apixaban.html [last accessed 12 August 2020].
- LAMA & LAMA/LABA for chronic obstructive pulmonary disease (COPD): Agency for Care Effectiveness, Ministry of Health, Singapore. Available from: https://www.ace-hta.gov.sg/our-guidance/lama-_-lama-laba-for-chronic-obstructive-pulmonary-disease.html [last accessed 12 August 2020].
- Sodium-glucose co-transporter 2 (SGLT2) inhibitors for type 2 diabetes mellitus: Agency for Care Effectiveness, Ministry of Health, Singapore. Available from: https://www.ace-hta.gov.sg/our-guidance/sodium-glucose-co-transporter-2-sglt2-inhibitors-for-type-2-diabetes-mellitus.html [last accessed 12 August 2020].
- Common Drug Review Pharmoeconomic Review Report For Nucala CADTH. Available from: https://www.cadth.ca/mepolizumab.
- Bermejo I, Stevenson M, Cooper K, Harnan S, Hamilton J, Clowes M, Carroll C, Harrison T, Saha S. Mepolizumab for treating severe eosinophilic asthma: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(2):131–144. doi:https://doi.org/10.1007/s40273-017-0571-8.
- Whittington MD, McQueen RB, Ollendorf DA, Tice JA, Chapman RH, Pearson SD, Campbell JD. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118(2):220–225. doi:https://doi.org/10.1016/j.anai.2016.10.028.
- Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks. Institute for Clinical and Economic Review, December 20, 2018.
- Clinical Review BLA 125526 NUCALA (mepolizumab). U.S Food & Drug Administration; 2015.
- Chew HC, Eng P. Asthma fatalities at the Emergency Department of the Singapore General Hospital. Eur J Emerg Med. 2007;14(1):32–34. doi:https://doi.org/10.1097/01.mej.0000224433.43999.ce.
- M810011 - Singapore Residents By Age Group, Ethnic Group And Sex, End June, Annual: Department of Statistics Singapore. Available from: https://www.tablebuilder.singstat.gov.sg/publicfacing/createDataTable.action?refId=14911.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22–27. doi:https://doi.org/10.3132/pcrj.2007.00002.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804. doi:https://doi.org/10.1177/0272989x11401031.
- Shuyu Ng C, Toh MP, Ko Y, Yu-Chia Lee J. Direct medical cost of type 2 diabetes in singapore. PloS One. 2015;10(3):e0122795. doi:https://doi.org/10.1371/journal.pone.0122795.
- Fee Benchmarks and Bill Amount Information: Ministry of Health Singapore. Available from: https://www.moh.gov.sg/cost-financing/fee-benchmarks-and-bill-amount-information.
- Starkie HJ, Briggs AH, Chambers MG, Jones P. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14(2):354–360. doi:https://doi.org/10.1016/j.jval.2010.09.011.
- Agency for Care Effectiveness: Ministry of Health, Singapore. Available from: https://www.ace-hta.gov.sg [last accessed 12 August 2020].
- Nguyen HV, Bose S, Mital S, Yii ACA, Ang SY, Lam SSW, Anantham D, Finkelstein E, Koh MS. Is bronchial thermoplasty cost-effective as treatment for problematic asthma patients? Singapore's perspective on a global model. Respirology. 2017;22(6):1102–1109. doi:https://doi.org/10.1111/resp.13027.
- Oh HC, Chow WL, Gao Y, Tiah L, Goh SH, Mohan T. Factors associated with inappropriate attendances at the emergency department of a tertiary hospital in Singapore. Singapore Med J. 2020;61(2):75–80. doi:https://doi.org/10.11622/smedj.2019041.
- Ng TP, Lim TK, Abisheganaden J, Eng P, Sin FL. Factors associated with acute health care use in a national adult asthma management program. Ann Allergy Asthma Immunol. 2006;97(6):784–793. doi:https://doi.org/10.1016/s1081-1206(10)60970-2.
- Lim SF, Wah W, Pasupathi Y, Yap S, Koh MS, Tan KL, Chay CJ, Ong ME. Frequent attenders to the ED: patients who present with repeated asthma exacerbations. Am J Emerg Med. 2014;32(8):895–899. doi:https://doi.org/10.1016/j.ajem.2014.04.052.
- Tan NC, Chen Z, Soo WF, Ngoh AS, Tai BC. Effects of a written asthma action plan on caregivers' management of children with asthma: a cross-sectional questionnaire survey. Prim Care Respir J. 2013;22(2):188–194. doi:https://doi.org/10.4104/pcrj.2013.00040.
- Wong AJW, Chan JJ, Koh MS, Lian SWQ, Fook SMC, Ong MEH. Compliance with asthma guidelines and association with outcomes in the Emergency Department of a Tertiary Care Teaching Hospital. J Acute Med. 2018;8(3):119–126.
- Zainudin BMZ, Kai Wei Lai C, Soriano JB, Jia-Horng W, De Guia TS, Asthma Insights and Reality in Asia-Pacific (AIRIAP) Steering Committee. Asthma control in adults in Asia-Pacific. Respirology. 2005;10(5):579–586. doi:https://doi.org/10.1111/j.1440-1843.2005.00753.x.
- Abdulwadud OA, Abramson MJ, Forbes AB, Walters EH . The relationships between patients' related variables in asthma: iplications for asthma management. Respirology. 2001;6(2):105–112. doi:https://doi.org/10.1046/j.1440-1843.2001.00316.x.
- Barry LE, Sweeney J, O'Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18(1):129. doi:https://doi.org/10.1186/s12931-017-0614-x.
- Bleecker ER, Menzies-Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, Alacqua M, Tran TN. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–293. doi:https://doi.org/10.1164/rccm.201904-0903SO.
- Dalal AA, Duh MS, Gozalo L, Robitaille MN, Albers F, Yancey S, Ortega H, Forshag M, Lin X, Lefebvre P. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. JMCP. 2016;22(7):833–847. doi:https://doi.org/10.18553/jmcp.2016.22.7.833.
- Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, Tran TN. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. JAA. 2018;11:193–204. doi:https://doi.org/10.2147/JAA.S176026.
- Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116.e7. doi:https://doi.org/10.1016/j.jaci.2017.04.009.
- Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, Chaudhuri R, Price D, Brightling CE, Heaney LG. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016;71(4):339–346. doi:https://doi.org/10.1136/thoraxjnl-2015-207630.
- Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. Epub 2018/09/08. doi:https://doi.org/10.1183/13993003.00703-2018.
- Voorham J, Xu X, Price DB, Golam S, Davis J, Zhi Jie Ling J, Kerkhof M, Ow M, Tran TN. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74(2):273–283. doi:https://doi.org/10.1111/all.13556.
- Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29(155):190151–190105. doi:https://doi.org/10.1183/16000617.0151-2019.
- Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25(2):161–172. doi:https://doi.org/10.1111/resp.13730.
- Harvey ES, Langton D, Katelaris C, Stevens S, Farah CS, Gillman A, Harrington J, Hew M, Kritikos V, Radhakrishna N, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5):1902420. doi:https://doi.org/10.1183/13993003.02420-2019.
- Llanos JP, Ortega H, Bogart M, Packnett ER, Manjelievskaia J, Bell CF, Hahn B. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. JAA. 2020;13:77–87. doi:https://doi.org/10.2147/jaa.s236609.
- Taille C, Chanez P, Devouassoux G, Didier A, Pison C, Garcia G, Charriot J, Bouee S, Gruber A, Pribil C, et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J. 2020;55(6):1902345. doi:https://doi.org/10.1183/13993003.02345-2019.
