Abstract
Objective:
The aim of this pilot validation study was to determine the accuracy of a smartphone (iPhone®) pedometer in adults with and without asthma.
Methods:
Ten adults with asthma and ten healthy controls underwent clinical assessment prior to completing two separate trials. Phase 1. standardized treadmill and self-paced walking tests. Total steps were recorded via: (i) Yamax Digiwalker™ SW800 pedometer positioned on the waistband, (ii) iPhone® pedometer positioned on the upper body, (iii) iPhone® pedometer positioned on the lower body and evaluated against a video-verified manual step-count. Phase 2. step-count was evaluated over seven-days during habitual free-living conditions via Yamax Digiwalker™ SW800 and iPhone® pedometers.
Results:
During treadmill walking, the iPhone® positioned on the lower body correlated strongly (r = 0.96) and produced the highest level of agreement (mean bias: −11 steps, LOA: −43 to 21 steps) in comparison to video-verified manual step-count. During self-paced walking, all devices provided an excellent step-count estimate. During free-living conditions, no difference was observed between the Yamax Digiwalker™ SW800 pedometer and iPhone® (P = 0.10) and a strong correlation (r = 0.94) and acceptable agreement (mean bias: −343, LOA: −1963 to 1276 steps) was observed.
Conclusion:
Our findings indicate that an in-built iPhone® pedometer offers a practical approach to physical activity assessment in adults with and without asthma. Future research is now required to further validate the precision of this approach and evaluate the efficacy and effectiveness of smartphone pedometers to monitor and promote physical activity when employed during medical consultation and/or clinical research trials.
Introduction
Asthma is a chronic inflammatory airways disease associated with airway hyperresponsiveness, bronchoconstriction and variable respiratory symptoms such as cough, wheeze, and dyspnea. The disease affects approximately 350-million people worldwide (Citation1) and remains a major source of global economic and societal burden (Citation2). Despite clear guidelines and even in the context of adequate health care provision, control is often suboptimal with asthma symptoms and exacerbations being common and under-reported (Citation3). For the most part, asthma management strategies focus on pharmacological intervention with a stepwise approach to treatment escalation according to local, national and international guidelines (Citation4). While this approach is effective in most cases, the adverse side effects associated with long-term therapy can be significant (Citation5,Citation6) and adjunctive treatments which can help improve asthma control and minimize complications should be considered when optimizing care.
Physical activity has the potential of providing affordable and accessible adjunct approach to asthma management and related co-morbidities (e.g. obesity and cardiovascular disease etc.) with recent systematic reviews and meta-analyses reporting an association between the duration of activity, improved symptomology, slower lung function decline, higher functional capacity and lower systemic inflammation (Citation7–9). However, previous studies have relied primarily on patient recall when evaluating physical activity in people with airways disease (i.e. self-report and/or questionnaires) and this can be prone to both over and underestimation (Citation10).
The optimal method of measuring physical activity currently remains debated (Citation11). Although objective devices such as accelerometers and pedometers (i.e. step-counters) are widely endorsed for assessment, they have substantial limitations. For example, measurement error can arise through participant reactivity (i.e. behavior changes in response to being observed). Equally, the high associated cost of the technology often precludes implementation in physical activity promotion programmes (i.e. pulmonary rehabilitation) and large-scale clinical trials.
Over the past decade there have been dramatic advances in mobile technologies and an exponential growth in smartphone devices (>3.5 billion users worldwide) (Citation12) with in-built activity sensors (i.e. functionality to monitor daily step-count) which provides a pragmatic alternative approach to physical activity assessment. Although the validity and application of smartphones pedometers have previously been reported in healthy cohorts (Citation13), there are little data on their use in people with asthma to measure habitual physical activity (i.e. total distance covered and time spent active) which is often significantly reduced and may impact and/or be an indirect measure of asthma control (Citation7).
The aim of this pilot validation study was therefore to determine the accuracy of a smartphone (Apple iPhone®—version 6 onwards) to quantify step-count in adults with and without asthma. We hypothesized that an in-built pedometer would provide a practical approach to physical activity assessment (i.e. valid step-count estimate) during laboratory and self-paced walking challenges and habitual free-living conditions.
Methods
Study population and experimental design
Twenty adults (male: n = 12) were enrolled into the study; ten adults with a prior physician-based asthma diagnosis prescribed ‘as-needed’ reliever and daily low dose ICS maintenance therapy and ten asymptomatic healthy controls with no prior history of airways disease or inhaler medication use (matched for age, gender and body mass index (BMI)). At study entry, all participants completed pre-participation American College of Sports Medicine (ACSM) health screening (Citation14) and underwent clinical assessment prior to completing two separate trials: treadmill and self-paced walking challenges (Phase 1) and habitual free-living conditions (Phase 2) (protocols detailed below). A standard Apple iPhone® (version 6 onwards) was used to quantify step-count for all trials. The study was approved by research ethics committee (ID: 61792) and all participants provided written informed consent.
Clinical assessment and baseline pulmonary function
The Asthma Control Questionnaire (ACQ) and Asthma Quality of Life Questionnaire (AQLQ) was completed by participants with asthma to evaluate symptom burden and disease specific health-related quality of life (Citation15,Citation16). The Dyspnea-12 (D-12) questionnaire was employed to characterize the physical and affective aspects of breathlessness and to ensure healthy controls had an entirely normal D-12 score (i.e. confirming their asymptomatic status) (Citation17). Lung function was assessed by maximal forced flow-volume spirometry (MicroLoop ML3535; Cardinal Health, Basingstoke, UK) and airway inflammation was evaluated via fractional exhaled nitric oxide (FeNO) using a hand-held measuring device (NIOX VERO; Aerocrine AB, Stockholm, Sweden) with established reference values employed in accordance with international guidelines (Citation18,Citation19). Body composition was determined via bioelectrical impedance analysis (Tanita, 330ST, Japan).
Phase 1: treadmill and self-paced walking challenges
Participants completed a standardized treadmill test (Woodway PPS55 Med, Germany) consisting of 3 × 3-min stages at predetermined speeds: low (2.5 kph); moderate (5.0 kph); high (7.5 kph) at a 1% gradient, followed by a self-paced 1-km walking test around a 400 m athletics track. For both tests, total steps were recorded using the following devices: (i) Yamax Digiwalker™ SW800 pedometer positioned on the waistband, (ii) iPhone® pedometer positioned on the upper body (armband), (iii) iPhone® pedometer positioned on the lower body (trouser pocket) and evaluated against a video-verified manual step-count conducted by the investigator (CR).
Phase 2: habitual free-living conditions
Total step-count was evaluated over a seven-day period during habitual free-living conditions via Yamax Digiwalker™ SW800 pedometer (criterion measure) and iPhone® pedometer. Participants were instructed to maintain their usual daily activities and were provided with a logbook to record hours spent active and total daily step-count. Participants were required to carry the devices on their person for a minimum of 10-h per day (Citation20).
Statistical analysis
Data are presented as mean (SD) or median (IQR) for continuous outcomes dependent on normality. An unpaired and paired samples t-test were employed to evaluate between and within group differences, respectively. Intra-class correlation coefficient (ICC) was calculated using a two-way mixed effect model with the mean single measure reported. Reproducibility was assessed (i.e. video-verified manual step-count vs. pedometer) using the method described by Bland and Altman with difference expressed as mean bias (i.e. mean difference between group measures) and upper and lower 95% limits of agreement (Citation21). Linear regression was conducted to evaluate proportional bias between mean differences in devices. Data was analyzed using SPSS Statistics 24 statistical software package (SPSS Inc., Version 26, Chicago, IL) and GraphPad Prism Version 8.0 (GraphPad Software, San Diego, California, USA). P < 0.05 was considered statistically significant.
Results
Respiratory symptoms and baseline pulmonary function
Of the ten participants with asthma, five had an ACQ score >1 (i.e. indicating inadequate asthma control). Of these, three had an AQLQ score <6 (i.e. indicating impaired quality of life). Two participants with an ACQ score <1 (i.e. well-controlled asthma) had an AQLQ score <6. The majority of participants (90%) had normal resting lung function with no evidence of expiratory airflow limitation (FEV1 predicted >80% and FEV1/FVC >70% predicted). Although participants with asthma had a lower FEV1% predicted (P = 0.008), no difference was observed for any other pulmonary function parameter. Clinical characteristics are presented in .
Table 1. Clinical characteristics and baseline lung function.
Phase 1: treadmill walking challenge
No difference was observed in video-verified total manual step-count between participants with asthma (1018 ± 54 steps) and healthy controls (1038 ± 58 steps) (P = 0.44) and therefore group data was pooled for analysis (mean: 1028 ± 56 steps). The Yamax Digiwalker™ SW800 pedometer correlated poorly (r = 0.37) with video-verified total manual step-count and produced wide limits of agreement (mean bias: −74 steps, LOA: −203 to 55 steps) at the lowest walking speed (2.5 kph) (P < 0.0001), yet provided an acceptable step-count estimate at moderate (5.0 kph) and high (7.5 kph) speeds.
In contrast, the iPhone® positioned on either the upper or lower body provided a valid step-count estimate across all walking speeds. Although the iPhone® under-reported for most test stages when compared with video-verified total manual step-count (P < 0.05), the absolute difference in step-count was negligible (range: −4 to −11 steps). For total step-count, the iPhone® (upper and lower) and Yamax Digiwalker™ SW800 pedometer had a percentage error of 2%, 1% and 8%, respectively. Specifically, the iPhone® positioned on the lower body (1017 ± 58 steps) correlated strongly (r = 0.96) and produced the highest level of agreement (mean bias: −11 steps, LOA: −43 to 21 steps) in comparison to video-verified manual step-count (). Proportional bias was identified for Yamax Digiwalker™ SW800 pedometer (F = 11.47, P = 0.003) but not for the iPhone® lower body (F = 0.36, P = 0.56) or iPhone® upper body (F = 1.24, P = 0.28) ().
Figure 1. Bland-Altman plots for iPhone® upper body: (a) 2.5 kph, (b) 5.0 kph, (c) 7.5 kph and (d) total steps during a standardized treadmill walking test. Closed black circles denote healthy controls; open circles denote adults with asthma.
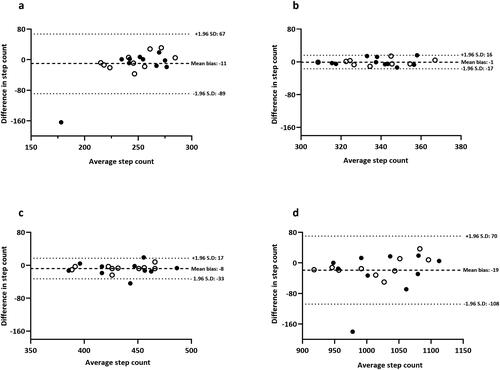
Figure 2. Bland-Altman plots for iPhone® lower body: (a) 2.5 kph, (b) 5.0 kph, (c) 7.5 kph and (d) total steps during a standardized treadmill walking test. Closed black circles denote healthy controls; open circles denote adults with asthma.
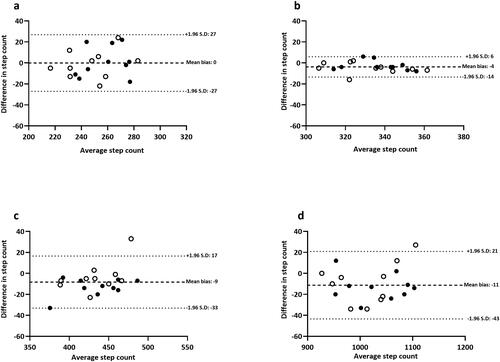
Figure 3. Bland-Altman plots for Yamax Digiwalker™ SW800: (a) 2.5 kph, (b) 5.0 kph, (c) 7.5 kph and (d) total steps during a standardized treadmill walking test. Closed black circles denote healthy controls; open circles denote adults with asthma.
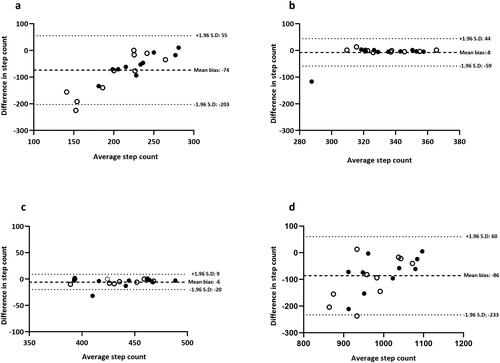
Table 2. Total step-count during standardized treadmill walking.
Phase 1: self-paced walking challenge
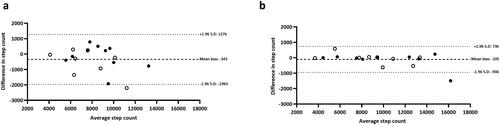
Visual inspection of the data indicated a significant outlier (>20% difference between devices and video-verified total manual step-count) and thus nineteen participants were included in the analysis (asthma: n = 9; healthy controls: n = 10). No significant difference was observed in video-verified total manual step-count between participants with asthma (1390 ± 168 steps) and healthy controls (1420 ± 131 steps) (P = 0.67) and therefore group data was pooled for analysis (mean: 1406 ± 146 steps). The iPhone® (upper and lower body) and Yamax Digiwalker™ SW800 pedometer had a percentage error of 1%, 0% and 1%, respectively. Although the strongest correlation (r = 0.99) and highest level of agreement was observed for iPhone® upper (mean bias: −4 steps, LOA: −49 to 41 steps), all devices provided an excellent step-count estimate during self-paced walking (). Proportional bias was not identified for any of the devices: Yamax Digiwalker™ SW800 pedometer (F = 0.02, P = 0.88), iPhone® lower body (F = 0.12, P = 0.74), iPhone® upper (F = 0.00, P = 0.96) ().
Figure 4. Bland Altman plots from 1-km self-paced walking test: (a) iPhone® upper body, (b) iPhone® lower body and (c) Yamax Digiwalker™ SW800. Closed black circles denote healthy controls; open circles denote adults with asthma.

Table 3. Total step-count during self-paced walking and habitual free-living conditions.
Phase 2: habitual free-living conditions
Over the seven-day period, visual inspection of the data indicated two significant outliers which corroborated with device noncompliance (as per logbook records) and thus eighteen participants were included in the analysis (asthma: n = 8; healthy controls: n = 10). No significant difference was observed in daily step-count (Yamax Digiwalker™ SW800 pedometer) between participants with asthma (7877 ± 2593 steps) and healthy controls (8809 ± 2326 steps) (P = 0.43) and therefore group data was pooled for analysis (mean: 8395 ± 2421 steps). No significant difference was observed between the Yamax Digiwalker™ SW800 pedometer and iPhone® (P = 0.10) and a strong correlation (r = 0.94) and acceptable agreement (mean bias: −343, LOA: −1963 to 1276 steps, error range: 4%) was observed. Importantly, analyzing data from the ‘best’ day of the week (i.e. highest compliance as per logbook records) resulted in a stronger correlation (r = 0.99) and even closer limits of agreement (mean bias: −105, LOA: −946 to 736 steps, error range: 0.5%) between devices (). Proportional bias was identified for the ‘best day’ (F = 5.18, P = 0.04) but not for ‘average days’ (F = 2.00, P = 0.18) ().
Discussion
The purpose of this pilot study was to evaluate the validity of modern smartphone technology for the assessment of physical activity in adults with and without asthma. In support of the initial hypothesis, our findings indicate that an in-built iPhone® pedometer provides an accurate step-count estimate during standardized treadmill and self-paced walking challenges and free-living conditions. The potential practical application of smartphone pedometers in the context of asthma management can therefore be considered three-fold. Firstly, as a simple approach to monitor or promote step-based physical activity during medical consultation (i.e. routine asthma review). Secondly, a reduction in physical activity may potentially represent an early marker of impending exacerbation that could be prevented through a self-management plan. Thirdly, as a research tool or outcome measure when utilized in clinical trials.
The measurement of physical activity is a complex task and thus the development and evaluation of scalable and low-cost methods of assessment remain an important research avenue (Citation22). In-keeping with previous comparable studies in healthy individuals (Citation13,Citation23) the present study confirms that the iPhone® (positioned on either the upper or lower body) provides an accurate step-count estimate during a standardized laboratory-based treadmill challenge at slow (2.5 kph), moderate (5.0 kph) and high (7.5 kph) walking speeds. Specifically, the iPhone® positioned on the lower body (i.e. trouser pocket) provided the closest agreement with video-verified manual total step-count, and importantly, either matched or outperformed the Yamax Digiwalker™ SW800 pedometer across all test stages.
It has previously been recommended that, to be considered ‘research-grade’ quality, pedometers should be within ±1% error range during standardized treadmill-based walking (Citation24). A key observation from this phase of the study was the significant step-count error (29%) for the Yamax Digiwalker™ SW800 pedometer at the slowest walking speed (2.5 kph). Many commercially available pedometers have a ‘acceleration dependent’ component, meaning they often underestimate step count at slower walking speeds (Citation25–27). In support of this concept, the error rate of the Yamax Digiwalker™ SW800 pedometer in the current study improved significantly during moderate (5.0 kph) and high (7.5 kph) walking speeds. Similarly, during self-paced walking (estimated average speed approximately 5.0 kph) all devices demonstrated excellent agreement with video-verified manual step-count. Importantly, regardless of positioning—on either the upper or lower body—the iPhone® accurately captured step count across all walking speeds. This highlights the potential benefit of using smartphone technology in patients recognized to avoid moderate to vigorous intensity physical activity (e.g. people with airways disease) (Citation28) or in cohorts with impaired mobility (i.e. elderly individuals and/or those with underlying musculoskeletal disorders) (Citation29).
The evaluation of pedometers in a real-world setting is important to determine device validity according to the specific demands (i.e. walking speeds) of daily tasks. A previous comprehensive assessment of 13 models of electronic pedometers, over a 24-h period, concluded that ± 10% variability was an acceptable error range during free-living conditions (Citation30). Specific to smartphone devices, Duncan et al. reported a significant under-estimation of steps when evaluating the iPhone® during free-living conditions in healthy individuals (±18% error range in comparison to the Actigraph GT3X) (Citation13). In contrast, the iPhone® exceeded the ±10% criterion (Citation30) (4% error range in comparison to the Yamax Digiwalker™ SW800 pedometer) in the current study and improved further when selecting the ‘best day’ according to participant compliance over a seven-day period (<1% error range). Although the disparity between studies remains unclear, our data indicate that, with optimal compliance (i.e. verifying time spent active with logbook records), the iPhone® pedometer can be considered a valid method to quantify habitual physical activity. Furthermore, using modern smartphone pedometers for this purpose may reduce the impact of participant reactivity given most people typically carry their device with them throughout the day. In addition, having reliable and accurate instruments to-hand, potentially helps patients to become powerful agents in behavioral self-management of their condition. Importantly, in-built smartphone pedometers report accurate scores, minute-by-minute, thus encouraging positive self-surveillance and habit formation.
Methodological considerations and future research
The present study has two key strengths. Firstly, the iPhone® pedometer was evaluated in both standardized and free-living conditions. Secondly, our results are directly compared to those of an asymptomatic group of healthy controls. It is important to recognize, however, that despite our encouraging findings, due to the current lack of a gold-standard comparator, evaluating the validity of any novel physical activity assessment method remains a challenge (Citation11). Although video-verified manual step-count was utilized to verify the accuracy of our data during laboratory and field-based walking challenges, a degree of measurement error is inevitable when evaluating step-count during habitual free-living conditions. In addition, the current study focused exclusively on the iPhone® pedometer (on the basis that it currently exceeds over half of the total smartphone UK market share (Citation31)) in a modest sample size and thus a logical extension of this work is to consider inter-device variability according to different smartphone brands/models and mobile operating systems in a large asthma cohort (i.e. factoring activity profiles according to disease severity and sub-types). Notwithstanding these limitations, our data provides the first evidence to suggest that in-built smartphone pedometers provide a reliable means of estimating step-count in adults with asthma. From a practical perspective, they may therefore be used in future prospective research trials and/or retrospective analysis of clinical records to determine the association between physical activity and asthma control and/or efficacy of physical activity promotion or behavior change programmes. This is particularly pertinent at present due to the SARS-CoV-2 pandemic where shielding and self isolation has had a negative impact on physical engagement for ‘high-risk’ groups and urgent need for remote monitoring.
Conclusion
In summary, our findings indicate that an in-built iPhone® pedometer offers a practical approach to physical activity assessment (i.e. valid step-count estimate under varying conditions) in adults with and without asthma. Future research is now required to further validate the precision of this approach and evaluate the efficacy and effectiveness of smartphone pedometers to monitor and promote physical activity when employed during medical consultation and/or clinical research trials.
Contribution statement
CR, ASK, IC, OJP designed the study. CR acquired the data. CR, ASK, OJP interpreted the data. CR, ASK, IC, JM, DP, OJP were involved in drafting and critical revision of manuscript; and final approval of the version to be published.
Guarantor statement
OJP confirms full responsibility for the content of the manuscript.
Acknowledgements
The authors wish to thank Professor Elizabeth Juniper for her permission to use the UK English version of the ACQ and AQLQ.
Declaration of interest
The authors have no real or perceived conflict of interest in respect of this manuscript.
References
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706.
- Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A, Heaven M, Fitzsimmons D, Bandyopadhyay A, Aftab C, Simpson CR, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14(1):113. doi:https://doi.org/10.1186/s12916-016-0657-8.
- Price D, Fletcher M, Van Der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24(1):1–10.
- Reddel HK, FitzGerald JM, Bateman ED, Bacharier LB, Becker A, Brusselle G, Buhl R, Cruz AA, Fleming L, Inoue H. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Resp J. 2019;53(6):1901046. doi:https://doi.org/10.1183/13993003.01046-2019.
- Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100(8):1307–1317. doi:https://doi.org/10.1016/j.rmed.2005.11.020.
- Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol. 2002;110(6 Suppl):S322–S328. doi:https://doi.org/10.1067/mai.2002.129966.
- Cordova-Rivera L, Gibson PG, Gardiner PA, McDonald VM. A systematic review of associations of physical activity and sedentary time with asthma outcomes. J Allergy Clin Immunol Pract. 2018;6(6):1968–1981. doi:https://doi.org/10.1016/j.jaip.2018.02.027.
- Eijkemans M, Mommers M, Draaisma JMT, Thijs C, Prins MH. Physical activity and asthma: a systematic review and meta-analysis. PloS One. 2012;7(12):e50775. doi:https://doi.org/10.1371/journal.pone.0050775.
- Xu M, Lodge CJ, Lowe AJ, Dharmage SC, Cassim R, Tan D, Russell MA. Are adults with asthma less physically active? A systematic review and meta-analysis. J Asthma. 2020;1–18. doi:https://doi.org/10.1080/02770903.2020.1810273.
- Pitta F, Troosters T, Probst V, Spruit M, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;27(5):1040–1055. doi:https://doi.org/10.1183/09031936.06.00064105.
- van Hees V. The challenge of assessing physical activity in populations. The Lancet. 2012;380(9853):1555–1556. doi:https://doi.org/10.1016/S0140-6736(12)61876-5.
- Statista. Number of smartphone users worldwide from 2014 to 2020 (in billions) eMarketer: eMarketer; 2016. https://www.statista.com/statistics/330695/number-of-smartphone-usersworldwide/.
- Duncan MJ, Wunderlich K, Zhao Y, Faulkner G. Walk this way: validity evidence of iphone health application step count in laboratory and free-living conditions. J Sports Sci. 2018;36(15):1695–1704. doi:https://doi.org/10.1080/02640414.2017.1409855.
- Price OJ, Tsakirides C, Gray M, Stavropoulos-Kalinoglou A. ACSM pre-participation health screening guidelines: a UK university cohort perspective. Med Sci Sports Exerc. 2019;51(5):1047–1053. doi:https://doi.org/10.1249/MSS.0000000000001868.
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King Dr. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:https://doi.org/10.1034/j.1399-3003.1999.14d29.x.
- Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115(5):1265–1270. doi:https://doi.org/10.1378/chest.115.5.1265.
- Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65(1):21–26. doi:https://doi.org/10.1136/thx.2009.118521.
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:https://doi.org/10.1164/rccm.201908-1590ST.
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin A-C, Plummer AL, Taylor DA; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi:https://doi.org/10.1164/rccm.9120-11ST.
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi:https://doi.org/10.1249/mss.0b013e31815a51b3.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;327(8476):307–310. doi:https://doi.org/10.1016/S0140-6736(86)90837-8.
- O’Reilly GA, Spruijt-Metz D. Current mHealth technologies for physical activity assessment and promotion. Am J Prev Med. 2013;45(4):501–507. doi:https://doi.org/10.1016/j.amepre.2013.05.012.
- Nolan M, Mitchell JR, Doyle-Baker PK. Validity of the Apple iPhone® /iPod Touch® as an accelerometer-based physical activity monitor: a proof-of-concept study. J Phys Act Health. 2014;11(4):759–769. doi:https://doi.org/10.1123/jpah.2011-0336.
- Tudor-Locke C, Sisson SB, Lee SM, Craig CL, Plotnikoff RC, Bauman A. Evaluation of quality of commercial pedometers. Can J Public Health. 2006;97(S1):S10–S16. doi:https://doi.org/10.1007/BF03405359.
- Beevi FHA, Miranda J, Pedersen CF, Wagner S. An evaluation of commercial pedometers for monitoring slow walking speed populations. Telemed J E Health. 2016;22(5):441–449. doi:https://doi.org/10.1089/tmj.2015.0120.
- Le Masurier GC, Tudor-Locke C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Med Sci Sports Exerc. 2003;35(5):867–871.
- Bassett DR, Jr., Ainsworth BE, Leggett SR, Mathien CA, Main JA, Hunter DC, Duncan GE. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28(8):1071–1077.
- Neale J, Orme MW, Majd S, Chantrell S, Singh SJ, Bradding P, Green RH, Evans RA. A comparison of daily physical activity profiles between adults with severe asthma and healthy controls. Eur Resp J. 2020;56(1):1902219. doi:https://doi.org/10.1183/13993003.02219-2019.
- Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health. 2012;9(7):1036–1048. doi:https://doi.org/10.1123/jpah.9.7.1036.
- Schneider PL, Crouter SE, Bassett DR. Pedometer measures of free-living physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36(2):331–335.
- Statista. Market share held by mobile operating systems in the United Kingdom (UK) from December 2011 to December 2019. https://www.statista.com/statistics/262179/market-share-held-by-mobile-operating-systems-in-the-united-kingdom/.