Abstract
Objective
PALLADIUM and IRIDIUM studies demonstrated efficacy and safety of indacaterol/mometasone (IND/MF) versus salmeterol/fluticasone (SAL/FLU). This post hoc analysis of pooled data from PALLADIUM and IRIDIUM studies evaluated efficacy and safety of IND/MF versus SAL/FLU in Asian patients with inadequately controlled asthma.
Methods
Both studies were Phase III, 52-week, randomized, double-blind, active-controlled that included patients with predicted pre-bronchodilator FEV1 (PALLADIUM, ≥50%–<85%; IRIDIUM, <80%), ACQ-7 score ≥1.5. Patients treated with IND/MF high- (150/320 μg) or medium-dose (150/160 μg) or SAL/FLU high-dose (50/500 μg) were included. Lung function, asthma control, and asthma exacerbations were evaluated.
Results
In total, 323 patients (IND/MF high-dose, n = 107; IND/MF medium-dose, n = 106, SAL/FLU high-dose, n = 110) were included. IND/MF high-dose showed improvement in trough FEV1 versus SAL/FLU high-dose at Weeks 26 (Δ, 42 mL; 95% CI, −35 to 120 mL), and 52 (Δ, 41 mL; 95% CI, −37 to 120 mL). IND/MF high-dose exhibited numerically greater improvement in ACQ-7 score versus SAL/FLU high-dose at Weeks 26 (Δ, −0.215; 95% CI, −0.385 to −0.044) and 52 (Δ, −0.176; 95% CI, −0.348 to −0.005). Improvements in trough FEV1 and ACQ-7 score were comparable between IND/MF medium-dose and SAL/FLU high-dose. IND/MF high- and medium-dose showed reductions in moderate or severe (45%; 30%), severe (39%; 41%), and all (9%; 25%) exacerbations, respectively, versus SAL/FLU high-dose over 52 weeks. All treatments were well tolerated.
Conclusions
Once-daily, single-inhaler IND/MF high-dose improved lung function with better asthma control, reduced asthma exacerbations with comparable safety versus twice-daily SAL/FLU high-dose. IND/MF medium-dose showed comparable outcomes to SAL/FLU high-dose at a reduced steroid dose.
Introduction
Asthma is a major global health problem, characterized by chronic inflammation of the entire bronchial tree that seriously affects the patient’s quality of life when uncontrolled (Citation1,Citation2). In Asia, the prevalence of asthma is increasing and has been reported to be in a range of 0.7% to 15.3% (Citation3). The primary approach of asthma management is to achieve and maintain disease control by reducing the symptoms and minimizing the risk of future exacerbations (Citation1,Citation4,Citation5).
Inhaled corticosteroid (ICS) therapy is the cornerstone of asthma management (Citation2,Citation6). In line with the global guidelines (Citation2), Asian guidelines (Japan (Citation7), Korea (Citation8), China (Citation9,Citation10), and India (Citation11)) recommend the addition of a long-acting β2-agonist (LABA) to medium- or high-dose ICS therapy as the preferred controller treatment for patients with inadequately controlled asthma on ICS alone or on a LABA/ICS low-dose combination.
The efficacy and safety of LABA/ICS combination in patients with inadequately controlled asthma on ICS therapy alone is also evident from literature (Citation1,Citation6,Citation12,Citation13). However, asthma control remains an elusive challenge despite the availability of current LABA/ICS therapies (Citation14,Citation15). There are numerous causes of poor asthma control, including poor medication adherence, poor inhaler technique, and inadequate medication dosing (Citation16).
Administration of LABA in combination with ICS in a single inhaler improved asthma management, cost effectiveness and better adherence (Citation17–20). Literature advocates that LABA provides symptom relief and reduces airflow limitation and thus, improves adherence of the single inhaler LABA/ICS compared with separate inhalers in patients with asthma (Citation20). It is evident that once-daily (o.d.) regimens might lead to better adherence compared with twice-daily (b.i.d.) regimens, and patients with asthma who showed improved adherence to the medications were associated with fewer exacerbations (Citation21,Citation22).
Once-daily fixed-dose combination of indacaterol acetate (IND, a LABA) and mometasone furoate (MF, an ICS), as high-dose (150/320 μg), medium-dose (150/160 μg), and low-dose (150/80 μg), delivered via the Breezhaler® device, is approved for maintenance treatment of patients with asthma. The efficacy and safety of IND/MF for management of asthma is well established in published studies (Citation23–25). The pooled analysis of PALLADIUM and IRIDIUM studies showed improvement in lung function, reduction in asthma exacerbations, and comparable asthma control with IND/MF high-dose and IND/MF medium-dose o.d. versus the standard-of-care treatment salmeterol xinafoate/fluticasone propionate (SAL/FLU) high-dose (50/500 μg) b.i.d. in patients with inadequately controlled asthma (Citation25).
This post hoc analysis of the pooled data from PALLADIUM (Citation23) and IRIDIUM (Citation24) studies in Asian patients with inadequately controlled asthma, assessed the efficacy and safety of IND/MF high- and medium-dose o.d. (via Breezhaler®) versus SAL/FLU high-dose b.i.d. (via Diskus®). This analysis also explored for any ethnic differences with the overall population from both studies.
Methods
Study design
Both the PALLADIUM (NCT02554786) (Citation23) and IRIDIUM (NCT02571777) (Citation24) studies were 52-week, multicenter, double-blind, triple- or double-dummy, parallel-group, active-controlled studies in patients with inadequately controlled asthma. The PALLADIUM study evaluated the efficacy and safety of IND/MF high- or medium-dose o.d. or MF high-dose (800 μg [400 μg b.i.d.]) or MF medium-dose (400 μg) o.d. and SAL/FLU high-dose b.i.d. in patients with inadequately controlled asthma, despite treatment with medium- or high-dose ICS or LABA/ICS low-dose. The IRIDIUM study evaluated the efficacy and safety of indacaterol acetate/glycopyrronium bromide/mometasone furoate high-dose o.d. (IND/GLY/MF; 150/50/160 μg) or medium-dose o.d. (150/50/80 μg) or IND/MF high- or IND/MF medium-dose o.d. and SAL/FLU high-dose b.i.d. in patients with inadequately controlled asthma despite treatment with LABA/ICS medium- or high-dose. The details of the study design, and methodology are described in the original manuscripts (Citation23,Citation24).
This pooled analysis included Asian patients from the PALLADIUM (Citation23) and IRIDIUM (Citation24) studies who received IND/MF high- or medium-dose o.d. or SAL/FLU high-dose b.i.d. for 52 weeks.
All data generated in this pooled analysis were based on previously published studies (PALLADIUM (Citation23) and IRIDIUM (Citation24)), which were approved by the Independent Ethics Committee or Institutional Review Boards and were conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent for inclusion in the studies as reported previously (Citation23,Citation24).
Patients
Patients with inadequately controlled asthma from China, Japan, Korea, Philippines, Thailand, and Vietnam, who were included in the IRIDIUM (Citation23) and PALLADIUM (Citation24) studies, were analyzed in this pooled analysis. Details of the inclusion and exclusion criteria are published in the original manuscripts (Citation23,Citation24). Patients aged ≥12 to ≤75 years (PALLADIUM (Citation23)) and ≥18 to ≤75 years (IRIDIUM (Citation24)) with a documented diagnosis of asthma and predicted pre-bronchodilator forced expiratory volume in 1 s (FEV1) (≥50% to <85% for PALLADIUM (Citation23) and <80% for IRIDIUM (Citation24)) with an Asthma Control Questionnaire-7 (ACQ-7) score ≥1.5, were included. IRIDIUM study included patients with a documented history of ≥1 asthma exacerbations that required systemic corticosteroid (SCS) treatment, emergency room (ER) visit, or hospitalization in the 12 months prior to screening (Citation24). Patients with an asthma attack/exacerbations requiring SCS or hospitalization, who inhaled tobacco products or had a > 10 pack-years smoking history, and an ER visit within 6 weeks of screening, were excluded from both studies. Patients with a history of chronic lung disease other than asthma, and clinically significant comorbidity or laboratory abnormality at the run-in visit, were also excluded (Citation23,Citation24).
Assessments
Trough FEV1 at Weeks 26 and 52, post-dose FEV1 at Day 1, peak expiratory flow (PEF) over Week 52, change in ACQ-7 score (Citation26,Citation27) over 52 weeks from baseline, and ACQ-7 responders (minimal clinically important difference [MCID] achievement; >0.5-point improvement from baseline in ACQ-7 scores) (Citation28,Citation29) at Weeks 26 and 52, were assessed with IND/MF high-dose and IND/MF medium-dose versus SAL/FLU high-dose. This pooled analysis also assessed asthma exacerbations (severe, moderate or severe, all [mild, moderate, severe]) and rescue medication use over 52 weeks with all treatment groups. The definitions of severe, moderate and mild asthma exacerbations are provided in supplementary material (). Patient’s safety over 52 weeks was also assessed.
Table 1. Baseline demographics and clinical characteristics (pooled Asian population from PALLADIUM and IRIDIUM studies; full analysis set).
Statistical analysis
Trough FEV1 and ACQ-7 scores were analyzed using a mixed model for repeated measures (MMRM). The MMRM contained treatment, study, visit, and treatment-by-visit interaction as fixed effects, with baseline FEV1/ACQ-7 measurement, baseline-by-visit interaction, FEV1 prior to inhalation and FEV1 within 15 to 30 min post-inhalation of salbutamol/albuterol (components of short acting β2-agonist [SABA] reversibility) as covariates, and center as a random effect. The mean morning and evening PEF over 52 weeks was assessed using analysis of covariance (ANCOVA) and MMRM models. The ANCOVA contained treatment and study, as fixed effects, with appropriate baseline value, FEV1 prior to inhalation and FEV1 within 15 to 30 min post-inhalation of salbutamol/albuterol (components of SABA reversibility) as covariates, and center as a random effect. ACQ responders were analyzed using a logistic regression model that included treatment, study, baseline ACQ-7, visit, treatment, visit interaction, baseline ACQ-7 visit interaction, FEV1 prior to inhalation of salbutamol/albuterol and FEV1 15 to 30 min after inhalation of salbutamol/albuterol. Annualized rates of asthma exacerbations were analyzed using a generalized linear model assuming the negative binomial distribution. The model included treatment, study, number of asthma exacerbations in the 12 months prior to screening, FEV1 prior to inhalation and FEV1 15 to 30 min after inhalation of salbutamol/albuterol. Log (exposure time in years) was used as an offset. Rescue medication endpoints including daily, daytime and nighttime number of puffs of rescue medication, and rescue medication-free days were analyzed using an ANCOVA model and MMRM similar to that used for mean morning/evening PEF. All efficacy analyses were assessed using the full analysis set (FAS), which included patients who were assigned a randomization number and had received at least one dose of study medication. Safety was evaluated using the safety analysis set, which included patients who received at least a single dose of study medication. Adverse events (AEs) were reported by using MedDRA Version 22.0. Analyses were performed using SAS Version 9.4. All p values from this pooled analysis should be considered as nominal, with all treatment comparisons being descriptive. Additional details of statistical analysis procedures are provided in supplementary material ().
Table 2. Incidence of adverse events by preferred term occurring in IND/MF high-dose, IND/MF medium-dose and SAL/FLU high-dose treatment groups over 52 weeks.
Results
Baseline demographics and clinical characteristics
A total of 323 patients (PALLADIUM, N = 169; IRIDIUM, N = 154) were included in this pooled analysis (IND/MF high-dose, n = 107 [PALLADIUM, 56; IRIDIUM, 51]; IND/MF medium-dose, n = 106 [PALLADIUM, 55; IRIDIUM, 51]; SAL/FLU high-dose, n = 110 [PALLADIUM, 58; IRIDIUM, 52]). Baseline demographic and clinical characteristics are provided in .
Lung function
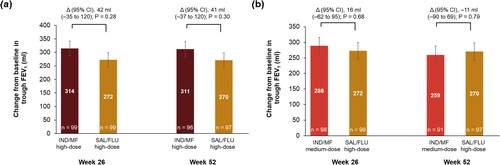
IND/MF high-dose showed numerical improvement in trough FEV1 compared with SAL/FLU high-dose at Week 26 (least square mean difference [Δ], 42 ml; 95% CI, −35 to 120 ml), and Week 52 (Δ, 41 ml; 95% CI, −37 to 120 ml; ) from baseline. The improvement from baseline in trough FEV1 with IND/MF medium-dose compared with SAL/FLU high-dose was similar at Week 26 (Δ, 16 ml; 95% CI, −62 to 95 ml) and Week 52 (Δ, −11 ml; 95% CI, −90 to 69 ml; ).
Figure 1. Improvement in trough FEV1 with (a) IND/MF high-dose and (b) IND/MF medium-dose compared with SAL/FLU high-dose at Weeks 26 and 52.
Data presented as LS mean ± SE, error bars represent SE values.
Participants received IND/MF high-dose (150/320 μg) o.d.; or IND/MF medium-dose (150/160 μg) o.d.; or SAL/FLU high-dose (50/500 μg) b.i.d.
n, number of patients analyzed.
Δ, LS mean treatment difference; b.i.d., twice-daily; FEV1, forced expiratory volume in 1 second; IND/MF, indacaterol acetate/mometasone furoate; LS, least square; o.d., once-daily; SAL/FLU, salmeterol xinafoate/fluticasone propionate
Improvement in post-dose FEV1 was seen with all treatments as early as 5 min and was maintained until 15 min, 30 min, and 1 h of drug administration on Day 1 (Supplementary figure; ).
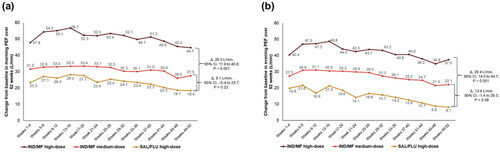
Both doses of IND/MF reported numerically greater morning and evening PEF values at each evaluated time point from Weeks 1 to 52 compared to SAL/FLU high-dose. IND/MF high-dose showed greater change in mean morning PEF (Δ, 26.3 L/min, 95% CI, 11.9 to 40.8 L/min; ) and evening PEF (Δ, 29.4 L/min, 95% CI, 14 to 44.7 L/min; ) compared with SAL/FLU high-dose over 52 weeks. However, the improvement in both morning and evening PEF was comparable between IND/MF medium-dose and SAL/FLU high-dose ().
Figure 2. Change in (a) morning PEF (b) evening PEF with IND/MF high- and medium-dose compared with SAL/FLU high-dose from Weeks 1‒52.
Data presented as LS mean.
Participants received IND/MF high-dose (150/320 μg) o.d.; or IND/MF medium-dose (150/160 μg) o.d.; or SAL/FLU high-dose (50/500 μg) b.i.d.
Δ, LS mean treatment difference; b.i.d., twice daily; IND/MF, indacaterol acetate/mometasone furoate; LS, least square; o.d., once daily; PEF, peak expiratory flow; SAL/FLU, salmeterol xinafoate/fluticasone propionate
Asthma control
Change in ACQ-7 score from baseline was observed with all treatment arms at each time point from baseline to Week 52 (Supplementary material, ). IND/MF high-dose exhibited greater improvement in ACQ-7 score from baseline compared with SAL/FLU high-dose at Week 26 (Δ, −0.215; 95% CI, −0.385 to −0.044, ). Similarly, IND/MF medium-dose showed numerical improvements in ACQ-7 score versus SAL/FLU high-dose, from baseline at Week 26 (Δ, −0.128, 95% CI, −0.298 to 0.042, ). The change in ACQ-7 score from baseline with IND/MF high-dose (Δ, −0.176; 95% CI, −0.348 to −0.005) and IND/MF medium-dose (Δ, −0.067; 95% CI, −0.238 to 0.105) versus SAL/FLU high-dose was sustained up to Week 52 (Supplementary material, ).
Figure 3. (a) Change from baseline in ACQ-7 score with IND/MF high-dose and IND/MF medium-dose versus SAL/FLU at Week 26 and (b) proportion of patients achieved MCID in ACQ-7 score with IND/MF high-dose and IND/MF medium-dose versus SAL/FLU from baseline at Week 26.
Data presented as LS mean (95% CI) for .
Participants received IND/MF high-dose (150/320 μg) o.d.; or IND/MF medium-dose (150/160 μg) o.d.; or SAL/FLU high-dose (50/500 μg) b.i.d.
n, number of patients analyzed.
ACQ, Asthma Control Questionnaire; b.i.d., twice daily; IND/MF, indacaterol acetate/mometasone furoate; LS, least square; o.d., once daily; OR, odds ratio; SAL/FLU, salmeterol xinafoate/fluticasone propionate.
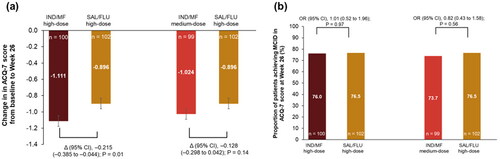
Proportion of patients achieving MCID in ACQ-7 score was comparable (>70%) in patients treated with IND/MF high-dose (odds ratio [OR], 1.01; 95% CI, 0.52 to 1.96) and medium-dose (OR, 0.82; 95% CI, 0.43 to 1.58) versus SAL/FLU high-dose at Week 26 (). Similar results in responder analysis for ACQ-7 score (>80%) were observed at Week 52 for all the treatment arms (Supplementary material, ).
Figure 4. Reductions in annualized rate of asthma exacerbations with IND/MF high- and medium-dose compared with SAL/FLU high-dose over 52 weeks.
Data presented as annualized rate (95% CI); error bars represent CI values.
Participants received IND/MF high-dose (150/320 μg) o.d.; or IND/MF medium-dose (150/160 μg) o.d.; or SAL/FLU high-dose (50/500 μg) b.i.d.
n, number of patients analyzed.
b.i.d., twice daily; IND/MF, indacaterol acetate/mometasone furoate; o.d., once daily; RR, rate ratio; SAL/FLU, salmeterol xinafoate/fluticasone propionate
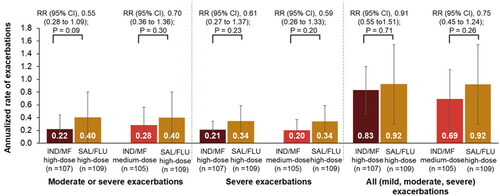
Asthma exacerbations
IND/MF high-dose showed 45% reduction in moderate or severe exacerbations (rate ratio [RR], 0.55; 95% CI, 0.28 to 1.09), 39% reduction in severe exacerbations (RR, 0.61; 95% CI, 0.27 to 1.37), and 9% reduction in all exacerbations (RR, 0.91; 95% CI, 0.55 to 1.51) compared with SAL/FLU high-dose over 52 weeks (). Similarly, IND/MF medium-dose showed 30% (RR, 0.70; 95% CI, 0.36 to 1.36), 41% (RR, 0.59; 95% CI, 0.26 to 1.33), and 25% (RR, 0.75; 95% CI, 0.45 to 1.24) reductions in moderate or severe, severe, and all exacerbations, respectively, versus SAL/FLU high-dose over 52 weeks ().
Rescue medication use
IND/MF high- and medium-dose showed comparable reduction in rescue medication use (reduction in number of puffs of rescue medication in daily, daytime, and nighttime use) versus SAL/FLU high-dose over 52 weeks (Table S3). The rescue medication-free days were comparable in patients receiving IND/MF high- and medium-dose versus SAL/FLU high-dose after 52 weeks of treatment.
Safety
The incidence of AEs was comparable between IND/MF high- (82, 76.6%) and medium-dose (75, 71.4%) and SAL/FLU high-dose (91, 83.5%) treatment groups over 52 weeks (). Asthma was the most frequently reported AE in all three treatment groups, followed by upper respiratory tract infection and nasopharyngitis. The incidence of serious AEs (SAEs) was reported to be low, and was comparable between treatment groups (IND/MF high-dose [10, 9.3%], IND/MF medium-dose [7, 6.7%], SAL/FLU high-dose [9, 8.3%]). Asthma was the most frequently reported SAE in all three treatment groups. The incidence of AEs suspected to be study drug-related, and patients with at least one AE leading to permanent discontinuation of study drug was generally low and similar across treatment groups. No deaths were reported, and all treatments were well tolerated. The AEs by preferred term, occurring in ≥1.0% of patients in any treatment group are presented in supplementary material (Table S4).
Discussion
In this post hoc analysis of the pooled data from PALLADIUM and IRIDIUM studies in Asian patients with inadequately controlled asthma, despite treatment with high-dose ICS or LABA/ICS medium- to high-dose combinations, we sought to determine the efficacy and safety of IND/MF high-dose (150/320 μg) o.d. versus SAL/FLU high-dose (50/500 μg) b.i.d. (at comparable ICS doses), and IND/MF medium-dose (150/160 μg) o.d. versus SAL/FLU high-dose (50/500 μg) b.i.d. (at lower ICS dose). This pooled analysis reported numerically greater improvements in lung function, greater improvement in asthma control, and greater reduction in annualized rates of asthma exacerbations with comparable safety profile with IND/MF high-dose versus SAL/FLU high-dose.
At a lower ICS dose, IND/MF medium-dose showed comparable improvements in lung function, asthma exacerbation reduction and asthma control with comparable safety profiles versus SAL/FLU high-dose. This indicates that IND/MF medium-dose might allow for treatment with minimum required dose of ICS, as recommended by guidelines, considering the AEs such as pneumonia, osteoporosis and adrenal insufficiency, caused due to long-term treatment with high- dose of ICS.
However, at a comparable ICS dose, the magnitude of improvement for some endpoints such as trough FEV1, ACQ-7 score, morning and evening PEF, and asthma exacerbations were greater with IND/MF high-dose versus SAL/FLU high-dose.
The baseline demographics and clinical characteristics were comparable between Asian population included in this pooled analysis and global population pooled from PALLADIUM and IRIDIUM studies (Citation25). However, patients in Asian sub-group had greater duration of asthma (16.8–18.6 years vs 15.9–16.8 years) with lower baseline ACQ-7 score (2.2 vs 2.4) compared with global pooled population (Citation25).
IND/MF high-dose reported 42 ml and 41 ml improvement in trough FEV1 compared with SAL/FLU high-dose at Weeks 26 and 52, respectively. IND/MF medium-dose reported comparable improvement in trough FEV1 (16 ml at Week 26, and −11 ml at Week 52) versus SAL/FLU high-dose. The results of this analysis are consistent with the outcome of the global pooled analysis (Citation25), where IND/MF high-dose (43 ml and 51 ml at Weeks 26 and 52, respectively) and IND/MF medium-dose (28 ml and 19 ml at Weeks 26 and 52, respectively) showed improvement in trough FEV1 compared with SAL/FLU high-dose. Similar improvements in trough FEV1 were also reported in PALLADIUM study with IND/MF high-dose versus SAL/FLU high-dose (36 ml and 48 ml at Weeks 26 and 52, respectively) (Citation23). The sub-analysis of the COSMOS study in Asian patients reported a 29 ml treatment difference in trough FEV1 with high-dose budesonide/formoterol (BUD/FORM) maintenance and reliever therapy (titrated to 320/9 μg b.i.d.) compared with SAL/FLU (titrated to 50/500 μg b.i.d.) in patients with uncontrolled asthma aged ≥16 years (Citation30). At a lower ICS dose, the improvement in trough FEV1 with IND/MF medium-dose was comparable with SAL/FLU high-dose at Week 52. However, greater improvements were observed with IND/MF high-dose versus SAL/FLU high-dose.
IND/MF high-dose reported clinically meaningful improvements in morning and evening PEF compared with SAL/FLU high-dose (Citation31). The PEF improvements in this Asian population are reported to be higher in comparison with the global pooled population (morning, 14.5 L/min; evening, 11 L/min) (Citation25), and PALLADIUM study population (morning, 13.8 L/min; evening, 9.1 L/min) (Citation23). These improvements in PEF with IND/MF were greater than those reported by Bousquet et al. where BUD/FORM as maintenance and reliever therapy 320/9 μg b.i.d. (mean BUD dose, 792 μg/day) showed a treatment difference of −0.8 L/min in morning PEF and 1.4 L/min in evening PEF versus SAL/FLU 50/500 μg b.i.d. (mean FLU dose, 1000 μg/day) in patients with uncontrolled asthma (Citation32). In a systematic review involving mixed treatment comparison of randomized controlled trials (n = 18), vilanterol/fluticasone furoate (VI/FF) 22/184 μg (predispensed dose of 25/200 μg) showed 11.3 L/min improvement in morning PEF versus SAL/FLU 50/500 μg in patients with uncontrolled asthma (Citation33); similar to values for IND/MF 150/320 μg versus SAL/FLU 50/500 μg reported in the global pooled population (Citation25). The results of our post hoc analysis suggest that single-inhaler IND/MF o.d. might be an appropriate alternative for improving lung function in patients with inadequately controlled asthma with high-dose ICS or other LABA/ICS formulations.
Furthermore, a dose-dependent improvement was observed in lung function for IND/MF high- and medium-dose in Asian patients, as observed in global population (Citation25). Results from a previous clinical study of different MF doses in patients with mild asthma also revealed dose-dependent improvement in trough FEV1 with different MF doses (Citation34).
In this analysis, >70% patients at Week 26, and >80% at Week 52, from all treatment groups exhibited MCID (0.5-point increase from baseline) in ACQ-7 score. IND/MF high- and medium-dose showed numerically greater improvement in ACQ-7 score from baseline at Weeks 26 (−0.215 to −0.128) and 52 (−0.176 to −0.067) versus SAL/FLU high-dose. These changes in ACQ-7 score are greater than those achieved in the global population (−0.015 to −0.091) (Citation25). Similar improvements in ACQ-7 score were also reported between IND/MF high-dose and SAL/FLU high-dose (−0.054) in the PALLADIUM study (Citation23). In addition, the sub-analysis of the COSMOS study in Asian population (Citation30) and Bosquet et al. (Citation32) showed a mean change of −0.047 (52 weeks) and −0.02 (26 weeks) in ACQ-5 score from baseline in favor of BUD/FORM compared with SAL/FLU high-dose. Greater improvements in ACQ-7 score and higher proportion of ACQ responders in this analysis compared with global population might be due to greater improvement in the morning PEF and reduction in asthma exacerbations in this population, which is an alternative way of assessing asthma control. These results might prove the beneficial effect of IND/MF in terms of asthma control in Asian patients with inadequately controlled asthma.
The annualized rate of asthma exacerbations (moderate or severe, severe, all) were reduced with both doses of IND/MF (9% to 45%) versus SAL/FLU high-dose in Asian patients with asthma compared with global pooled population (6% to 26%) (Citation25), and patients included in the PALLADIUM study (7% to 11%) (Citation23). The reductions in asthma exacerbations reported in this analysis are consistent with those reported in the sub-analysis of the COSMOS Study in Asian patients (Citation30).
The use of rescue medications and rescue medication-free days were comparable between the three treatment arms, and in alignment with the global pooled population (Citation25). This result implies that IND/MF might provide better asthma control with similar use of rescue medication compared with SAL/FLU. The results of this analysis can be considered to be in line with the Japanese asthma guideline that emphasizes the reduction in the number of inhalations with improved efficacy with the use of single-inhaler LABA/ICS therapy (Citation7).
Safety profile was comparable across the three treatments. All treatments were well tolerated with no new or unexpected safety findings. The safety results of this analysis are consistent with the global population (Citation25), PALLADIUM study (Citation23), and the published LABA/ICS studies (Citation30,Citation32,Citation35).
This pooled analysis is based on a subset of patients (n = 323) compared with the global pooled analyses of PALLADIUM and IRIDIUM studies (N = 3154) (Citation25), and therefore was not powered to detect differences between treatment. The smaller sample size may also lead to greater uncertainty in treatment effects and potentially deviations from the global population in the results of this subpopulation on some endpoints. All treatment comparisons assessed in this analysis were descriptive.
Conclusions
To best of our knowledge, this is the first pooled analysis from large randomized controlled studies in Asian patients to report consistent efficacy benefits (improvement in lung function, reduction in exacerbations with better asthma control, and comparable rescue medication use) and comparable safety profiles with any LABA/ICS o.d. compared with widely used SAL/FLU high-dose b.i.d. At a similar ICS dose, single-inhaler IND/MF high-dose o.d. showed numerically greater improvement in lung function with better asthma control, reduction in asthma exacerbations versus SAL/FLU high-dose b.i.d. These outcomes were comparable between IND/MF medium-dose o.d. (at lower ICS dose) and SAL/FLU high-dose b.i.d. in Asian patients with inadequately controlled asthma, at a reduced steroid dose. All treatments were well tolerated with acceptable safety profiles.
Contributorship statement
Yasuhiro Gon and Yoichi Nakamura supervized this study. Tsuyoshi Ishii and Ivan Nikolaev conducted conceptualization of this study. David Lawrence was responsible for the analysis of the data. Dong Wang and Kazuya Sumi performed validation of this study. All authors contributed to the interpretation of the results. The authors contributed to the preparation of the manuscript draft, along with critical review and approval of manuscript for submission to the journal. All authors contributed to intellectual content of the manuscript and approved for publication.
Abbreviations:
| Δ | = | least square mean treatment difference |
| ACQ | = | Asthma Control Questionnaire |
| AE | = | adverse event |
| ANCOVA | = | analysis of covariance |
| b.i.d. | = | twice-daily |
| BDP | = | beclomethasone dipropionate |
| BUD | = | budesonide |
| CI | = | confidence interval |
| ER | = | emergency room |
| FAS | = | full analysis set |
| FEV1 | = | forced expiratory volume in 1 second |
| FF | = | fluticasone furoate |
| FLU | = | fluticasone propionate |
| FORM | = | formoterol |
| GINA | = | Global Initiative for Asthma |
| HR | = | hazard ratio |
| ICS | = | inhaled corticosteroid |
| IND | = | indacaterol acetate |
| LABA | = | long-acting β2-agonist |
| MCID | = | minimal clinically important difference |
| MF | = | mometasone furoate |
| MMRM | = | mixed model for repeated measures |
| o.d. | = | once daily |
| OR | = | odds ratio |
| PEF | = | peak expiratory flow |
| RR | = | rate ratio |
| SAE | = | serious adverse event |
| SAL | = | salmeterol xinafoate |
| SCS | = | systemic corticosteroid |
| VI | = | vilanterol |
Supplementary_material__PALLADIUM-IRIDIUM_pooled_analysis.docx
Download MS Word (340.5 KB)Acknowledgements
Under the direction of authors, Rabi Narayan Panigrahy and Vatsal Vithlani (professional medical writers; Novartis) assisted in the preparation of this article in accordance with the third edition of Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Declaration of interest
Authors received no compensation related to development of the manuscript. Yasuhiro Gon reports receiving personal fees from Novartis, GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim, outside the submitted work. Yoichi Nakamura received lecture fees (honoraria) from AstraZeneca, GlaxoSmithKline, Mylan, Novartis, and Sanofi. Tsuyoshi Ishii, Dong Wang, and Kazuya Sumi are employees of Novartis Pharma K.K. David Lawrence and Ivan Nikolaev are employees of Novartis Pharma AG.
Data sharing
Novartis is committed to sharing access to patient-level data and supporting documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
Additional information
Funding
References
- Aalbers R, Vogelmeier C, Kuna P. Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respir Med. 2016;111:1–7. doi:https://doi.org/10.1016/j.rmed.2015.11.002.
- Global Initiative for Asthma (GINA). 2020. Global Strategy for asthma management and prevention. Updated 2020. Available from: https://ginasthma.org/gina-reports/. Accessed: 30 September 2020.
- Song WJ, Kang MG, Chang YS, Cho SH. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy. 2014;4(2):75–85. doi:https://doi.org/10.5415/apallergy.2014.4.2.75.
- Devillier P, Humbert M, Boye A, Zachgo W, Jacques L, Nunn C, West S, Nicholls A, Antoun Z, Spinu L, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting beta2-agonists (ICS/LABA) in patients with uncontrolled asthma: An open-label, randomized, controlled trial. Respir Med. 2018;141:111–120. doi:https://doi.org/10.1016/j.rmed.2018.06.009.
- Balki A, Balamurugan S, Bardapurkar S, Dalal S, Singh A, Singh BP, Vaidya A, Gogtay JA. Comparison of fluticasone/formoterol with budesonide/formoterol pMDI in adults with moderate to severe persistent asthma: Results from a 12-week randomized controlled trial. Pulm Pharmacol Ther. 2018;48:28–36. doi:https://doi.org/10.1016/j.pupt.2017.09.001.
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010;(5):CD005535. doi:https://doi.org/10.1002/14651858.CD005535.pub2.
- Nakamura Y, Tamaoki J, Nagase H, Yamaguchi M, Horiguchi T, Hozawa S, Ichinose M, Iwanaga T, Kondo R, Nagata M, et al. Japanese guidelines for adult asthma 2020. Allergol Int. 2020;69(4):519–548. doi:https://doi.org/10.1016/j.alit.2020.08.001.
- Kim DK, Park YB, Oh YM, Jung KS, Yoo JH, Yoo KH, Kim KH, Steering and Scientific Committee of Asthma Study Group and Guideline Control Committee in The Korean Academy of Tuberculosis and Respiratory Diseases (KATRD). Korean Asthma Guideline 2014: Summary of Major Updates to the Korean Asthma Guideline 2014. Tuberc Respir Dis (Seoul). 2016;79(3):111–120. doi:https://doi.org/10.4046/trd.2016.79.3.111.
- Asthma Workgroup, Chinese Thoracic Society, Chinese Societ of General Practitioners. Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version). J Thorac Dis. 2013;5:667–677.
- Respiratory Diseases Branch of the Chinese Medical Association Bronchial asthma therapeutic guidelines. Definition, diagnosis, treatment, education and management of bronchial asthma. Chin J Tuberc Respir Dis. 2003;26:132–138. (Chinese).
- Agarwal R, Dhooria S, Aggarwal AN, Maturu VN, Sehgal IS, Muthu V, Prasad KT, Yenge LB, Singh N, Behera D, et al. Guidelines for diagnosis and management of bronchial asthma: Joint ICS/NCCP (I) recommendations. Lung India. 2015;32(Suppl 1):S3–S42. doi:https://doi.org/10.4103/0970-2113.154517.
- Dal Negro RW, Bonadiman L, Turco P. Fluticasone furoate/Vilanterol 92/22μg once-a-day vs Beclomethasone dipropionate/Formoterol 100/6 µg b.i.d.: a 12-month comparison of outcomes in mild-to-moderate asthma. Multidiscip Respir Med. 2018;13(1):18. doi:https://doi.org/10.1186/s40248-018-0131-x.
- Averell CM, Stanford RH, Laliberte F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2021;58(1):102–110. doi:https://doi.org/10.1080/02770903.2019.1663429.
- Buhl R, Heaney LG, Loefroth E, Larbig M, Kostikas K, Conti V, Cao H. One-year follow up of asthmatic patients newly initiated on treatment with medium- or high-dose inhaled corticosteroid-long-acting β2-agonist in UK primary care settings. Respir Med. 2020;162:105859. doi:https://doi.org/10.1016/j.rmed.2019.105859.
- Schatz M, Meckley LM, Kim M, Stockwell BT, Castro M. Asthma exacerbation rates in adults are unchanged over a 5-year period despite high-intensity therapy. J Allergy Clin Immunol Pract. 2014;2(5):570–574. doi:https://doi.org/10.1016/j.jaip.2014.05.002.
- Shimizu Y, Shiobara T, Arai R, Chibana K, Takemasa A. Real-life effectiveness of fluticasone furoate/vilanterol after switching from fluticasone/salmeterol or budesonide/formoterol therapy in patients with symptomatic asthma: Relvar Ellipta for Real Asthma Control Study (RERACS study). J Thorac Dis. 2020;12(5):1877–1883. doi:https://doi.org/10.21037/jtd-19-3913.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Long-acting beta(2)-agonist and inhaled corticosteroid combination therapy for adult persistent asthma: systematic review of clinical outcomes and economic evaluation. CADTH Technol Overv. 2010;1:e0120.
- Rogala B, Majak P, Gluck J, Dębowski T. Asthma control in adult patients treated with a combination of inhaled corticosteroids and long‑acting β2‑agonists: a prospective observational study. Pol Arch Intern Med. 2017;127(2):100–106. doi:https://doi.org/10.20452/pamw.3899.
- Price D, David-Wang A, Cho SH, Ho JC, Jeong JW, Liam CK, Lin J, Muttalif AR, Perng DW, Tan TL, et al. Asthma in Asia: Physician perspectives on control, inhaler use and patient communications. J Asthma. 2016;53(7):761–769. doi:https://doi.org/10.3109/02770903.2016.1141951.
- Global Asthma Network. The global asthma report 2018. Auckland, New Zealand: Global Asthma Network, 2018.
- Wells KE, Peterson EL, Ahmedani BK, Williams LK. Real-world effects of once vs greater daily inhaled corticosteroid dosing on medication adherence. Ann Allergy Asthma Immunol. 2013;111(3):216–220. doi:https://doi.org/10.1016/j.anai.2013.06.008.
- Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, Chowdhry VK, Favro D, Lanfear DE, Pladevall M. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–1191 e2. doi:https://doi.org/10.1016/j.jaci.2011.09.011.
- van Zyl-Smit RN, Krüll M, Gessner C, Gon Y, Noga O, Richard A, de Los Reyes A, Shu X, Pethe A, Tanase AM, et al. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respir Med. 2020;8(10):987–999. doi:https://doi.org/10.1016/S2213-2600(20)30178-8.
- Kerstjens HAM, Maspero J, Chapman KR, van Zyl-Smit RN, Hosoe M, Tanase AM, Lavecchia C, Pethe A, Shu X, D’Andrea P. IRIDIUM trial investigators. Once-daily, single-inhaler mometasone–indacaterol–glycopyrronium versus mometasone–indacaterol or twice-daily fluticasone–salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000–1012. doi:https://doi.org/10.1016/S2213-2600(20)30190-9.
- Chapman KR, van Zyl-Smit RN, Maspero JF, Kerstjens HAM, Gon Y, Hosoe M, Tanase AM, Pethe A, Shu X, D’Andrea P. Once-daily mometasone/indacaterol fixed-dose combination versus twice-daily fluticasone/salmeterol in patients with inadequately controlled asthma: pooled analysis from PALLADIUM and IRIDIUM studies. BMJ Open Resp Res. 2021;e000819.
- Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-constrolled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi:https://doi.org/10.1016/j.rmed.2005.08.012.
- Juniper EF, O′Byrne PM, Guyatt Gh, Ferrie Pj, King Dr. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:https://doi.org/10.1034/j.1399-3003.1999.14d29.x.
- Jia CE, Zhang HP, Lv Y, Liang R, Jiang YQ, Powell H, Fu JJ, Wang L, Gibson PG, Wang G. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131(3):695–703. doi:https://doi.org/10.1016/j.jaci.2012.08.023.
- Barnes PJ, Casale TB, Dahl R, Pavord ID, Wechsler ME. The Asthma Control Questionnaire as a clinical trial endpoint: past experience and recommendations for future use. Allergy. 2014;69(9):1119–1140. doi:https://doi.org/10.1111/all.12415.
- Vogelmeier C, Naya I, Ekelund J. Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged ≥16 years) with asthma: a sub-analysis of the COSMOS study. Clin Drug Investig. 2012;32(7):439–449. doi:https://doi.org/10.2165/11598840-000000000-00000.
- Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23–27. doi:https://doi.org/10.1034/j.1399-3003.1999.14a06.x.
- Bousquet J, Boulet L-P, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, Carlsheimer Å. Å. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–2446. doi:https://doi.org/10.1016/j.rmed.2007.07.014.
- Svedsater H, Stynes G, Wex J, Frith L, Leather D, Castelnuovo E, Detry M, Berry S. Once-daily fluticasone furoate/vilanterol versus twice daily combination therapies in asthma-mixed treatment comparisons of clinical efficacy. Asthma Res Pract. 2016;2:4. doi:https://doi.org/10.1186/s40733-015-0016-0.
- Inman M D, Watson R M, Rerecich TACY, Gauvreau G M, Lutsky B N, Stryszak PAUL, O’Byrne P M. dependent effects of inhaled mometasone furoate on airway function and inflammation after allergen inhalation challenge. Am J Respir Crit Care Med. 2001;164(4):569–574. doi:https://doi.org/10.1164/ajrccm.164.4.2007063.
- Jacques L, Bakerly ND, New JP, Svedsater H, Lay-Flurrie J, Leather DA. Effectiveness of fluticasone furoate/vilanterol versus fluticasone propionate/salmeterol on asthma control in the Salford Lung Study. J Asthma. 2019;56(7):748–757. doi:https://doi.org/10.1080/02770903.2018.1490751.
