Abstract
Objective
For patients with severe asthma (SA), overestimation of asthma control may lead to poorer outcomes. The objective of this study was to assess concurrent patient and specialist assessments of asthma control and treatment effectiveness among a large US cohort of SA patients.
Methods
CHRONICLE is an ongoing observational study of patients with SA treated by US subspecialists. Asthma control was assessed using the patient-completed Asthma Control Test™ (ACT™) and specialist clinical assessment of control. Treatment effectiveness was measured using the Global Evaluation of Treatment Effectiveness (GETE) completed by patients and specialists.
Results
1109 patients who completed online surveys at enrollment were included. 14%, 28%, 25%, and 33% of patients had ACT™ scores of 5–9, 10–15, 16–19, and 20–25, respectively. Compared with 67% of patients with uncontrolled asthma by ACT™, 44% were uncontrolled by specialist assessment. 54% of patients who were uncontrolled according to the ACT™ were rated as controlled by specialists, demonstrating overestimation of asthma control. Based on ACT™ score, asthma control was more frequent among patients treated with biologics compared to other treatments. Using the GETE, 90% of patients reported treatment effectiveness compared with 71% of specialists. Patient and specialist treatment effectiveness categorizations agreed 73% of the time.
Conclusion
Specialists commonly overestimated asthma control relative to ACT™ scores. Patients reported treatment effectiveness more frequently than specialists. These findings emphasize the importance of validated instruments to assess asthma control and reduce potential treatment gaps associated with patient-specialist discordance.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT03373045.
Introduction
For patients with asthma, airflow limitations and other symptoms can vary in intensity over time (Citation1). This is particularly true for the 5–10% of patients with asthma who have severe asthma (SA) (Citation2). Effective management of SA requires a partnership between the patient and health care provider (HCP), as well as continual adjustment of treatment to achieve disease control and minimize symptoms, activity limitations, exacerbation risk, persistent airflow limitation, and mortality risk (Citation1). As such, accurate assessments of asthma control and treatment effectiveness by HCPs are vital to optimal disease management.
Previous studies have reported discordance between HCP and patient assessments of asthma symptom control (Citation3). In a study of 73 patients and 9 clinicians at an asthma specialist center in the United Kingdom, clinicians routinely overestimated asthma symptom improvement and underestimated asthma deterioration following a medication change (Citation4). Subsequent studies in Spain, the United Kingdom, and Japan revealed that HCPs frequently underestimated asthma impact and patient treatment satisfaction (Citation5), and both patients and HCPs overestimated asthma control (Citation3,Citation6). Overestimation of asthma control by HCPs has been associated with asthma-related fatalities (Citation7). Despite the importance of asthma control and treatment effectiveness assessments, no large studies in the United States have examined the accuracy of HCP and patient assessments.
The CHRONICLE study (ClinicalTrials.gov Identifier: NCT03373045) is an ongoing, non-interventional, US-based study of a large, contemporary sample of patients with SA and their treating subspecialists. The objective of the current analysis of the CHRONICLE study was to evaluate concordance between patient and treating subspecialist assessments of asthma control and treatment effectiveness.
Methods
Study design and enrollment criteria
CHRONICLE is an ongoing observational study of patients with SA treated by US allergists or pulmonologists (Citation8,Citation9). Eligible patients have SA, are aged ≥18 years, and are receiving biologics and/or maintenance systemic corticosteroids (mSCS) or uncontrolled per European Respiratory Society/American Thoracic Society criteria (Citation2) while receiving high-dosage inhaled corticosteroids with additional controllers (HD ICS+). To maximize the real-world characteristics of the study sample, sites are directed to approach all eligible patients for potential recruitment. Patients who are not fluent in English or Spanish; are unable to complete study follow-up or web-based patient-reported outcomes (PROs); or are receiving investigational therapy for asthma, allergy, atopic disease, or eosinophilic disease as part of a clinical trial during the 6 months prior to enrollment are excluded. Patients enrolled in CHRONICLE are permitted to subsequently enroll in trials of investigational therapies. The current analysis is based on patients with SA who were enrolled in CHRONICLE between February 2018 and February 2020 and evaluations by their subspecialist physician. The CHRONICLE study protocol received central institutional review board (Advarra, Columbia, MD) approval on November 3, 2017, and was registered on ClinicalTrials.gov on December 14, 2017 (NCT03373045).
Asthma control and treatment assessments
In CHRONICLE, patient and specialist reports of asthma control and treatment effectiveness are collected at enrollment (Citation8,Citation9). Patients and specialists are blinded to each other’s responses on all assessments. For the current analysis, two different characterizations of asthma control were analyzed. Each patient was asked to complete an online Asthma Control Test™ (ACT™) immediately following study enrollment. The ACT™ is a validated, five-item, five-option Likert-type questionnaire, with possible scores ranging from 5 (poor asthma control) to 25 (complete asthma control) (Citation10). ACT™ scores of 20–25 indicated asthma that was “well controlled,” 16–19 “not well controlled,” and 5–15 “very poorly controlled.” (Citation11) Second, at enrollment, the specialist physician treating the patient described the patient’s asthma as controlled or uncontrolled based on their own assessment. Specialists were also asked what information they utilized to inform their assessment (e.g. patient verbal reports, lung function testing, in-office ACT™ results, or recent exacerbations).
Treatment effectiveness assessments
The Global Evaluation of Treatment Effectiveness (GETE) was completed by both patients and specialists at enrollment. The GETE is a five-point categorical scale of treatment effectiveness that has demonstrated good construct validity and inter-rater reliability in prior studies of patients with moderate-to-severe asthma and their physicians (Citation12). It is based on patient- and HCP-specific versions of the question “How effective has [your/the] treatment been in controlling [your/the patient’s] asthma?” and selecting one of the following categories: complete control, marked improvement, discernible but limited improvement, no appreciable change, or worsening [of asthma] (Citation12). Using these categories, “some improvement” was defined as patients in GETE categories of complete control, marked improvement, or limited improvement, and “no improvement” as patients in GETE categories of no appreciable change or worsening.
Statistical analyses
Patient characteristics at enrollment and assessments of asthma control and treatment effectiveness were summarized using descriptive statistics for patients with non-missing data. No imputation of missing data was conducted given the cross-sectional, non-longitudinal nature of the analysis and the risk of bias from imputation. Where feasible, results were analyzed by treatment subgroups based on whether patients were treated with HD ICS+, biologic agents, and/or mSCS at the time of enrollment. To evaluate concordance between patient and specialist assessments of control, the relative distributions of ACT™ asthma control categories were plotted on 100% stacked horizontal bar graphs against patient- and specialist-reported binary classification of asthma control. To evaluate concordance of patient and specialist assessments of treatment effectiveness, relative distributions of GETE categorical scores from specialist assessments were plotted on 100% stacked horizontal bar graphs against the results from patients. Because of the descriptive nature of this analysis, no statistical testing was performed.
Results
Of 1884 patients enrolled, 1109 (59%) completed at least one online survey at enrollment; 67% were receiving treatment with biologic agents, 13% were receiving mSCS, and 27% were receiving HD ICS + only without biologics or mSCS (7% were receiving both biologics and mSCS). Characteristics of patients who completed surveys were generally similar to the overall study population (), although patients who completed the surveys were slightly more likely to be White (80% of those completing vs 75% of the overall study population), receive care from an allergist/immunologist (43% vs 39%), and have commercial health care insurance (64% vs 60%).
Table 1. Patient demographics and clinical characteristics.Table Footnote a
Determination of patient asthma control
Complete ACT™ scores were evaluable for 1038 patients at enrollment. Of these, 14%, 28%, 25%, and 33% of patients had scores of 5–9, 10–15, 16–19, and 20–25, respectively. As a result, 67% of patients were classified as having uncontrolled asthma (25% not well controlled and 42% very poorly controlled; ). Prevalence of uncontrolled asthma by patient ACT™ was higher among patients treated with mSCS (91%) and HD ICS + only (84%) and lower for patients on biologic therapy (58%).
Figure 1. Specialist and ACT™ assessments of controla,b A) for the overall population and B) by treatment category.
ACT™, Asthma Control Test™; HD ICS+, high-dosage inhaled corticosteroids with additional controllers; mSCS, maintenance systemic corticosteroids.
aPer ACT™, “well-controlled” includes scores 20–25; “not well controlled” includes scores 16–19; “very poorly controlled” includes scores 5–15.
bPercentages may not total 100.0% due to rounding.
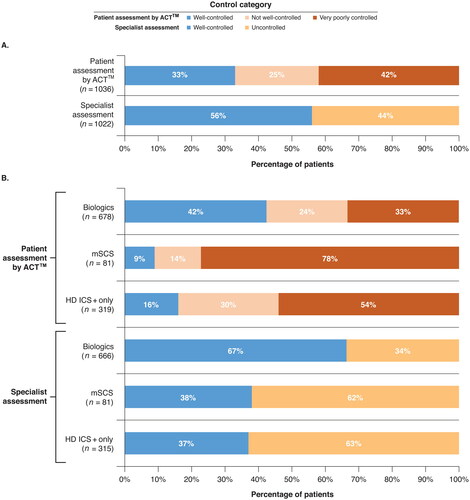
Based on 1022 specialist assessments, 453 (44%) patients were determined to have uncontrolled asthma. The prevalence of uncontrolled asthma based on specialist assessment was higher among patients treated with HD ICS + only (63%) and mSCS (62%) and lower for patients on biologic therapy (33%). Based on site report, specialist-reported control was informed by the provider’s clinical impression (85% of patients), patient verbal reports without a standardized survey (49%), in-office ACT™ (43%), lung function testing (39%), and recent exacerbations (37%).
Specialist assessment versus ACT™ scores
Comparing specialist assessments and patient ACT™ scores, 85% of patients who were considered uncontrolled by specialists were also uncontrolled per ACT™ score, whereas 46% of patients considered controlled by specialists were controlled per ACT™ (). Among patients deemed controlled by specialists, 26% were not well controlled and 28% were very poorly controlled according to the ACT™. Results were similar by patient sex (female vs male) and race/ethnicity (non-Hispanic White vs all others). The only patient characteristics associated with this specialist overestimation of control were Medicare insurance (28% of patients deemed controlled by specialist but uncontrolled by ACT™ versus 22% of patients overall) and retired employment status (27% vs 22%, respectively).
Figure 2. Concordance between specialist assessment of control and patient ACT™. Specialist assessment of patients’ asthma control status is compared with patients’ ACT™ resultsa A) for the overall population and B) by treatment category.
ACT™, Asthma Control Test™; HD ICS+, high-dosage inhaled corticosteroids with additional controllers; mSCS, maintenance systemic corticosteroids.
aPercentages may not sum to 100% as a result of rounding.
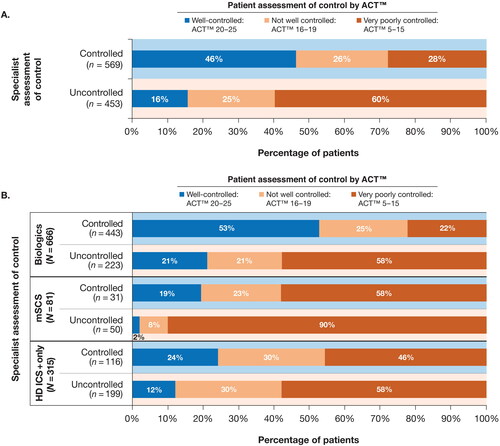
The highest concordance between specialist assessments and ACT™ scores for uncontrolled asthma occurred among patients treated with mSCS, as 98% who were considered uncontrolled by specialist were uncontrolled per ACT™. The highest concordance for controlled asthma occurred among patients treated with biologics; 53% of those considered controlled by specialists were well controlled per ACT™ ().
Ratings of treatment effectiveness
Among patients who completed the GETE at enrollment and had a specialist assessment (N = 1009), patients were generally more likely to report treatment effectiveness than their specialists. Specialists reported some improvement for 71% of patients and no improvement or worsening for 29% of patients (). In contrast, 90% of patients reported some improvement, whereas 10% reported no improvement or worsening. Of the patients who reported no improvement, specialists reported some improvement in 6%. For patients whose specialists reported no improvement, 71% reported some improvement themselves. Results were similar by patient sex and race/ethnicity. The low concordance between patient and specialist assessments of treatment effectiveness were examined in more detail by plotting the percentages of each category scored by specialists within each category scored by patients (). Results by treatment class generally aligned with the overall results (). Similar to asthma control assessment results, concordance between patient and specialist determinations of treatment effectiveness was greatest among patients treated with biologics.
Figure 3. Concordance between specialist and patient assessments of treatment effectiveness. Specialist ratings of treatment effectiveness using the five-point GETE assessment are compared with patients’ self-assessmentsa A) for the overall population and B) by treatment category.
GETE, Global Evaluation of Treatment Effectiveness; HD ICS+, high-dosage inhaled corticosteroids with additional controllers; mSCS, maintenance systemic corticosteroids.
aPercentages may not sum to 100% as a result of rounding.
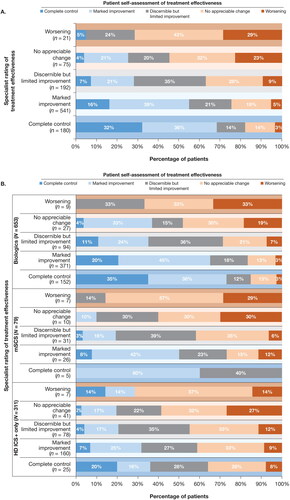
Table 2. Concordance for individual categories on the specialist and patient GETE (N = 1009).Table Footnote a
Discussion
This study represents the first large, real-world assessment of simultaneously reported asthma control and perceived treatment effectiveness among US patients with SA and their treating subspecialists. In this population, treating subspecialist physicians tended to overestimate asthma control relative to control determined by the patient’s ACT™, particularly among patients not receiving biologic therapy. This trend was evident even among those who were very poorly controlled per ACT™ score. These effects were consistent across all patient subgroups evaluated, although they were somewhat more prevalent among retired patients and those with Medicare insurance, which could reflect increased acceptance of asthma symptoms among older, retired adults. Although physician judgment is typically used in clinical practice, our findings demonstrate that a validated instrument like the ACT™ adds value through its quantitative assessment of specific patient-reported symptoms and impairments over the preceding 4 weeks. Additionally, the current findings underscore that patient assessments with validated instruments should be conducted more routinely and given greater consideration in specialists’ assessments of asthma control. According to US National Asthma Education and Prevention Program guidelines, providers should use the ACT™ or similar validated instruments to guide therapy in SA (Citation13).
It is important to note that asthma control is composed of two domains: symptom impairment and exacerbation risk (Citation14). The ACT™ only addresses the impairment domain of asthma control and does not assess the risk domain. Although it was not available at the time this study was initiated, additional tools, such as the Asthma Impairment and Risk Questionnaire (AIRQ®), are now available that measure both domains of asthma control with greater sensitivity and specificity than the ACT™ (Citation15). During the ACT™ validation, specialists rated patients’ control based on the patient’s medical history, physical examination, and forced expiratory volume in 1 s (FEV1, percent predicted and relation to prior maxima). The ACT™ threshold of <20 for uncontrolled asthma resulted in 74% of patients being correctly classified, with 69% sensitivity and 76% specificity (Citation10). This result indicates that the ACT™ classification is imperfect but balances sensitivity and specificity. However, the specialist assessment of control, by which the ACT™ was assessed, differs considerably from a real-world clinical assessment that could be more hastily performed, potentially with more limited inputs. In contrast with the ACT™, a score cut point of ≥2 on the AIRQ® had a sensitivity of 90% and a specificity of 79% for separating well-controlled asthma from all others.
A different pattern was observed with assessments of treatment effectiveness. Patients were more likely to report treatment effectiveness than specialists; most patients surveyed reported some improvement when no improvement was reported by the specialist. This result suggests that patient reports of treatment effectiveness should be balanced against specialist opinions that are informed by objective measures and data from validated PROs.
Although there have been no large studies evaluating concordance between patient and specialist assessments of asthma control and treatment effectiveness among patients with asthma in the United States, several studies have been conducted in the United Kingdom, Europe, and Japan (Citation3,Citation5,Citation6,Citation16). Overestimation of asthma control by providers was first described in a small UK cohort as an unexpected finding during an attempt to quantify the minimal important difference of the Asthma Control Questionnaire (ACQ) (Citation4). The first large, real-world study examining concurrent specialist and patient assessments was conducted in Spain among 1160 patients with asthma (68% with mild-to-moderate asthma) and 300 pulmonologists. Concordance for asthma impact and overall treatment satisfaction were 57% and 56%, respectively; specialists underestimated both factors relative to patient assessments in 26% of patients (Citation5). This study differed slightly from the current study because it measured asthma impact (normal activities, social activities, and overall health-related quality of life) and treatment satisfaction (symptoms, attacks, and several other metrics) rather than asthma control and treatment effectiveness. A subsequent study in Spain evaluated concordance between patient and specialist (97% were subspecialists) assessments of the impact of asthma on daily living among 2902 patients with moderate-to-severe asthma and demonstrated similar findings (Citation17). In the United Kingdom, a study of 234 patients with asthma (all severities) in primary, secondary, and tertiary care centers evaluated patient and specialist assessment of control relative to the ACT™ and found that patients and providers both overestimated control: 84% of patients and 74% of specialists reported asthma control, whereas ACT™ scores classified only 55% as controlled (ACT™ score ≥20)6; there was a larger discrepancy for patients with more severe asthma. Similarly, a study of 1697 patients with asthma in primary and secondary care centers in Japan found that 52% of patients were controlled based on ACQ score, whereas physicians reported that 80% were controlled (Citation3). Patient overestimation of control was also previously demonstrated in three studies conducted in Europe and the United Kingdom. A study of 4274 adults in Europe with asthma who were prescribed ICS/long-acting β2-agonist therapy reported that approximately 71% of patients who characterized their asthma as well controlled did not meet the Global Initiative for Asthma (GINA)–defined criteria for well-controlled asthma (Citation16). In the AIRE study, Rabe et al. (Citation18) and Vermeire et al. (Citation19) reported that approximately 50% of patients with severe persistent symptoms classified their disease as well controlled or completely controlled. And in the REALISE European survey, >80% of patients with asthma who were classified as uncontrolled by GINA criteria described their disease as controlled (Citation20).
A limitation of the current study is that patient surveys were not completed by 40% of patients enrolled in the CHRONICLE study; however, it is reassuring that the characteristics of patients completing surveys and the overall study population were generally similar. In this study, provider assessments of asthma control were based on a binary choice of controlled versus uncontrolled. There were no options for providers to indicate not well controlled or very poorly controlled. Although this decision provided less granularity regarding the specialist’s assessment of control, having only two choices was advantageous as it eliminated an ambiguous middle option and provided clarity on whether the specialist felt that action was warranted to improve asthma control. Patients with SA who are not treated by a subspecialist are not enrolled in the CHRONICLE study and thus were not evaluable in this analysis. For patients with SA who are not treated by a subspecialist, the discordance in assessments of asthma control could differ, particularly as primary care providers are responsible for assessing multiple conditions simultaneously.
Conclusions
In this large, real-world, US study of simultaneously reported asthma control and treatment effectiveness among patients with SA and their treating subspecialists, subspecialists overestimated asthma control relative to patient ACT™ scores. In contrast, patients were more likely to report treatment effectiveness than specialists. To reduce potential treatment gaps associated with this discordance, providers should utilize and give greater consideration to validated instruments, such as the ACT™ or AIRQ®, to guide decisions about disease management and treatment changes in patients with SA.
Author contributions
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript and provided final approval of the version to be published.
Prior presentation
Selected data from this article were presented as an oral presentation titled “Comparison of asthma control and treatment effectiveness assessments between US patients with asthma and treating subspecialists: real-world data from the CHRONICLE study” at the 2019 American College of Chest Physicians (CHEST) Annual Meeting (published abstract: Panettieri RA, et al. Chest. 2019;156(4 suppl):A211–A214. https://doi.org/10.1016/j.chest.2019.08.1450).
Acknowledgements
The investigators and staff at CHRONICLE study sites are gratefully acknowledged for their contributions to this study. Medical writing support was provided by Shane Walton, PhD, of Cello Health Communications/MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA).
Declaration of interest
Reynold A. Panettieri Jr. has served on advisory boards and received grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis. Bradley E. Chipps has served on advisory boards, as a speaker, and as a consultant for AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Regeneron, and Sanofi Genzyme. Wendy C. Moore has served on advisory boards for AstraZeneca, Sanofi, Genentech, and GlaxoSmithKline. Weily Soong has served as a consultant for AstraZeneca, Genentech, Regeneron, Sanofi, Teva, and Pack Health; as a speaker for AstraZeneca, Regeneron, GSK, Sanofi, and Optinose; and as a clinical trial investigator for AstraZeneca, Novartis, Regeneron, Sanofi, Teva, Genentech, and Avillion. Warner W. Carr has served as a speaker for AstraZeneca, Teva, Boehringer Ingelheim, Regeneron, and Sanofi; and as a consultant for AstraZeneca, Teva, Boehringer Ingelheim, Regeneron, Sanofi, Circassia, CSL Behring, Genentech, GlaxoSmithKline, Horizon Pharma, Kaleo, Mylan, Pfizer, Shire, Meda, Baxalta, Novartis, Greer Laboratories, Alcon Laboratories, Valeant Pharmaceuticals, Grifols, and Aerocrine. James L. Kreindler, Sean O’Quinn, Frank Trudo, and Christopher S. Ambrose are employees and shareholders of AstraZeneca.
Funding
The CHRONICLE study, including its design, data collection, analysis, and interpretation was funded by AstraZeneca. Employees of AstraZeneca served as authors and thus were involved in the writing of and decision to publish this report.
Data availability statement
CHRONICLE is an ongoing study; individual de-identified participant data cannot be shared until the study concludes. The full study protocol is available upon request of the corresponding author. Individuals who were or were not involved in the study may submit publication proposals to the study’s Publication Steering Committee by contacting the corresponding author.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention. [accessed 2019 Aug 7]. Available from: http://www.ginasthma.org/.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:https://doi.org/10.1183/09031936.00202013.
- Matsunaga K, Hamada K, Oishi K, Yano M, Yamaji Y, Hirano T. Factors associated with physician-patient discordance in the perception of asthma control. J Allergy Clin Immunol Pract. 2019;7(8):2634–2641. doi:https://doi.org/10.1016/j.jaip.2019.04.046.
- Juniper EF, Chauhan A, Neville E, Chatterjee A, Svensson K, Mörk A-C, Ståhl E. Clinicians tend to overestimate improvements in asthma control: an unexpected observation. Prim Care Respir J. 2004;13(4):181–184. doi:https://doi.org/10.1016/j.pcrj.2004.04.003.
- Urrutia I, Plaza V, Pascual S, Cisneros C, Entrenas LM, Luengo MT, Caballero F. Asthma control and concordance of opinions between patients and pulmonologists. J Asthma. 2013;50(8):877–883. doi:https://doi.org/10.3109/02770903.2013.819886.
- Menzies-Gow A, Chiu G. Perceptions of asthma control in the United Kingdom: a cross-sectional study comparing patient and healthcare professionals’ perceptions of asthma control with validated ACT scores. NPJ Prim Care Respir Med. 2017;27(1):48. doi:https://doi.org/10.1038/s41533-017-0050-x.
- Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry report. London: Royal College of Physicians; 2014.
- Ambrose CS, Chipps BE, Moore WC, Soong W, Trevor J, Ledford DK, Carr WW, Lugogo N, Trudo F, Tran TN, et al. The CHRONICLE study of US adults with subspecialist-treated severe asthma: objectives, design, and initial results. Pragmat Obs Res. 2020;11:77–90. doi:https://doi.org/10.2147/POR.S251120.
- Moore WC, Panettieri RA, Trevor J, Ledford DK, Lugogo N, Soong W, Chipps BE, Carr W, Belton L, Gandhi H, et al. Biologic and maintenance systemic corticosteroid therapy among US subspecialist-treated patients with severe asthma. Ann Allergy Asthma Immunol. 2020;125(3):294–303.e1. doi:https://doi.org/10.1016/j.anai.2020.04.004.
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:https://doi.org/10.1016/j.jaci.2003.09.008.
- Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi:https://doi.org/10.1016/j.jaci.2006.01.011.
- Lloyd A, Turk F, Leighton T, Walter Canonica G. Psychometric evaluation of Global Evaluation of Treatment Effectiveness: a tool to assess patients with moderate-to-severe allergic asthma. J Med Econ. 2007;10(3):285–296. doi:https://doi.org/10.3111/13696990701478856.
- National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Full report 2007. Bethesda, MD: NHLBI Health Information Center; 2007.
- Chipps BE, Corren J, Israel E, Katial R, Lang DM, Panettieri RA, Peters SP, Farrar JR. Asthma yardstick: practical recommendations for a sustained step-up in asthma therapy for poorly controlled asthma. Ann Allergy Asthma Immunol. 2017;118(2):133–142.e3. doi:https://doi.org/10.1016/j.anai.2016.12.010.
- Murphy KR, Chipps B, Beuther DA, Wise RA, McCann W, Gilbert I, Eudicone JM, Gandhi HN, Harding G, Coyne KS, et al. Development of the Asthma Impairment and Risk Questionnaire (AIRQ): a composite control measure. J Allergy Clin Immunol Pract. 2020;8(7):2263–2274.e5. doi:https://doi.org/10.1016/j.jaip.2020.02.042.
- Kritikos V, Price D, Papi A, Infantino A, Ställberg B, Ryan D, Lavorini F, Chrystyn H, Haughney J, Lisspers K, et al. A multinational observational study identifying primary care patients at risk of overestimation of asthma control. NPJ Prim Care Respir Med. 2019;29(1):43. doi:https://doi.org/10.1038/s41533-019-0156-4.
- Crespo-Lessmann A, Plaza V, Gonzalez-Barcala FJ, Fernandez-Sanchez T, Sastre J. Concordance of opinions between patients and physicians and their relationship with symptomatic control and future risk in patients with moderate-severe asthma. BMJ Open Respir Res. 2017;4(1):e000189. doi:https://doi.org/10.1136/bmjresp-2017-000189.
- Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–807. doi:https://doi.org/10.1183/09031936.00.16580200.
- Vermeire PA, Rabe KF, Soriano JB, Maier WC. Asthma control and differences in management practices across seven European countries. Respir Med. 2002;96(3):142–149. doi:https://doi.org/10.1053/rmed.2001.1241.
- Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi:https://doi.org/10.1038/npjpcrm.2014.9.
