Abstract
Objective
Conventional inhaler devices have a low efficacy in targeting small airways. Smart nebulizers can be used to increase deposition to small airways by adjusting the flow and depth of each inhalation based on patients ‘individual inspiratory capacity. We investigated whether targeting of high dose inhaled corticosteroids (ICS) to small airways with a smart nebulizer could reduce exacerbation rate in children with severe asthma (SA).
Methods
We conducted a retrospective study in children with SA using a smart nebulizer (Akita® Jet nebulizer) for the administration of high dose ICS in our outpatient clinic at the Erasmus MC — Sophia Children’s Hospital. Clinical data before and after start of treatment were collected. The primary outcome was exacerbation rate, defined as: number of asthma exacerbations for which oral corticosteroid courses (OCS) were prescribed. The exacerbation rate 1 year before treatment was compared with the exacerbation rate 1 year after start of treatment. Secondary outcomes were changes in spirometry parameters, hospital admissions and medication use.
Results
Data on OCS use was available for 28/31 patients. Median number of asthma exacerbations requiring OCS courses 1 year before decreased from 2 (interquartile range(IQR) 2) to 0.5 (IQR 3) 1 year after treatment (p = 0.021). Hospital admission decreased from 1 (IQR 3) to 0 (IQR 1)(p = 0.028). FEV1, FEF25-75 and FEF75 were not significantly improved after one year of treatment with the smart nebulizer (p = 0.191; p = 0.248; p = 0.572).
Conclusion
Targeting small airways with high dose ICS using a smart nebulizer resulted in a significant reduction in exacerbations requiring OCS after one year of treatment.
Introduction
The prevalence of severe asthma (SA) is approximately 2–4% of all asthma patients (Citation1,Citation2). Despite this low prevalence, SA greatly affects quality of life and accounts for high healthcare expenditure and resource utilization (Citation3–5). Mild to moderate asthma can be effectively treated with dry powder inhalers (DPI’s) or pressurized metered dose inhalers (pMDI’s) with inhaled corticosteroids (ICS). In patients with SA the step-up treatment advised by international guidelines fails to control disease despite optimal treatment of modifiable factors (Citation6). Knowledge about the pathogenesis of SA has led to the development of monoclonal antibodies (biologicals) targeting specific inflammatory pathways. The introduction of biologicals has greatly improved asthma control in the majority of children with SA (Citation7). However, treatment is costly and approximately 8% of the patients with SA are not eligible for biological treatment (Citation8). In addition, approximately 25% of SA patients need to switch to a different biological after initial treatment fails to control their disease (Citation9,Citation10). Other patients, in particular children, fear receiving injections and therefore need an alternative treatment.
Small airways play a major role in asthma pathophysiology as structural and functional alterations are frequently observed (Citation11). The reported prevalence of small airways involvement in asthma is between 50% and 90% depending on asthma severity (Citation12,Citation13). Small airways disease is associated with uncontrolled asthma and the future loss of asthma control (Citation14,Citation15). Treatment of SAD in asthma patients could therefore be a potential target for therapeutic intervention. Based on computational fluid dynamic modeling and in vitro studies, it is suggested that conventional inhaler devices, such as pMDIs and DPIs, have relatively low efficacy and are highly variable in targeting small airways (Citation16,Citation17). Efficient deposition of aerosols in the small airways is highly dependent on slow and deep inhalation (Citation18). Smart nebulizers allow for more efficient delivery of medication to the small airways (Citation19). By coaching patients to inhale with a slow (flow 12 L/min) and deep inhalation maneuver, higher doses are deposited into the small airways. The information on patients’ individual inspiratory capacity is recorded on a smart card. This card also records adherence, making it possible to monitor and discuss adherence to treatment.
In adults with oral corticosteroid (OCS)-dependent asthma and in children with cystic fibrosis, small airways targeted treatment with smart nebulizer technology (e.g. Akita®Jet) has proved to be highly effective (Citation20,Citation21). A pilot study in children with mild asthma showed a slightly higher increase in asthma control and lung function in patients using the Akita® Jet in comparison to a conventional nebulizer (Citation22). However, to date, there is little experience in clinical practice on the use of smart nebulizer technology for treatment of SA in children. At the Erasmus MC—Sophia Children’s Hospital, we started treatment with high dose ICS using the Akita® jet nebulizer in children with SA who had uncontrolled asthma despite step 4 or 5 treatment according to Global Initiative for Asthma (GINA) guidelines (Citation23), as a ‘last resort’ treatment before starting OCS maintenance treatment. We performed a retrospective study in this group to examine the effects of small airways targeted treatment with the Akita® jet nebulizer at population level. We hypothesized that high-dose ICS treatment targeted to the small airways would decrease exacerbations rate, decrease number of hospital admissions and would improve small airways function measured using spirometry.
Methods
Study design
We conducted an investigator initiated retrospective study in children with SA who received treatment with the Akita® Jet nebulizer (from now on referred to as Akita®) between January 2010 and December 2019. All patients were treated at the outpatient clinic of the department of pediatric pulmonology of Erasmus MC—Sophia Children’s Hospital (Rotterdam, the Netherlands). Diagnosis of SA was confirmed by an experienced pediatric pulmonologist after a thorough work-up and treatment of modifiable factors (Citation23). All patients were either on step 4 or 5 treatment (GINA guidelines) before start of treatment with the Akita®. All children had persistent asthma symptoms, and/or frequent exacerbations. Inclusion criteria for the retrospective analysis were: diagnosis of SA according to GINA criteria (Citation23) and age 4–18 years at initiation of treatment with the Akita®. Exclusion criteria were: active smoking and age <4 and >18 years. The local institutional review board reviewed the study (MEC-2019–0554) and judged that the rules laid down in the Medical Research Involving Human subjects Act does not apply to this research (Citation24). Before start of data acquisition written informed consent was obtained from all subjects and/or legal representatives for the use of clinical data for this study. Vectura Group Plc. supported the research by an unconditional grant for a PhD research program. However, Vectura Group Plc. did not initiate the study nor were they involved in any part of the conduct of this study.
Data collection
Patient records were reviewed for: clinical demographics, allergies, medication use, OCS courses, admissions, adherence, and spirometry measurements. Data were collected at predefined consecutive visits: 12, 9, 6, and 3 months before and 1, 3, 6, 9, and 12 months after start of treatment with ICS with the Akita®. If multiple visits were present, the visit closest to the predefined visit was chosen. Three independent investigators (WB, SK and RG) collected data from the electronic patient records using a study specific protocol. Extracted data were de-identified and coded to ensure anonymization and blinding for the analyses. Missing data in the patient records were marked as missing in the database. Data monitoring and validation were conducted by the departments research coordinator.
Outcome measures
The primary outcome was exacerbation rate defined as: number of asthma exacerbations for which OCS were prescribed. The exacerbation rate 1 year before was compared with the exacerbation rate 1 year after initiation of treatment with the Akita®. The secondary outcomes were: changes in spirometry parameters, hospital admissions and daily nominal dose of ICS. Forced expiratory volume in 1 s (FEV1), Forced expiratory flow between 25% and 75% of forced vital capacity (FEF25-75) and at 75% of forced vital capacity (FEF75) were calculated according to the formulas reported by Global Lung function Initiative (Citation25) and presented as %predicted (%pred) values.
Smart nebulizer
The Akita® Jet inhalation system (Vectura Group Plc., UK) was the smart nebulizer used in this study. The Akita® consists of a smart compressor combined with a modified jet nebulizer handset (derived from a Pari handset). The smart compressor is breath-actuated and controls the fraction of the inspiration time in which an aerosol is generated based on the patients’ individual inspiratory capacity. The nebulizer coaches patients in performing a slow and deep inhalation maneuver. This breath-actuated medication release in combination with the slow and deep inhalation has shown to result in a lung deposition of approximately 60% of the delivered dose and more efficient small airway deposition (Citation19,Citation26). During nebulization the Akita® provides feedback on the inhalation technique which results in less variability. Data on inspiratory time and adherence is recorded on a smart card.
Drug regimen
Patients started with nebulization of budesonide 1 mg twice daily or fluticasone propionate 2 mg twice daily depending on physician’s choice and availability of the drug formulation. Before nebulization of the selected ICS, patients nebulized salbutamol 2.5 mg and/or ipratropium bromide 0.5 mg. The emitted dose is approximately 40% of the nominal or loading dose. Of the emitted dose, 60% is deposited in the lung and 40% extrathoracic (Citation19). At initiation of treatment all maintenance asthma medication was continued. ICS administered with pMDI or DPI were tapered down or stopped when sufficient asthma control was achieved, according to the patient and treating physician. ICS dose administered with the Akita® was decreased once ICS administered through pMDI or DPI was stopped. Nominal daily average pMDI or DPI ICS dose for every patient was calculated and converted to budesonide pMDI/DPI equivalent dose (250 μg fluticasone propionate = 400 μg budesonide, 500 μg beclomethasone standard particle size = 400 μg budesonide, 200 μg beclomethasone extra‐fine = 400 μg budesonide and 160 μg ciclesonide= 400 μg budesonide) (Citation27).
Statistical analysis
Descriptive statistical analyses were performed on patient characteristics. Categorical data are shown as counts and proportions. Continuous normally distributed data are shown as mean ± standard deviation and not normally distributed data are shown as median and interquartile range (IQR). Data distribution was tested for normality using Shapiro-Wilk and Kolmogorov-Smirnov test. The change in number of exacerbations requiring OCS and the change in number of hospital admissions 1 year before and 1 year after treatment were compared using the Wilcoxon signed-ranked test. The mixed-effects modeling frame work was used to investigate the evolution of the spirometry measurements (FEV1, FEF25-75, and FEF75) over time during treatment with the Akita®. Mixed models consist of random and fixed effects (Citation28). The benefit of the mixed effects model for assessing the differences in spirometry measurements over time is that it accounts for multiple measurements within patients (random effect). Fixed effects are factors that are assumed to have the same effect across subjects. In this model we entered hospital visit (resembling effect of treatment), age at baseline, sensitization to inhaled allergens, BMI at baseline and the change in exacerbation rate (number of exacerbations 1 year after initiation of treatment – number of exacerbations 1 year before start of treatment) as fixed effects. In the model, hospital visit 1, 2, 3, and 4 were, respectively, 12, 9, 6, and 3 months before start of treatment. Hospital visit 5, 6, 7, 8, 9, and 10 were respectively start of treatment and 1, 3, 6, 9, and 12 months after treatment. We assumed linear evolutions over time (visits). We, furthermore, assume a random intercept which allows each subject to start at a different spirometer baseline value. We included interaction terms into the model to study whether patients with sensitization to allergens and patients with changes in exacerbation rate had different effect of treatment on the spirometry outcomes. Significance level was set to p < 0.05. Statistical analyses were performed with SPSS 25 (Chicago, USA), Graphpad Prism 8 and the statistical software package R Version 3.6.1.
Results
Patient characteristics
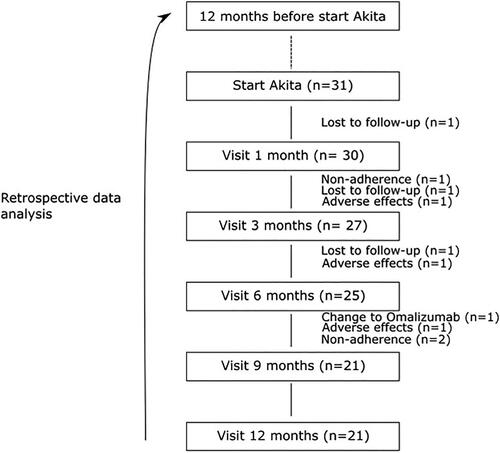
In total 35 patients with SA used the Akita® between January 2010 and December 2019. Four patients did not provide informed consent for the use of clinical data therefore 31 patients were included in the study. Of these 31, three were lost to follow up and seven patients did not use the Akita® for 1 year because they were non adherent (n = 3), experienced adverse events (n = 3) or switched to another therapy (n = 1) (). The adverse events were: suppressed growth velocity, weight gain, gingivitis and development of a skin rash. Of the patients who reported adverse events 2 were on budesonide and 1 on fluticasone propionate. In total 21 patients used the Akita® for at least 1 year. Baseline characteristics are presented in . Mean age was 11.2 ± 3.8 years. Gender was evenly distributed. In the work up of their SA 61.3% of patients had a bronchoscopy performed and all patients had a chest computed tomography scan done.
Table 1. Population characteristics.
Primary outcome
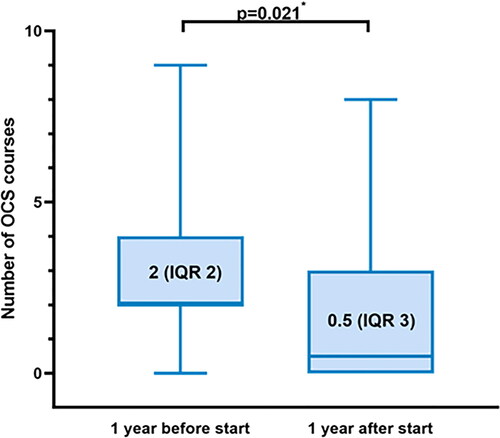
Median number of exacerbations requiring OCS decreased from 2 (IQR 2) to 0.5 (IQR 3) during treatment with the Akita® (n = 28, p = 0.021) (). No data on number of exacerbations were available for the patients (n = 3) that were loss to follow up, therefore these patients were omitted from the analysis. The difference in exacerbation rate remained significant when the patients that did not fulfill 1 year of treatment with the Akita® were left out of the analysis (n = 21, p = 0.013).
Figure 2. This boxplot shows the median number of OCS courses 1 year before start of treatment and 1 year after treatment(n = 28) including the minimum/maximum and first/third quartile. The difference remained statistically significant(p = 0.013) after leaving out the patients that did not fulfill one year of treatment(n = 21). * Wilcoxon signed rank test.
Secondary outcomes
Median number of hospital admissions decreased from 0.5 (IQR 2) to 0.0 (IQR 1) during treatment with the Akita®, which was not significant (n = 28, p = 0.088). However, if patients that did not fulfill 1 year of treatment with the Akita® were left out of the analysis the median number of hospital admission decreased from 1 (IQR 3) to 0 (IQR 1)(n = 21, p = 0.028). No data on number of hospital admissions were available for the patients (n = 3) that were lost to follow, therefore these patients were omitted from the analysis. shows median spirometry values of FEV1, FEF25-75, and FEF75 before and after start of treatment at the predefined time points. The median %pred of FEV1, FEF25-75, and FEF75 at baseline were 78.23%, 46.58%, and 40.52% (). After 12 months of treatment they increased up to 85.65%, 57.36%, and 54.32%, respectively. The highest increase for FEV1 and FEF75 from baseline was seen in the first month of treatment. The highest increase for FEF25-75 was seen at 6 months of treatment. Results were similar when only the patients (n = 21) that did fulfill one year of treatment were included in the analysis (). Linear mixed model analysis showed no significant result for the main fixed effects on FEV1, FEF25-75, and FEF75 (). This indicates that no significant overall change in spirometry parameters were observed during one year of treatment with the Akita® nebulizer. Allergy, BMI, age, and change in exacerbation rate were also not strongly associated with spirometry parameters. To see whether patients with sensitization to allergens and patients with a decrease in exacerbation rate had different effect of treatment on the spirometry outcomes we studied their interaction. We observed that the interaction between hospital visit and change in exacerbation rate was significant for FEV1, FEF25-75, and FEF75. This implies that patients who had less exacerbations after one year of treatment also experienced improvement in spirometry and patients that had an increase in exacerbations had a decrease in spirometry. The interaction for allergen sensitization was not significant.
Figure 3. Shows median spirometry measurements with interquartile range of FEV1, FEF25-75 and FEF75. The bold colored line are all the patients that used the Akita® Jet nebulizer (n = 28). The dotted line shows the patients that have fulfilled one year of treatment with the Akita® Jet nebulizer (n = 21).
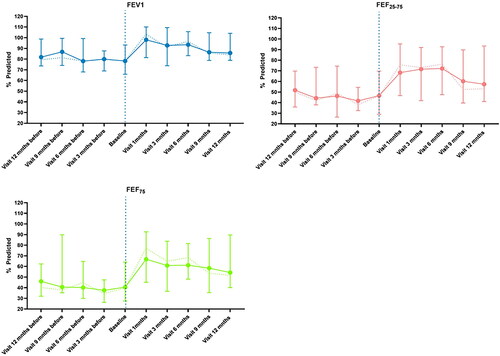
Table 2. Shows the median %predicted values and interquartile range (IQR) for FEV1, FEF25-75, and FEF75. Mo = months.
Table 3. Shows the linear mixed effects model studying the evolution of spirometry parameters over time.
Quantitative data on adherence were only available for several patients because these data were discussed with patients but not always recorded in the patient records. More recent data were not recorded due to a technical failure of the card reader needed for subtraction of the logged data. Therefore, data on adherence were not included in our analyses.
Pharmacological therapy
and show asthma medication use before, during, and after treatment with the Akita®. Before start of treatment 16 patients were using high dose ICS with a long-acting β agonist (LABA) and/or leukotriene modifier (LTRA), 14 patients were using combination inhalers (ICS/LABA) and one patient was using high dose ciclesonide without LABA/LTRA and received methylprednisolone injections. All patients administered their asthma medication using a pMDI with valved holding chamber. At the start of treatment 1 patient received budesonide 0.5 mg twice daily instead of 1 mg twice daily. For fluticasone propionate two patients received 1 mg twice daily and one patient 1 mg once daily instead of 2 mg twice daily. During treatment with the Akita® tapering of budesonide and fluticasone proprionate dose resulted in a 185.1 µg and 196.4 µg decrease respectively. After one year of treatment 5/21 patients were still using additional ICS treatment (): 3/5 ICS monotherapy and 2/5 combination inhalers. Before initiation of treatment three patients used omalizumab but stopped because their asthma remained uncontrolled. During treatment with the Akita® one patient was on omalizumab and another patient started with omalizumab 3 months after start of treatment.
Figure 4. Gives a graphical overview of medication use. The horizontal black line represent the use of the Akita® treatment. Each pill indicates an OCS course. The red bars shows the use of ICS administered through pressurized metered dose inhaler or dry powder inhaler. The green bar indicates omalizumab use.
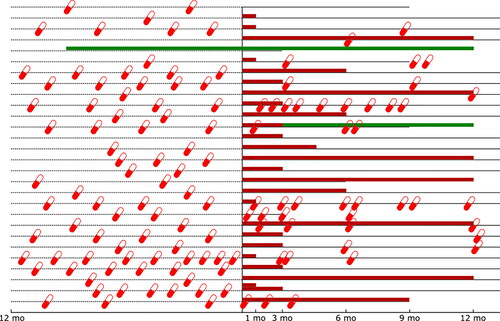
Table 4. Use of asthma medication before and after treatment with the Akita® Jet nebulizer.
Discussion
In this retrospective study we observed that targeted treatment of small airways with a smart nebulizer in children with SA is associated with a significant reduction of asthma exacerbations and hospital admissions after one year of treatment compared to the year before start. Spirometry parameters did not significantly improve during smart nebulizer treatment. The median exacerbation rate decreased by more than 50% after treatment with the Akita®. Our results are in line with those found in a study conducted in adults with OCS-dependent asthma who were given small airways-targeted treatment with budesonide administered with the Akita® (Citation20). In this study, a significant OCS dose reduction of ≥50% and an increase of FEV1 in comparison to baseline was found. Although our population differed in age and none of the children used OCS maintenance treatment, this previous study does support our hypothesis that small airways targeted treatment is beneficial in patients with SA.
The concept of small airways-targeted treatment is not new, but has regained interest due to studies that showed high prevalence of small airways involvement in asthma (Citation12,Citation13). Drug delivery to small airways is challenging because most drugs administered with conventional inhaler devices are deposited in the larger airways (Citation16). Fine particle pMDI’s were developed to increase the amount of drugs delivered to the small airways. In this study most patients were using fine particle pMDI’s but despite this their asthma remained uncontrolled. The inhalation maneuver is believed to be the most important factor for efficient aerosol deposition in small airways (Citation18). Smart nebulizers were developed to coach the patient in performing a correct inhalation maneuver (Citation19). Several studies have shown the added value of small airways targeted treatment with a smart nebulizer in pulmonary diseases (Citation20,Citation21,Citation29,Citation30). It is therefore reasonable to believe that the rapid improvement in FEF25-75 and FEF75 observed in the first months is caused by more efficient small airways targeted treatment. There is debate whether spirometry is the best way to assess small airway function. In this retrospective analysis small airway function was assessed using spirometry because this was the only small airway function parameter available. Alternative lung function methods such as multiple breath washout, impulse oscillometry and imaging might be more sensitive in detecting changes in small airway function (Citation31).
Several factors, other than small airways targeted treatment could also have contributed to the observed improvement in exacerbation rate and hospital admissions. First, administration of high dose ICS through the Akita® in combination with the temporary continuation of conventional treatment resulted in patients receiving very high doses of ICS. In patients with OCS-dependent asthma, it was shown that administration of high dose ICS has a similar effect to that of low dose OCS treatment as a result of systemic effects of high dose ICS (Citation32). The observed improvement could therefore be a result of high dose ICS treatment rather than small airways targeted treatment. We agree that the administered ICS dose is high in this population and that it exceeds the recommendation by international guidelines. Most patients in this study were severe (therapy resistant) asthmatics with frequent exacerbations despite treatment of modifiable factors and maximal pharmacological treatment. With no alternative therapies available at that time, we treated patients with high dose ICS administered with the Akita®. The difference in starting dose between budesonide and fluticasone propionate was due to a difference in concentration of the drug formulation available.
A second factor that might also explain the observed improvement in exacerbation rate, hospital admissions and the initial improvement in spirometry is the improvement in adherence. Studies have shown that adherence to treatment is generally poor in patients with asthma (Citation33). The possibility of monitoring and discussing adherence in clinical practice is an important feature of the Akita®. In the first weeks of treatment, patients frequently visited and contacted specialized asthma nurses. This intensive follow-up likely resulted in improved adherence and subsequently less exacerbations, hospital admissions and initial improvement in spirometry parameters. In addition, reduction in adherence after longer use of the Akita®, might also explain why the initial improvement in spirometry parameters decreases over time. Unfortunately, almost no objective data on adherence could be retrieved in our study. Therefore we were forced to leave these data out. This is a limitation of our study. Nevertheless, we believe that the improvement observed in this study cannot only be attributed to improved adherence but rather to a combination of small airways targeted treatment with high dose ICS and improved adherence.
The third factor that could also explain the association between Akita® treatment and decreased exacerbation rate and amount of hospital admissions is the aging of the population. Generally, as children get older their asthma will improve, therefore assessing the children before and after treatment will include this natural change. However, this improvement will be gradual, modest, and less likely to occur in patients with severe asthma. Lastly, It could be argued that the improvements observed are due to a phenomenon called “regression to the mean” (Citation34). In this analysis, patients who started with small airways-targeted treatment, most likely started the treatment during a period of very poor asthma control. Hence, these patients were likely to improve over time.
Increased lung deposition as a result of small airways targeted treatment might not only be beneficial in decreasing asthma exacerbations and hospital admissions, but also in reducing side effects. Lower oropharyngeal deposition (Citation19,Citation30) may decrease local side effects such as cough, hoarseness, dysphonia, and oral candidiasis. In addition, with increased lung deposition higher drug dose reaches the target area, which might allow for lower ICS administration. In our study, we observed that during treatment with the Akita® nominal daily ICS dose could be reduced in the majority of patients (). Increased small airway deposition might on the other hand result in higher alveolar uptake, causing the systemic concentration to increase, subsequently leading to more side effects (Citation35). In this retrospective study, three patients ceased ICS treatment with the Akita® because they reported side effects. As maintenance treatment with conventional inhaler devices was continued alongside Akita® treatment it is uncertain whether these side effects were due to the administration of higher doses of ICS or to increased small airway deposition.
There are limitations in this study that are inherently related to the observational retrospective single-center design. The lack of a randomized setting with a control group being the most important limitation. The absence of this randomized setting with a control group is important because the true effect of small airways treatment in this study could be obscured through confounding by unknown or unmeasured factors. Another limitation related to the study design was that in our study not all patients used the Akita® for a whole year. However, sensitivity analysis including and excluding the patients that did not use the Akita for the entire year did not change the primary outcome. Due to the missing data on adherence, the continuation of conventional treatment alongside and the study design related limitations the generalizability of our findings are limited. Therefore we cannot make any strong recommendation regarding the true effect of small airways targeted treatment with the Akita. To cope with the aforementioned limitations of a retrospective design and to further investigate the effect of small airways targeted treatment in severe asthma a randomized controlled trial should be conducted.
Conclusion
With this retrospective observational study we wanted to share our experience with small airways targeted treatment in children with severe (therapy resistant) asthma. In this study we observed that small airways targeted treatment with smart nebulizer technology decreased the amount of asthma-exacerbations and hospital admission after one year of treatment, compared to the year before. However, due to the retrospective design of the study we can only speculate on the causality of this difference. Notwithstanding these limitations, the results in this study may support the potential role of small airways targeted treatment with a smart nebulizer in SA. International guidelines currently indicate biologicals are the preferred and recommended treatment option for patients with SA and type 2 inflammation. However, various factors, such as high costs, limited eligibility for treatment, the need for use of injections or intravenous infusion and unresponsiveness of disease in certain patients, mean that biologicals are not a suitable treatment for all patients. Treatment with the Akita® nebulizer or another smart nebulizer that can target the small airways could therefore be a possible alternative or add-on in patients with steroid responsive severe therapy resistant asthma.
Author contributions
WB contributed to the design of the work, data collection, analyses, drafted the manuscript till the final version. SK contributed to the design of the work. SK and RG contributed to the data collection. EA contributed to the statistical analyses. HJ initiated the study, contributed to the design of the work, data analyses, drafted the manuscript. SK, RG, EA, MP, HT and HJ critically revised the manuscript and gave final approval to the manuscript.
Acknowledgements
We would like to thank the specialized asthma nurses J. Overweel, Y. den Boer and A. Hogeboom for taking care of asthma patients using the Akita®. In addition, we would like to thank E. van der Wiel for the validation of the data.
Declaration of interest
S. Kloosterman, R. Greidanus and E. Andrinopoulou report no disclosures. W. van den Bosch reports grants from Vectura Group Plc. in the form of an unconditional grant for PhD research programme, during the conduct of the study. M. Pijnenburg reports to have obtained fees for institution from SANOFI genzyme outside the submitted work. H. Janssens reports grants and personal fees from Vertex oustide the submitted work. H. Tiddens reports grants from Vectura Group Plc., during the conduct of the study; personal fees from Vertex, grants and personal fees from Novartis, personal fees from Insmed, grants and personal fees from Thirona, outside the submitted work; In addition, Dr. Tiddens has a patent PRAGMA-CF issued and is heading the Erasmus MC-Sophia Children’s Hospital core laboratory Lung Analysis.
Funding
This study was funded by an unrestricted research grant of Vectura Group Plc. in 2018. The sponsor was not involved in the conduct of the study.
References
- Larsson K, Ställberg B, Lisspers K, Telg G, Johansson G, Thuresson M, Janson C. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res. 2018;19(1):12. doi:10.1186/s12931-018-0719-x.
- Ahmed H, Turner S. Severe asthma in children-a review of definitions, epidemiology, and treatment options in 2019. Pediatr Pulmonol. 2019;54(6):778–787. doi:10.1002/ppul.24317.
- Jansson S-A, Backman H, Andersson M, Telg G, Lindberg A, Stridsman C, Lundbäck B, Rönmark E. Severe asthma is related to high societal costs and decreased health related quality of life. Respir Med. 2020;162:105860. doi:10.1016/j.rmed.2019.105860.
- Hossny E, Caraballo L, Casale T, El-Gamal Y, Rosenwasser L. Severe asthma and quality of life. World Allergy Organ J. 2017;10(1):28. doi:10.1186/s40413-017-0159-y.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1).
- Pijnenburg MW, Fleming L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir Med. 2020;8(10):1032–1044. doi:10.1016/S2213-2600(20)30399-4.
- Johnson N, Varughese B, De La Torre MA, Surani SR, Udeani G. A review of respiratory biologic agents in severe asthma. Cureus. 2019;11(9):e5690. doi:10.7759/cureus.5690.
- Jeimy S, Tsoulis MW, Hachey J, Kim H. Eligibility of monoclonal antibody-based therapy for patients with severe asthma: a Canadian cross-sectional perspective. Allergy Asthma Clin Immunol. 2018;14:68. doi:10.1186/s13223-018-0301-6.
- Reihman AE, Liu C, Cruse MH, Freid L, Perez N, Smith VL, Ferguson L, Wechsler M, Holguin F, Manka L, Sharma S. Clinical predictors of treatment failure to anti-IL5 therapy in severe eosinophilic asthma. B101 New biological treatments for asthma. American Thoracic Society International Conference Abstracts: American Thoracic Society; 2020. p. A4263–A4263. doi:10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A4263.
- Ortega G, Tongchinsub P, Carr T. Combination biologic therapy for severe persistent asthma. Ann Allergy Asthma Immunol. 2019;123(3):309–311. doi:10.1016/j.anai.2019.06.013.
- van den Bosch WB, James AL, Tiddens HAWM. Structure and function of small airways in asthma patients revisited. Eur Respir Rev. 2021;30(159):200186. doi:10.1183/16000617.0186-2020.
- Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, Papi A, Van der Molen T, Rabe KF, Siddiqui S, ATLANTIS study group, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7(5):402–416. doi:10.1016/S2213-2600(19)30049-9.
- Usmani OS, Singh D, Spinola M, Bizzi A, Barnes PJ. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir Med. 2016;116:19–27. doi:10.1016/j.rmed.2016.05.006.
- Manoharan A, Anderson WJ, Lipworth J, Lipworth BJ. Assessment of spirometry and impulse oscillometry in relation to asthma control. Lung. 2015;193(1):47–51. doi:10.1007/s00408-014-9674-6.
- Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul W. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. J Asthma. 2012;49(6):586–592. doi:10.3109/02770903.2012.690481.
- Walenga RL, Longest PW. Current inhalers deliver very small doses to the lower tracheobronchial airways: assessment of healthy and constricted lungs. J Pharm Sci. 2016;105(1):147–159. doi:10.1016/j.xphs.2015.11.027.
- de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143–151. doi:10.1016/j.ejpb.2015.07.016.
- Tiddens HAWM, Bos AC, Mouton JW, Devadason S, Janssens HM. Inhaled antibiotics: dry or wet? Eur Respir J. 2014;44(5):1308–1318. doi:10.1183/09031936.00090314.
- Munro S, Vos W, Mignot B. P166 Use of in silico deposition modelling to evaluate the lung deposition patterns of budesonide delivered from different inhaler devices in patients with severe asthma. Thorax. 2018;73(Suppl 4):A192–A192. doi:10.1136/thorax-2018-212555.324.
- Vogelmeier C, Kardos P, Hofmann T, Canisius S, Scheuch G, Muellinger B, Nocker K, Menz G, Rabe KF. Nebulised budesonide using a novel device in patients with oral steroid-dependent asthma. Eur Respir J. 2015;45(5):1273–1282. doi:10.1183/09031936.00152014.
- Bakker EM, Volpi S, Salonini E, van der Wiel-Kooij EC, Sintnicolaas CJJCM, Hop WCJ, Assael BM, Merkus PJFM, Tiddens HAWM. Improved treatment response to dornase alfa in cystic fibrosis patients using controlled inhalation. Eur Respir J. 2011;38(6):1328–1335. doi:10.1183/09031936.00006211.
- Mainz J, Canisius S, Scheuch G, et al. An open-label randomized pilot trial to evaluate tolerability, safety and applicability of budesonide inhalation suspension (BIS) delivered via akita jet in children aged 3-11 years with mild to moderate asthma. J Aerosol Med Pulmonary Drug Deliv. 2013;26A:A45.
- Asthma GIf. Global Strategy for Asthma Management and Prevention 2020. 2020.
- Scott AM, Kolstoe S, Ploem MCC, Hammatt Z, Glasziou P. Exempting low-risk health and medical research from ethics reviews: comparing Australia, the United Kingdom, the United States and the Netherlands. Health Res Policy Syst. 2020;18(1):11. doi:10.1186/s12961-019-0520-4.
- Cooper BG, Stocks J, Hall GL, Culver B, Steenbruggen I, Carter KW, Thompson BR, Graham BL, Miller MR, Ruppel G, et al. The Global Lung Function Initiative (GLI) Network: bringing the world’s respiratory reference values together. Breathe (Sheff). 2017;13(3):e56–e64. doi:10.1183/20734735.012717.
- Brand P, Schulte M, Wencker M, Herpich CH, Klein G, Hanna K, Meyer T. Lung deposition of inhaled alpha1-proteinase inhibitor in cystic fibrosis and alpha1-antitrypsin deficiency. Eur Respir J. 2009;34(2):354–360. doi:10.1183/09031936.00118408.
- National Institute for Health and Care Excellence (NICE) Guideline. Inhaled corticosteroid doses for NICE’s asthma guideline Last updated July 2018.
- Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315(4):407–408. doi:10.1001/jama.2015.19394.
- Bakker EM, van der Wiel-Kooij EC, Müllinger B, Kroneberg P, Hop WCJ, Tiddens HAWM. Small-airways deposition of dornase alfa in children with asthma and persistent airway obstruction. J Allergy Clin Immunol. 2013;132(2):482–485 e10. doi:10.1016/j.jaci.2013.02.006.
- Fischer A, Stegemann J, Scheuch G, Siekmeier R. Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur J Med Res. 2009;14 Suppl 4(Suppl 4):71–77. doi:10.1186/2047-783x-14-s4-71.
- Konstantinos Katsoulis K, Kostikas K, Kontakiotis T. Techniques for assessing small airways function: Possible applications in asthma and COPD. Respir Med. 2016;119:e2–e9. doi:10.1016/j.rmed.2013.05.003.
- Maijers I, Kearns N, Harper J, Weatherall M, Beasley R. Oral steroid-sparing effect of high-dose inhaled corticosteroids in asthma. Eur Respir J. 2020;55(1):1901147. doi:10.1183/13993003.01147-2019.
- Kaplan A, Price D. Treatment adherence in adolescents with asthma. J Asthma Allergy. 2020;13:39–49. doi:10.2147/jaa.S233268.
- Weinstein JN. How do we move beyond regression to the mean? Improving health and health care. Spine (Phila Pa 1976). 2018;43(2):73–75. doi:10.1097/BRS.0000000000002504.
- Stoloff SW, Kelly HW. Updates on the use of inhaled corticosteroids in asthma. Curr Opin Allergy Clin Immunol. 2011;11(4):337–344. doi:10.1097/ACI.0b013e328348a813.