Abstract
Objective
A systematic review was performed to determine if the continuous laryngoscopy exercise test (CLE) has been used in the diagnostics of exercise dyspnea in adults with asthma, and whether inducible laryngeal obstruction (ILO) is found in those with asthma or with severe or difficult-to-treat asthma.
Data Sources
We used Scopus and PubMed databases. The articles published up to 13 August 2019 were considered.
Study Selections
We excluded manuscripts that did not contain information about adult patients with asthma. We included six studies from 59 search results in Scopus and none from the 17 search results in PubMed.
Results
The articles included 455 study individuals. Of these, 229 (50.3%) had diagnosed asthma or were treated with asthma medication. Altogether 31/229 (13.5%) subjects with diagnosis of asthma or previous asthma treatment had exercise-induced laryngeal obstruction (EILO) as comorbidity. The CLE test was performed on 229 patients with asthma. The method was used only for differential diagnosis of exercise-induced dyspnea to confirm EILO. At least 10/455 (2.2%) out of the 455 subjects experienced adverse events.
Conclusions
This systematic review revealed that only a small proportion of patients with asthma had undergone the CLE test to assess exercise-induced dyspnea. None of the selected manuscripts reported severity of asthma. Whether CLE provides a valuable diagnostic tool for patients with severe or difficult-to-treat asthma cannot be determined according to this review.
Introduction
Rationale
The continuous laryngoscopy exercise (CLE) test has been examined as an intervention to determine if exercise-induced dyspnea is caused by exercise-induced laryngeal obstruction (EILO). The CLE test has become the gold-standard method for diagnosing this condition. The method constitutes contemporary ergo-spirometry and laryngoscopy continuously performed during subject exercise to exhaustion and was first described by Heimdal et al. in 2006 (Citation1).
EILO is often misdiagnosed as asthma as the symptoms are similar. Probably the most remarkable difference is the timing of symptoms; in EILO symptoms appear at peak exercise and resolve in minutes, whereas in asthma symptoms usually appear after exercise and resolve more slowly. Furthermore, inspiratory stridor is atypical for patients with asthma and it is more usual for inducible laryngeal obstruction (ILO).
ILO is an umbrella term for laryngeal obstruction manifested by different inducers such as exercise, irritants, or emotional stress. ILO does not include conditions with obstruction in airways distal to the larynx, for instance asthma (Citation2).
It is postulated that exercise-induced bronchoconstriction results from transient dehydration of the airway surface when breathing dry air with increased rate during exercise. This leads to eosinophil and mast-cell activation and degranulation and release of inflammatory mediators (e.g. histamine, leukotrienes, tryptase, and prostaglandins) that may cause bronchoconstriction (Citation3). Alternatively, cooling of the airways has been suggested as a mechanism for exercise-induced bronchoconstriction, especially for example in skiers (Citation4).
Published studies on subjects with asthma involving the CLE test are lacking, and such studies mainly focus on dysfunctional breathing problems (Citation1,Citation5–9). In a recent study, the participants could not be separated into airway hyperreactivity and EILO groups only by symptoms (Citation5). Therefore, further examinations of the CLE test in patients with asthma are needed.
Objectives
Our systematic review aimed to investigate whether continuous laryngoscopy has been performed in adult patients with suspicion of asthma, diagnosed asthma, or severe or difficult-to-treat asthma (Citation10) in the assessment of exercise dyspnea. Furthermore, we aimed to explore if ILO is found in this CLE-tested population.
Materials and methods
The search strategy consisted of (asthma OR dyspnea) AND (cle OR “continuous laryngoscopy” OR “video laryngoscopy”) AND exercise. Dyspnea was involved in the search strategy since there was lack of search results if only asthma was included.
The participants and the examined condition involved patients with asthma symptoms, asthma suspicion, or diagnosis of asthma. The intervention constituted the CLE test. The context integrated identifying the underlying cause of dyspnea in patients with asthma-like symptoms or asthma. The main outcomes were number of study participants with asthma, and ILO findings in the CLE test in adults. We also determined if the CLE test was used in patients with severe or difficult-to-treat asthma and the feasibility of the CLE test in patients with asthma or suspicion of asthma.
In Scopus database we employed the filters article, English, human, and adult, which were automatically inserted in the search strategy with Boolean operator “AND” separating each criterion.
The same search strategy was performed in PubMed database. In PubMed we applied filters English, Humans, Adult: 19+ years, Case reports, Classical article, Clinical study, Clinical trial, Clinical trial phase I-IV, Comparative study, Controlled clinical trial, Evaluation studies, Journal article, Multicenter study, Observational study, Randomized controlled study, Twin study, Validation studies.
Only the original articles published up to August 13, 2019 were considered in both databases. Reviews were excluded. Two of the authors evaluated the search results and selected relevant publications to this topic based on abstracts. Qualitative synthesis and semi-quantitative synthesis were performed on whether the CLE test is feasible in adult patients with asthma.
Individual characteristics
Demographic outcomes included age, gender, lung-function results, smoking and use of asthma medication. Data for descriptive statistics was collected to and analyzed in Microsoft Excel for Mac version 16.53 (Microsoft 2021) software.
Definitions
Diagnosis of asthma was based on presence of symptoms and a positive bronchoprovocation test (BPT) or bronchodilator reversibility test (GINA guidelines) (Citation11). Alternatively, diagnosis of asthma was based on history and treatment of asthma in the previous year.
According to GINA guidelines, difficult-to-treat asthma is uncontrolled asthma despite treatment with medium- or high-dose inhaled corticosteroids with a second controller or with maintenance oral corticosteroid. Furthermore, severe asthma is considered uncontrolled despite adherence with maximal optimized therapy and worsens when high-dose treatment is decreased (Citation11).
Diagnosis of EILO is based on visual estimation of the laryngeal obstruction. In the Norwegian standardized scoring system, diagnosis of EILO rested on grade score at least 2 (moderate) for either glottic or supraglottic obstruction.
Outcomes
Clinical outcomes comprised number of subjects with diagnostic glottic obstruction, number of subjects with diagnostic supraglottic obstruction, comorbidity of asthma and EILO, adverse events and method of exercise.
Bias of the analyses was estimated by the following details: size of the study population, age of the study population, gender of the study population, diagnosed asthma or asthma suspicion, methods of asthma diagnostics, and whether asthma drugs were withdrawn before the test. Two researchers assessed bias and discrepancies were resolved by discussion. Possible bias was considered in the qualitative synthesis of the data. The PRISMA criteria were applied in this systematic review and in presentation of the results (Citation12).
Results
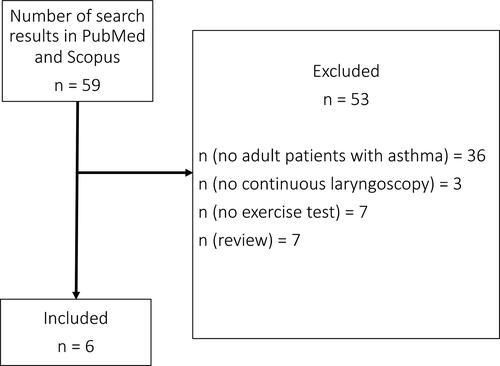
Six articles were included in this systematic review (). We obtained 59 search results in Scopus database and 17 search results in PubMed, of which all 17/17 were included in Scopus search results. Therefore, we obtained altogether 59 search results in this systematic review. The publications investigated CLE in exercise-induced dyspnea mostly in adult populations. When we excluded manuscripts that did not investigate asthma or adults, six publications remained for this systematic review (–) (Citation1,Citation5–9).
Table 1. Summary of the included studies involving objectives, study design, study population and methods.
Table 2. Summary of the included studies involving summary measures, main results, and bias.
Table 3. Summary of the included studies involving lung function.
Individual characteristics
These publications included 455 study individuals; of these, 229 (50.3%) had diagnosed asthma or were treated with asthma medication and 63.1% were women. Median age varied from 17 to 33 years and age range was 15 to 69 years (Citation1,Citation5–9).
Walsted et al. examined 23 patients with asthma (62.2% of the 37 subjects) and 5/23 of them (21.7%) had EILO (Citation9). Respectively, Nielsen et al. described 38 patients with asthma (43.2% of the 88 subjects), of which 12/38 had EILO (31.6%) (Citation6). Furthermore, Tervonen et al. analyzed 13 study individuals with asthma (43.3% of the 30 subjects) (Citation8). In 10/21 (47.6%) patients with asthma, EILO was confirmed by Christensen et al. (Citation5). Røksund et al. observed that asthma treatment during the year preceding the CLE test was reported by 128/151 (84.8%) of patients. Additionally, 113/151 (74.8%) patients had a normal laryngoscopy at rest and a moderate or severe adduction of laryngeal structures in parallel with increasing inspiratory distress during exercise (Citation7). Heimdal et al. reported 3/4 cases were treated with β2-agonists and 1 with inhaled corticosteroids. In maximal exercise, moderate supraglottic adduction appeared in all 4 cases and slight glottis adduction in 2, moderate in 1, and severe in 1 case (Citation1).
Definitions
When baseline FEV1 > 70% of expected, diagnosis of asthma was based on consistent symptoms and positive result in methacholine or mannitol bronchoprovocation test in the study of Walsted et al. In cases with baseline FEV1 ≤ 70% of expected, diagnosis of asthma was based on symptoms, history of asthma, bronchodilator reversibility, and peak flow variation >20% (Citation9). Nielsen et al. based diagnosis of asthma on symptoms and positive bronchoprovocation test or bronchodilator reversibility test (Citation6), whereas Tervonen et al. applied the Global Initiative for Asthma (GINA) criteria (Citation8). In the study of Christensen et al. diagnosis of asthma was mainly based on self-reported data when patients reported that they have or had had asthma in a questionnaire before testing (Citation5). In the study of Røksund et al. this was absence of information if diagnosis of asthma was confirmed in those with treatment for asthma (Citation7). Information on asthma test results was not provided by Heimdal et al. (Citation1).
Diagnosis of EILO was based on symptoms and glottic and supraglottic obstruction. Glottic and supraglottic laryngeal obstruction was graded utilizing the Norwegian standardized 0–3 scoring system (0 normal, 1 mild, 2 moderate, 3 severe) described by Maat et al. (Citation13) and Røksund et al. (Citation7) in four studies (Citation5–7,Citation9) and the EILOMEA method in two studies (Citation5,Citation6). The assessment of EILO consisted of grade score ≥2 (at least moderate) for either glottic or supraglottic obstruction assessed by Norwegian scoring system. Tervonen et al. visually diagnosed EILO when glottic narrowing was at least 90% (Citation8).
In the study of Tervonen et al. the glottic narrowing (collapse of the posterior supraglottic structures and aryepiglottic folds, and an adduction of the vocal cords) was visually evaluated by an otorhinolaryngologist; the authors expressed the need for a quantitative measurement (Citation8).
Heimdal et al. and Walsted et al. described separately the level of glottic and supraglottic obstruction (Citation1,Citation9).
Mode of exercise
One of the studies was performed on ergometer (Citation8) and the others on treadmill (Citation1,Citation5–9).
Outcomes
The CLE test was performed on 229 patients with asthma. Altogether, 31 (13.5%) diagnosed subjects with asthma had EILO as comorbidity ( and ) (Citation1,Citation5–9). Data on exercise-induced asthma was absent. The CLE test method was used only for differential diagnosis of exercise-induced dyspnea to confirm EILO but not for assessment of asthma. EILO was diagnosed in 44.6% (N = 203) of all subjects (N = 455) ( and ). Diagnosis of EILO was evident except from the study of Tervonen et al. in which four cases were highly suspicious of EILO but did not fulfill the EILO diagnosis criteria (Citation8). EILO was considered as moderate or severe in 43.5% of all study individuals (Citation1,Citation5–9). In the two studies by Heimdal et al. and Walsted et al. glottic obstruction was mild in 2/53 (3.8%), moderate in 1/53 (1.9%), and severe in 1/53 (1.9%) study individuals (Citation1,Citation9). Respectively, supraglottic obstruction was mild in 13/53 (24.5%), moderate in 4/53 (7.5%), and severe in 0/53 study individuals. Laryngeal (supraglottic or glottic) obstruction was moderate or severe altogether in 12/53 (22.6%) study individuals.
The number of study individuals suffering both asthma and EILO and experiencing supraglottic or glottic obstruction (or both) was missing (Citation5,Citation6,Citation9). Heimdal et al. reported that 100% of patients with asthma and EILO had moderate supraglottic obstruction at maximal effort. Respectively, 50% had slight, 25% moderate and 25% severe glottic obstruction (Citation1).
Only in one (Citation1) of the six studies that included subjects suffering from both asthma and EILO, separate lung function data for patients with asthma were presented (Citation1,Citation5–9). Heimdal et al. reported normal lung function values on average for patients with asthma before exercise test (Citation1) (). Furthermore, Røksund et al. reported normal lung function data on average both for study and asymptomatic control group (Citation7). Walsted et al. found no statistically significant difference in the number of patients with asthma between the EILO and non-EILO (subjects without EILO) groups (62% vs 63%). However, the FEV1/FVC ratio was slightly lower (0.83) in the non-EILO than in the EILO group (0.89) (p = 0.01). Exhaled nitric oxide (FeNO) exceeded 25 ppb in 12 non-EILO (43%) and 2 EILO study individuals (25%). Normal median FEV1 and FVC were reported in both groups (Citation9). The study population consisted of athletes in the study by Nielsen et al. Asthma was diagnosed in 38 subjects (43.2%). FeNO exceeded 50 ppb in two non-EILO study individuals. FEV1 mean values were reported as normal in both EILO and non-EILO groups (Citation6). Two studies did not present lung function data or severity of asthma of the subjects (Citation5,Citation8). However, in the study of Tervonen et al. 97% were reported as nonsmokers in study group in comparison to 6.7% in the controls (Citation8).
At least 10 (2.2%) out of the 455 subjects experienced adverse events. The FEV1 measurement triggered a laryngeal spasm in one patient, one experienced a panic attack during preparation phase, and one had vasovagal collapse during local anesthesia of the nose. These events caused termination of the test. An asthma-like attack appeared in one patient, the oxygen saturation decreased to <90% temporarily in five study individuals, and a ST level decrease was observed in one subject (coronary artery disease was revealed in subsequent tests) (Citation8). Furthermore, procedural anxiety and hyperventilation attacks or frank panic reactions in certain study individuals were observed (Citation7).
Bias and limitations
Bias across studies may have been due to overrepresentation of young athletic Caucasian individuals without lung diseases or severe lung diseases (Citation1,Citation5–9). Sample size was frequently limited. In three studies, asthma drugs were withdrawn before bronchoprovocation test (Citation5,Citation6,Citation9). β sympathomimetics were given to subjects before the CLE test in two of these studies (Citation5,Citation6). In addition, two studies presented usage of asthma medication instead of diagnosis of asthma (Citation1,Citation7).
Discussion
Individual characteristics
In this review, 229 (50%) of the 455 study individuals from the six included studies had diagnosed asthma (Citation1,Citation5–9). Altogether 45% of the study participants had diagnosis of EILO. Altogether 31 (14%) subjects with diagnosed asthma had EILO as comorbidity. According to the lung function values, which were provided in four of the six selected articles (Citation1,Citation6,Citation7,Citation9), there was a lack of subjects with severe asthma (Citation1,Citation5–9).
The subjects consisted of mostly athletic and young adults (mean or median age between 16–31 years old). Overall, the study population lacked the elderly and patients with lower physical fitness or more severe overall morbidity. Of the study individuals, 63.1% were female. Although the severity of asthma was not reported in detail, we assume that most of the patients of asthma were young and otherwise rather healthy, and their severity of asthma was mild-to-moderate according to overall young age of the study individuals.
The study populations were predominantly small but had a large range in size (range 16–186). Patients with asthma were well represented (50.3%, n = 229) in the populations studied, and 13.5% (n = 31) had coexisting EILO.
Definitions
Not every EILO patient experiences maximal exercise-induced symptoms when cycling or running, and this likely applies also to patients with asthma. Additionally, environmental factors were missing (such as dry or cold air or allergens), which can also provoke symptoms during exercise in patients with asthma. A recent study reported the possibility of performing the CLE test while swimming (Citation14). This reflects the importance of examining the patient in a relevant environment and during their usual exercise method. In addition to different environments and exercise types that may affect the development of EILO, day-to-day variation may also affect the progress of symptoms and EILO, which should be considered when interpretating CLE test results (Citation6).
In two of the studies, diagnosis of asthma was based on symptoms of asthma and reported lung function data. The data consisted of peak flow monitoring, methacholine or mannitol challenge test, β2-agonist reversibility in spirometry, or eucapnic hyperventilation test (EVH) (Citation6,Citation9). Tervonen et al. employed the same diagnostic criteria according to GINA in 2009 (8).
In three studies, asthma drugs were withdrawn before the bronchoprovocation test and timing of the withdrawal in relation to the CLE test was unclear (Citation5,Citation6,Citation9). Furthermore, occasionally β sympathomimetics were given to subjects before the CLE test, which can mask possible symptoms of exercise-induced asthma and prevent EIB (Citation5,Citation6). It is possible that the number of patients with symptoms of exercise-induced asthma and the findings would have been more notable if asthma drugs were withdrawn before the test in all the studies. We assume that administration or withdrawal of asthma drugs should not have an effect on laryngeal obstruction (EILO).
We aimed to explore if the CLE test could be used in the differential diagnosis of severe asthma. However, none of the selected studies described asthma severity. Thus, we were unable to assess whether patients with asthma had severe asthma or whether patients with severe asthma were even included in these studies. The highest median age of study individuals in one study was 27.8 years (range 15–69 years) (Citation8). Furthermore, the FEV1/FVC ratio was 0.832 in the non-EILO group and 0.892 in the EILO group (Citation9). Thus, we assume that these studies excluded patients with severe asthma or the number of these patients was minimal (Citation1,Citation5–9). Moreover, the data on whether patients with difficult-to-treat asthma were included are absent.
EILO was estimated by visual scoring in all the studies (Citation1,Citation5–9). Most of the studies adopted the Norwegian standardized scoring system for estimating the level of laryngeal obstruction and placed patients with moderate to severe grade in the EILO group (Citation1,Citation5–9). Mild laryngeal obstruction was not diagnostic for EILO. In the study of Tervonen et al. diagnosis of EILO was based on findings of inspiratory stridor, supraglottic collapse of arytenoids and aryepiglottic folds toward the aditus of the larynx, and vocal cord adduction (Citation8). In two manuscripts, moderate-to-severe EILO were grouped together (Citation5,Citation8). Additionally, in two other studies the number of study individuals suffering from glottic or supraglottic obstruction or both was not clearly expressed for each subgroup (Citation6,Citation7). It was not possible to estimate how many study individuals experienced either severe glottic or supraglottic obstruction, as these often coexist and the same subjects may be included in both subgroups.
Outcomes
Separate results for and the number of patients with moderate and severe obstruction were infrequently reported. Only two (Citation1,Citation9) from the six studies presented the level of laryngeal obstruction separately for glottic and supraglottic obstruction (Citation1,Citation5–9) in the manner that number of study individuals in each subgroup of severity degree was evident.
In the four studies providing lung function values further information about the medication of each study individual with asthma was missing (Citation1,Citation6,Citation7,Citation9). Therefore, the degree of laryngeal obstruction in patients with asthma or with severe or difficult-to-treat asthma could not be estimated. None of the examinations applied the CLE test for diagnosing asthma or determining its severity. All the analyses performed the CLE test to specifically diagnose EILO (Citation1,Citation5–9).
Furthermore, none of the studies was performed systematically in patients with severe asthma. The clinical background to the exercise tests was the differential diagnosis of exercise dyspnea to evaluate whether the symptoms were caused by exercise asthma or EILO.
It is likely that the studied populations with asthma were biased with a greater proportion of EILO findings than would be expected in the general population or general asthma population (Citation1,Citation5–9). However, according to this review, we cannot estimate how frequent EILO might be among patients with severe asthma.
Although CLE is considered especially when a patient experiences atypical asthma or exercise-associated respiratory symptoms that are suspected as laryngeal, the study of Christensen et al. revealed that none of the symptoms was specific to bronchial hyperreactivity or to EILO (Citation5). This highlights the importance of considering CLE e.g. when response to asthma medication is insufficient. Additionally, rarer conditions, such as exercise-induced anaphylaxis (EIA), should be considered if exercise-induced symptoms are atypical and include pruritus, urticaria, and hypotension (Citation3).
Conclusions
This systematic review revealed that the CLE test has been applied to analyze exercise-induced dyspnea among patients with asthma only with a limited number of patients. Most of the study individuals were young athletes. Day-to-day variation of symptoms and EILO should be considered when interpreting the results of a single CLE test (Citation6). Simultaneous presentation of asthma and EILO is possible; in this review, 13.5% of the subjects with diagnosed asthma had EILO as comorbidity. Whether the CLE test is a valuable diagnostic tool for severe or difficult-to-treat asthma patients cannot be assessed according to our review.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Heimdal JH, Røksund OD, Halvorsen T, Skadberg BT, Olofsson J. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope. 2006;116(1):52–57. doi:10.1097/01.mlg.0000184528.16229.ba.
- Christensen PM, Heimdal JH, Christopher KL, Bucca C, Cantarella G, Friedrich G, Halvorsen T, Herth F, Jung H, Morris MJ, et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions. Eur Respir Rev. 2015;24(137):445–450. doi:10.1183/16000617.00006513.
- Weiler JM, Brannan JD, Randolph CC, Hallstrand TS, Parsons J, Silvers W, Storms W, Zeiger J, Bernstein DI, Blessing-Moore J, et al. Exercise-induced bronchoconstriction update-2016. J Allergy Clin Immunol. 2016;138(5):1292–1295 e36. doi:10.1016/j.jaci.2016.05.029.
- Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, Storms WW, Weiler JM, Cheek FM, Wilson KC, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187(9):1016–1027. doi:10.1164/rccm.201303-0437ST.
- Christensen PM, Thomsen SF, Rasmussen N, Backer V. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol. 2011;268(9):1313–1319. doi:10.1007/s00405-011-1612-0.
- Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc. 2013;45(11):2030–2035. doi:10.1249/MSS.0b013e318298b19a.
- Røksund OD, Maat RC, Heimdal JH, Olofsson J, Skadberg BT, Halvorsen T. Exercise induced dyspnea in the young. Larynx as the bottleneck of the airways. Respir Med. 2009;103(12):1911–1918. doi:10.1016/j.rmed.2009.05.024.
- Tervonen H, Niskanen MM, Sovijarvi AR, Hakulinen AS, Vilkman EA, Aaltonen LM. Fiberoptic videolaryngoscopy during bicycle ergometry: a diagnostic tool for exercise-induced vocal cord dysfunction. Laryngoscope. 2009;119(9):1776–1780. doi:10.1002/lary.20558.
- Walsted ES, Hull JH, Sverrild A, Porsbjerg C, Backer V. Bronchial provocation testing does not detect exercise-induced laryngeal obstruction. J Asthma. 2017;54(1):77–83. doi:10.1080/02770903.2016.1195843.
- Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–976. doi:10.1056/NEJMra1608969.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021. Available from: www.ginasthma.org.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi:10.1016/j.jclinepi.2021.03.001.
- Maat RC, Røksund OD, Halvorsen T, Skadberg BT, Olofsson J, Ellingsen TA, Aarstad HJ, Heimdal JH. Audiovisual assessment of exercise-induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol. 2009;266(12):1929–1936. doi:10.1007/s00405-009-1030-8.
- Walsted ES, Swanton LL, van Someren K, Morris TE, Furber M, Backer V, Hull JH. Laryngoscopy during swimming: a novel diagnostic technique to characterize swimming-induced laryngeal obstruction. Laryngoscope. 2017;127(10):2298–2301. doi:10.1002/lary.26532.