Abstract
Objective
To evaluate the real-world impact of mepolizumab on the incidence of asthma exacerbations, oral corticosteroid (OCS) use and asthma exacerbation-related costs in patients with high-burden severe asthma.
Methods
This was a retrospective study of the MarketScan Commercial and Medicare Databases in patients with high-burden severe asthma (≥80th percentile of total healthcare expenditure and/or significant comorbidity burden). Patients were ≥12 years of age upon mepolizumab initiation (index date November 1, 2015–December 31, 2018) and had ≥2 mepolizumab administrations during the 6 months post-index. Asthma exacerbation frequency (primary outcome), use of OCS (secondary outcome), and asthma exacerbation-related costs (exploratory outcome) were assessed during the 12 months pre-index (baseline) and post-index (follow-up).
Results
In total, 281 patients were analyzed. Mepolizumab significantly reduced the proportion of patients with any asthma exacerbation (P < 0.001) or exacerbations requiring hospitalization (P = 0.004) in the follow-up versus baseline period. The mean number of exacerbations decreased from 2.5 to 1.5 events/patient/year (relative reduction: 40.0%; P < 0.001). The proportion of patients with ≥1 OCS claim also decreased significantly from 94.0% to 81.9% (relative reduction: 12.9%; P < 0.001), corresponding to a decrease from 6.6 to 4.7 claims/person/year (P < 0.001). Of the 264 patients with ≥1 OCS claim during baseline, 191 (72.3%) showed a decrease in mean daily OCS use by ≥50% in 117 patients (61.3%). Total asthma exacerbation-related costs were significantly lower after mepolizumab was initiated (P < 0.001).
Conclusions
Mepolizumab reduced exacerbation frequency, OCS use and asthma exacerbation-related costs in patients with high-cost severe asthma. Mepolizumab provides real-world benefits to patients, healthcare systems and payers.
Introduction
The standard of care for patients with severe asthma is treatment with high-dose inhaled corticosteroids (ICS) alongside a second controller and/or systemic corticosteroid (SCS) (Citation1). However, despite being part of the standard of care in these patients, chronic SCS use is associated with several well-documented side effects, including increased risk of infections, diabetes, osteoporosis, and psychiatric disorders (Citation2). In addition, growing evidence shows that even short bursts of SCS treatment (<30 days) may be associated with adverse effects, including an increased risk of gastrointestinal ulcers/bleeding, loss of bone density and serious impacts on mental health (Citation3).
Even with continuous use or bursts of SCS, patients with severe asthma may still experience frequent exacerbations (Citation1). These events are the main contributor to direct costs in asthma (Citation4), meaning that patients with severe asthma have significantly increased costs compared with patients with less severe asthma (Citation5–8). In addition, patients with severe asthma frequently have serious comorbidities (Citation9–11), which can contribute to poor asthma control (Citation12) and increased healthcare resource utilization and costs (Citation13,Citation14). The combination of frequent exacerbations and high comorbidity burden makes patients with severe asthma among the most costly to treat, and this group accounts for approximately half of all annual asthma-related costs (Citation5,Citation15).
A recent study estimated the annual direct medical costs of severe asthma in the US to be $9175 per year. On top of this the indirect costs of missed work and reduced productivity amounted to a further $1000 for patients with severe asthma (Citation16). Thus, there remains an unmet need for cost-effective treatments for severe asthma that lower the frequency of exacerbations, and the requirement for SCS and asthma-related healthcare costs.
Mepolizumab is a humanized monoclonal antibody that binds to and inactivates interleukin (IL)-5, thereby blocking the proliferation, activation and survival of eosinophils (Citation17). It is approved for the treatment of severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis in multiple regions worldwide, and for hypereosinophilic syndrome, and chronic rhinosinusitis with nasal polyps in the USA (Citation18–20). In patients with severe asthma, mepolizumab has reduced exacerbation rates and SCS use in both clinical trials and real-world studies (Citation9,Citation10,Citation21–27) Additionally, real-world analyses have demonstrated the positive clinical effectiveness of mepolizumab in patients with severe and life-threatening asthma (Citation28–31). However, there remain limited data on the real-world effect of mepolizumab in the subset of patients with asthma who incur the largest proportion of asthma-related healthcare expenses (e.g. patients who require the most healthcare resource use and are most likely to frequently attend emergency room) and who have high comorbidity burden.
The aim of this study was to compare the rate of asthma exacerbations, OCS use and asthma exacerbation-related costs in the 12 months before and after initiating mepolizumab in patients in the USA with high-cost severe asthma (i.e. those comprising the highest 20% of total medical and pharmacy costs and/or with a high comorbidity burden).
Methods
Study design and data source
This was a retrospective study of data from the MarketScan Commercial Database and Medicare Supplemental Database (GSK ID: 212693, HO-20–19952). Between 1995 and 2018, this database was estimated to contain the healthcare experience of approximately 198.9 million privately insured individuals in the USA covered under a variety of fee-for-service, partially capitated and fully capitated health plans. Patients were tracked longitudinally across the two databases in order to prevent patients being counted twice.
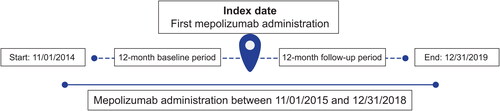
Patients with asthma costly to treat who were newly prescribed mepolizumab from November 1, 2015, to December 31, 2018, were identified; the index date was the date of the first medical or pharmacy claim for this treatment. Data were examined for patients during the 12 months prior to and including the index date (baseline period) and in the 12 months following the index date (follow-up period) (). This study used previously collected and fully deidentified data and as such was not classified as research involving human participants. Therefore, institutional review board approval was not required.
Study population
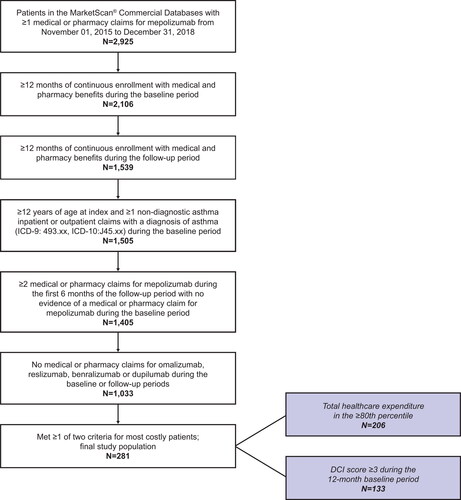
Eligible patients were ≥12 years of age and had evidence of mepolizumab use between November 1, 2015, and December 31, 2018. Patients were required to have ≥12 months of continuous enrollment with medical and pharmacy benefits during the baseline period and the 12-month follow-up period. During the baseline period, patients must have had ≥1 nondiagnostic inpatient or outpatient claim with a diagnosis code for asthma (International Classification of Diseases [ICD]-9: 493.xx, ICD-10:J45.xx) in any position on the claim. Severe asthma was defined according to GINA, where patients required Step 4 or 5 treatment to control their asthma, had asthma that was uncontrolled despite this treatment, or received maintenance OCS for >50% of the prior year (Citation12). Additionally, eligible patients with asthma were required to have (i) total healthcare expenditure in the ≥80th percentile (i.e. those who had the top 20% of costs in the database) and/or (ii) a Deyo Charlson Comorbidity (DCI) score ≥3 (Table S1). Patients must also have received ≥2 administrations of mepolizumab during the first 6 months of the follow-up period. Individuals with evidence of omalizumab, reslizumab, benralizumab or dupilumab use during the baseline or follow-up periods, or mepolizumab during the baseline period were excluded (Table S2).
Table 1. Patient demographics and baseline characteristics.
Outcomes
The primary outcome was the change in asthma exacerbations (any exacerbation or an exacerbation resulting in hospitalization) during the follow-up period versus the baseline period, measured as the proportion of patients experiencing an exacerbation and total number of exacerbations. An exacerbation was defined as an event that required healthcare utilization and met one of the following criteria: (i) ≥1 outpatient or emergency room claim with a diagnosis of asthma (ICD-9: 493.xx, ICD-10: J45.xx) in any position and ≥1 claim for SCS (intramuscular, intravenous, or oral) within −4/+5 days of the event, or (ii) an inpatient hospital admission with a primary diagnosis of asthma (ICD-9: 493.xx, ICD-10: J45.xx). The start of the exacerbation period was the date of the outpatient or emergency room claim, and the end of the exacerbation period was 13 days following the exacerbation start. Exacerbation episodes lasted a minimum of 14 days, and exacerbations occurring within 14 days following the end of a prior exacerbation were considered a single episode. Claims for scheduled administration of mepolizumab were excluded.
The secondary outcome was change in OCS use in the follow-up versus baseline period, measured as: (i) the proportion of patients with ≥1 OCS claim; (ii) the mean number of OCS claims per patient; (iii) the change in mean daily OCS dose, calculated as the sum of OCS doses across all pharmacy claims/365 (days), and standardized using prednisone equivalents; (iv) the number of patients with chronic OCS use, defined in two ways to measure chronic use over the full baseline and follow-up periods as well as use of higher doses of OCS over a 90-day period (first, at a mean daily dose of ≥5 mg over the 12-month baseline or follow-up period and, secondly, a mean daily dose of ≥10 mg for the final 90 days of the baseline period and for each 90 days during the follow-up period); (v) the proportion of patients with ≥1 OCS burst, and; (vi) the mean number of OCS bursts per patient. An OCS burst was defined as an OCS pharmacy claim with a mean daily dose of ≥20 mg prednisone equivalents for 3–28 days and ≥1 outpatient or emergency room claim with a diagnosis of asthma in any position within −7/+6 days of this OCS pharmacy claim.
Change in asthma exacerbation-related costs during the follow-up versus baseline period was an exploratory outcome. Costs included were inpatient claims with a primary diagnosis of asthma, outpatient claims with an asthma diagnosis in any position, or medical or pharmacy claims for asthma ‘rescue’ medications during an exacerbation. Mepolizumab acquisition and administration costs and the cost of all inhaled maintenance therapies (including ICS, long-acting β2-agonists [LABA], long-acting muscarinic antagonists [LAMA], leukotriene receptor antagonists [LTRA], mast cell stabilizer [MCS] and methylxanthines) were excluded (Citation10).
Additional outcomes assessed in this study included the number of mepolizumab claims and the proportion of patients with ≥10 mepolizumab claims (both during the follow-up period), and the proportion of patients using asthma medications and the number of medication claims during the baseline and follow-up periods.
Statistical analysis
Patient demographics (examined on the index date) and clinical characteristics (examined during the baseline period) were analyzed descriptively. Differences in outcomes between the baseline and follow-up periods were analyzed using McNemar’s tests (nominal and categorical variables) and paired t-tests (interval and continuous variables).
Results
Patient population
In total, 281 patients met the criteria for inclusion in this analysis (). Of these patients, 75 (26.7%) had a DCI score ≥3, 148 (52.7%) had total costs in the top 20% and 58 (20.6%) met both criteria (). The mean age of this sample was 53.9 years, and most (59.4%) patients were female. The most common DCI conditions (excluding chronic pulmonary disease experienced by all patients) were mild or moderate diabetes in 78 patients (27.8%) and a malignancy in 56 (19.9%) (Table S1). The most common comorbid conditions were allergic rhinitis (65.8%), hypertension (57.7%), sinusitis (56.9%), respiratory infections (56.2%) and chronic obstructive pulmonary disease (COPD) (50.2%) (). Overall, 65.2% of patients were receiving medium or high dose ICS or ICS/LABA at baseline.
Primary outcome: asthma exacerbations
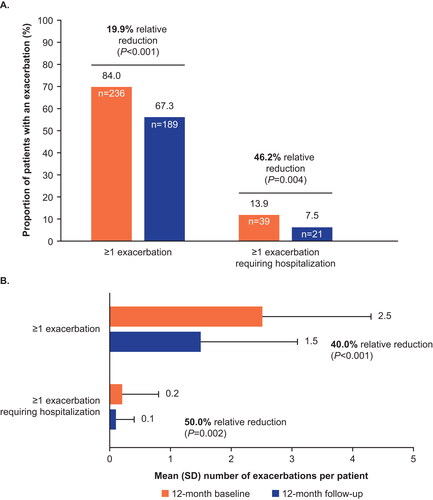
The proportion of patients with any asthma exacerbation significantly decreased from 84.0% in the baseline period to 67.3% in the follow-up period (P < 0.001), corresponding to a 19.9% relative reduction. Similarly, there was a significant relative reduction of 46.0% (from 13.9% to 7.5%) in the proportion of patients with exacerbations requiring hospitalization (P = 0.004) (). The mean number of any exacerbation per patient decreased from 2.5 in the baseline period to 1.5 during the follow‑up period, corresponding to a relative reduction of 40.0% (P < 0.001). A significant relative reduction (50.0%) was also seen in the number of exacerbations requiring hospitalization (from 0.2 to 0.1; P = 0.002) ().
Figure 3. Proportion of patients with exacerbations (A), and mean number of exacerbations per patient (B) during the baseline and follow-up periods.
Any exacerbation was defined as ≥1 outpatient or emergency room claim with a diagnosis of asthma (ICD-9: 493.xx, ICD-10: J45.xx) and ≥1 claim for a SCS (intramuscular, intravenous, or oral) within −4/+5 days of the encounter; an exacerbation requiring hospitalization was defined as inpatient hospital admissions with a primary diagnosis of asthma.
ICD, International Classification of Diseases; SCS, systemic corticosteroid; SD, standard deviation.
Secondary outcome: use of OCS
The proportion of patients with ≥1 OCS claim reduced significantly from 94.0% (n = 264) in the baseline period to 81.9% (n = 230) during the follow-up period (a relative reduction of 12.9%; P < 0.001). This corresponded to a significant decrease in the mean number of OCS claims of 28.8% (from 6.6 to 4.7 per year; P < 0.001) (). Of the 264 patients with ≥1 OCS claim during the baseline period, 191 (72.3%) showed a reduction in mean daily OCS use, and for 117 patients (61.3%) OCS use dropped to half of baseline levels or lower (Figure S1). The mean (standard deviation [SD]) daily dose of OCS fell from 10.6 (30.2) mg/day in the baseline period to 7.5 (21.7) mg/day in the follow-up period (P = 0.09).
Figure 4. Number of OCS claims and OCS bursts per patient (A), patients with OCS claims and OCS use at a dose of ≥5mg/day (B), and OCS use at a dose of ≥10 mg/day (C) during the follow-up period.
OCS bursts were defined as mean ≥20 mg/day prednisone equivalents for a duration of 3–28 days and 1 outpatient or emergency room claim with a diagnosis of asthma (ICD-9: 493.xx, ICD-10: J45.xx) ± 7 days of the pharmacy claim.
ICD, International Classification of Diseases; OCS, oral corticosteroids; SD, standard deviation.
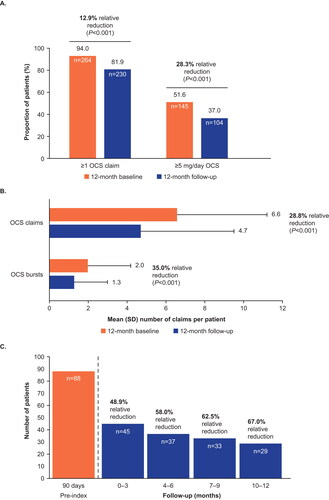
In terms of chronic OCS use, the proportion of patients using OCS at a mean daily dose of ≥5 mg decreased significantly from 51.6% during the baseline period to 37.0% in follow-up, corresponding to a relative reduction of 28.3% (P < 0.001) (). Between the 90 days pre-index date and the first 90 days of the follow-up period, the number of patients using OCS at a mean daily dose of ≥10 mg decreased from 88 to 45 patients (a relative reduction of 48.9%) and continued to decrease over the remaining 9 months of the follow-up period, culminating in a 67.0% relative reduction in the final 3 months of follow-up versus pre-index ().
The number of patients with ≥1 OCS burst fell from 191 (68.0%) at baseline to 147 (52.3%) at follow-up. The mean number of OCS bursts per patient reduced significantly from 2.0 in the baseline period to 1.3 in the follow-up period (a relative reduction of 35.0%; P < 0.001) ().
Exploratory outcome: exacerbation-related costs
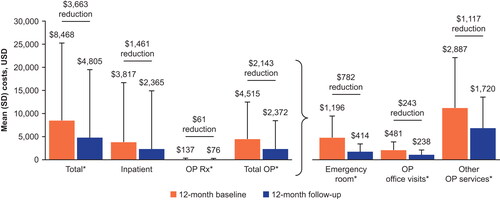
Total asthma exacerbation-related costs (excluding mepolizumab, administration and inhaled maintenance therapy costs) were reduced from $8,468 during the baseline period to $4,805 during the follow-up period, a significant saving of $3,663 (P < 0.001) (). Costs were significantly (P < 0.05) lower during the follow-up versus baseline period across all cost component categories with the exception of inpatient costs, which demonstrated a non-significant decrease during the follow-up versus baseline period ().
Figure 5. Asthma exacerbation-related healthcare costs excluding maintenance therapy costs.
*P < 0.05. Costs for mepolizumab and other maintenance medications are excluded. All costs were adjusted for inflation using the Consumer Price Index and standardized to 2019 US dollars. Claims with asthma exacerbation-related costs were identified as inpatient claims with a primary diagnosis of asthma, outpatient claims with an asthma diagnosis in any position, or medical or pharmacy claims for systemic corticosteroids and "rescue" medications during the exacerbation episode.
OP, outpatient; Rx, medical prescription; SD, standard deviation; USD, US dollars.
Additional outcomes: mepolizumab claims and use of therapies
Patients had a mean (SD) of 10.2 (4.1) mepolizumab claims during the follow-up period with an average of 37.3 days (17.4) between claims. In all, 62.3% (n = 175) of patients had ≥10 mepolizumab claims. The proportion of patients who had a gap of >12 weeks between claims was 22.1%.
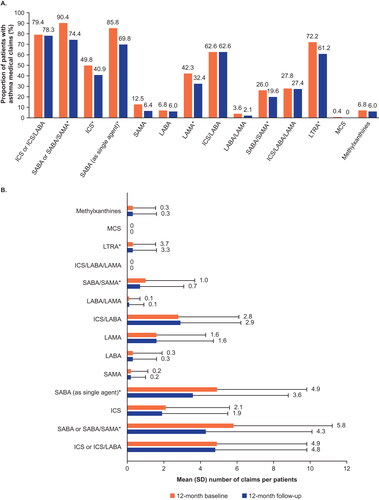
The proportion of patients using asthma therapies was lower during the follow-up compared with the baseline period for all categories except ICS/LABA (). The mean number of medication claims per patient did not change remarkably in the follow-up period compared with the baseline period for most therapies, with the exception of LTRA, which were significantly reduced (P < 0.05) ().
Figure 6. Proportion of patients with asthma medication claims (A) and the mean number of claims (B) during the baseline and follow-up periods.
Mean ICS/LABA/LAMA use could not be calculated as usage was determined based on claims for ICS/LABA/LAMA therapy or overlapping ICS, LABA, and LAMA claims; *P < 0.05
ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; MCS, mast cell stabilizer; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation.
Discussion
To the best of our knowledge, this is the first real-world study to examine the effect of mepolizumab in patients with high-burden severe asthma, i.e. those with total medical and pharmacy costs in the top 20% and/or those with a high comorbidity burden. Mepolizumab treatment was associated with a significant 40–50% reduction in annual asthma exacerbation frequency, in addition to a significant reduction in the proportion of patients requiring OCS use and mean number of OCS claims per patient. Mepolizumab treatment also led to significant savings in total asthma exacerbation-related healthcare costs in all cost component categories except for inpatient costs, which demonstrated a non-significant reduction. The results from this study align with reports from clinical studies and prior observational studies, and support the real-world effectiveness of mepolizumab in treating patients with severe asthma (Citation9,Citation10,Citation21–26,Citation29–31). The findings are also consistent with studies that have shown switching from omalizumab to mepolizumab reduced annual costs to patients; with the increased cost of mepolizumab offset by savings to all other costs (reduced exacerbations and OCS costs) (Citation32). In this study, initiation of mepolizumab significantly reduced the incidence of asthma exacerbations, particularly those requiring hospitalization. Preventing exacerbations in patients with this phenotype is clinically important, not only for short-term alleviation of disease burden, but because it may also help to avoid longer-term issues associated with severe and frequent exacerbations, including irreversible lung function decline (Citation33,Citation34). The results presented here support those from clinical trials (Citation21,Citation22,Citation35,Citation36) and real-world studies in populations with severe (eosinophilic) asthma (Citation8,Citation9,Citation24,Citation25,Citation29–31,Citation37) in demonstrating the clinical benefit of mepolizumab in patients with severe asthma in clinical practice. Additionally, although not assessed in this study, improved asthma control may also translate into more general improvements in health such as improved health-related quality of life, as demonstrated in randomized controlled trials (Citation21,Citation22).
Mepolizumab led to a significantly reduced need for OCS. Importantly, almost two-thirds of patients showed a decrease in mean daily OCS use of ≥50% versus baseline levels, and chronic use of OCS was also reduced according to both definitions. High-dose and/or long-term OCS have been associated with numerous adverse effects, including increased risk of infections, diabetes, osteoporosis, and psychiatric disorders (Citation2), but are necessary for some patients with severe asthma to manage their disease (Citation1,Citation38). Therefore, by lessening the reliance on OCS, mepolizumab treatment may reduce treatment burden and lessen the risk of OCS-related adverse events in this cohort. The significant reduction in OCS bursts in this study following initiation of mepolizumab is also highly relevant. Although OCS bursts are not defined consistently in the literature, and vary from the definition used in this study, there is robust and increasing evidence associating bursts with risks similar to those of long-term OCS use, such as gastrointestinal ulcers/bleeding, loss of bone density and serious impacts on mental health (Citation3). The OCS-sparing effect of mepolizumab has also been seen in previous clinical trials and real-world studies (Citation9,Citation22,Citation27,Citation29–31).
Use of maintenance medications such as ICS decreased somewhat following mepolizumab initiation. This complements the findings of two previous retrospective studies of patients receiving mepolizumab, the first found that nearly 50% of patients reduced or discontinued ICS for at least 3 months following mepolizumab initiation (Citation39) and the second observed a step-down of maintenance treatment in ∼40% of patients following at least 6 months of mepolizumab treatment (Citation29). Similarly, another retrospective study in patients with life-threatening asthma found that patients with the highest cost and comorbidity burden are likely to benefit from mepolizumab treatment through reduced use of most other maintenance medications (Citation28), which may be physician-recommended or patient-initiated. Together, this indicates that mepolizumab may reduce treatment burden beyond its established OCS reducing effects.
In this study, the clinical benefits of mepolizumab translated into lower exacerbation-related costs. Mean exacerbation-related costs were reduced by almost half, from $8,468 to $4,805, and significant decreases in costs were seen across all components of healthcare utilization except inpatient costs. The non-significant decrease in inpatient costs may be attributable to the low absolute incidence of exacerbations requiring hospitalization during both the baseline (0.2/year) and follow-up (0.1/year) periods, or to the fact that the cost of each hospitalization, unlike the overall number of hospitalizations, is unlikely to change. Additionally, it is possible that a small number of patients, each with a high number of admissions, continued to drive inpatient costs during the follow-up period. Overall, these results suggest that in addition to reducing the burden of severe asthma on patients, mepolizumab also provides benefits to healthcare systems and payers.
The patients included in this real-world study likely represented a wider, more heterogeneous population than that included in the mepolizumab clinical development program, which included randomized controlled trials with stringent eligibility criteria in which patients with concurrent respiratory disease and other comorbidities were excluded (Citation21,Citation22). Additionally, approximately half the patients included in the current study had COPD, higher than the 9% included in early initiators of treatment analysis for the real-world REALITI-A study, which evaluated mepolizumab effectiveness in clinical practice (Citation26). Consequently, although the efficacy of mepolizumab in patients with COPD remains to be fully established (Citation40,Citation41), the results of the current study, along with a recent retrospective real-world study conducted in the United States (Citation42), suggest that patients with severe asthma and comorbid COPD may benefit from mepolizumab. The broad population of patients in the current study may also have contributed to a lower mean baseline exacerbation rate (2.5/year) than observed in some previous randomized controlled trials and real-world studies (2.9–4.6/year) with more limited study populations (Citation21,Citation22,Citation24,Citation26,Citation29,Citation30,Citation37); the current study selected for patients with high healthcare costs and/or comorbidity burden compared with previous studies, which selected for patients with frequent exacerbations or did not require high costs or comorbidity burden.
The claims in the MarketScan Commercial Database are used as a proxy for medication use, as is the case for other similar studies. A number of limitations are inherent to this approach. As pharmacy claims do not contain a diagnosis, it is not possible to determine whether the steroid use data extracted for the current analysis was specifically for asthma or a comorbid condition. To control for this, the study was designed to compare changes within individuals pre- and post-mepolizumab treatment rather than that at a population-level. Multiple metrics of OCS use were evaluated as both chronic and acute OCS use are important markers of asthma control. These included mean daily doses and number of claims which did not have to be linked to an asthma diagnosis and OCS bursts, which had to be associated with a diagnosis of asthma within −7/+6 days of the claim. Adherence with prescriptions cannot be established, and the total number of patients who claimed for asthma treatments is therefore likely to have been overestimated. In addition, data are subject to coding limitations and entry error (Citation43), although these are likely to be rare and minimal when they occur because of accuracy requirements on medical claims. Furthermore, the results presented here may not be generalizable beyond the private and Medicare insurance population, particularly because of international differences in epidemiology of severe asthma and healthcare systems. Finally, because comorbidities were only assessed during the baseline period, the contribution of time-varying risk factors (Citation44) to exacerbation frequency were not accounted for. However, because of the self-paired pre-and-post approach study design used here, time-fixed confounding factors were accounted for and allowed comparison of changes in outcome before and after mepolizumab initiation, an advantage over cross-sectional study designs. The MarketScan Commercial Database contains a large volume of data on patients in a real-world setting, most of which are standardized, meaning comparisons are robust and can be performed with a reasonably large sample size without the impact of missing data, which is common in other types of real-world studies. Finally, this study comprises a wider population of patients in real-world clinical practice than are usually assessed in randomized clinical trials because of more strict eligibility criteria.
Conclusions
Mepolizumab treatment was associated with reductions in exacerbation frequency, OCS use and asthma-exacerbation-related costs in patients with high-cost severe asthma, i.e. those with the highest total healthcare expenditures and/or significant comorbidity burden. These results complement existing clinical trial data and real-world studies, and further demonstrate that mepolizumab provides important clinical benefits to patients and cost benefits to healthcare systems and payers.
Supplemental Material
Download EPS Image (1.5 MB)HO-20-19952_1Y_MS_PRR_Supplement_submission_clean.docx
Download MS Word (134 KB)Acknowledgements
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Katie Crossland, PhD, at Fishawack Indicia, part of Fishawack Health, UK, and was funded by GSK.
Declaration of interests
NLL received consulting fees for advisory board participation from Amgen, AstraZeneca, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GSK and AstraZeneca; and travel support from AstraZeneca; her institution received research support from Amgen, AstraZeneca, Avillion, Gossamer Bio, Genentech, GSK, Regeneron, Sanofi, and Teva. TC is a current employee of GSK and holds stocks/shares in GSK. ERP and JW are employees of IBM Watson Health, which received funds from GSK for this study. MB and BH are former employees of GSK and hold stocks/shares in GSK.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicastudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The data that support the findings of this study are available from the MarketScan Commercial Claims Database, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission, including appropriate data use agreements and potential licenses, of the MarketScan Commercial Claims database.
Additional information
Funding
References
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018.
- Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29(155):190151. doi:10.1183/16000617.0151-2019.
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi:10.1186/1471-2466-9-24.
- Chastek B, Korrer S, Nagar SP, Albers F, Yancey S, Ortega H, Forshag M, Dalal AA. Economic burden of illness among patients with severe asthma in a managed care setting. JMCP. 2016;22(7):848–861. doi:10.18553/jmcp.2016.22.7.848.
- Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, Suruki RY, Kawatkar AA. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4(1):120–129 e3. doi:10.1016/j.jaip.2015.08.003.
- Kerkhof M, Tran TN, Soriano JB, Golam S, Gibson D, Hillyer EV, Price DB. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116–124. doi:10.1136/thoraxjnl-2017-210531.
- Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi:10.1186/s12890-017-0409-3.
- Llanos JP, Ortega H, Bogart M, Packnett ER, Manjelievskaia J, Bell CF, Hahn B. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. JAA. 2020;ume 13:77–87. doi:10.2147/JAA.S236609.
- Ortega H, Hahn B, Bogart M, Bell CF, Bancroft T, Chastek B, Llanos JP. Impact of mepolizumab on exacerbations in severe asthma: results from a U.S. insurance claims data base. Allergy Asthma Proc. 2020;41(5):341–347. doi:10.2500/aap.2020.41.200043.
- Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, Busby J, Jackson DJ, Pfeffer PE, Rhee CK, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053.
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2021. [accessed 2022 Mar]. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
- Chen W, Safari A, FitzGerald JM, Sin DD, Tavakoli H, Sadatsafavi M. Economic burden of multimorbidity in patients with severe asthma: a 20-year population-based study. Thorax. 2019;74(12):1113–1119. doi:10.1136/thoraxjnl-2019-213223.
- Chen W, Lynd LD, FitzGerald JM, Marra CA, Balshaw R, To T, Tavakoli H, Sadatsafavi M, Canadian Respiratory Research Network. Excess medical costs in patients with asthma and the role of comorbidity. Eur Respir J. 2016;48(6):1584–1592. doi:10.1183/13993003.01141-2016.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3(1):1.
- Song HJ, Blake KV, Wilson DL, Winterstein AG, Park H. Medical costs and productivity loss due to mild, moderate, and severe asthma in the United States. JAA. 2020;ume 13:545–555. doi:10.2147/JAA.S272681.
- Emma R, Morjaria JB, Fuochi V, Polosa R, Caruso M. Mepolizumab in the management of severe eosinophilic asthma in adults: current evidence and practical experience. Ther Adv Respir Dis. 2018;12:1753466618808490. doi:10.1177/1753466618808490.
- GSK. Mepolizumab US prescribing information. 2020. [accessed 2020 Oct 7]. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF.
- European Medicines Agency. Mepolizumab (NUCALA) summary of product characteristics 2019 [accessed 2021 Mar]. Available from: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf.
- GSK. Mepolizumab Japan prescribing information. 2020. [accessed 2021 Jun 21]. Available from: https://gskpro.com/ja-jp/products-info/nucala/index/.
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/S2213-2600(17)30125-X.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID, Investigators S, SIRIUS Investigators Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Yancey SW, Ortega HG, Keene ON, Mayer B, Gunsoy NB, Brightling CE, Bleecker ER, Haldar P, Pavord ID. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol. 2017;139(4):1167–1175 e2. doi:10.1016/j.jaci.2016.08.008.
- van Toor JJ, van der Mark SC, Kappen JH, In ‘t Veen J, Braunstahl GJ. Mepolizumab add-on therapy in a real world cohort of patients with severe eosinophilic asthma: response rate, effectiveness, and safety. J Asthma. 2020;58(5):651–658.
- Schleich F, Graff S, Nekoee H, Moermans C, Henket M, Sanchez C, Paulus V, Guissard F, Donneau AF, Louis R. Real-world experience with mepolizumab: does it deliver what it has promised? Clin Exp Allergy. 2020;50(6):687–695. doi:10.1111/cea.13601.
- Harrison T, Canonica GW, Chupp G, Lee J, Schleich F, Welte T, Valero A, Gemzoe K, Maxwell A, Joksaite S, et al. Real-world mepolizumab in the prospective severe asthma REALITI–a study – initial analysis. Eur Respir J. 2020;56(4):2000151. doi:10.1183/13993003.00151-2020.
- Taille C, Chanez P, Devouassoux G, Didier A, Pison C, Garcia G, Charriot J, Bouee S, Gruber A, Pribil C, et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J. 2020;55(6):1902345. doi:10.1183/13993003.02345-2019.
- Silver J, Bogart M, Molfino N, Packnett E, McMorrow D, Wu J, Hahn B. Impact of mepolizumab in patients with life-threatening asthma. Chest. 2020;158(4)Supplement:A3–A4. doi:10.1016/j.chest.2020.08.053.
- Sposato B, Camiciottoli G, Bacci E, Scalese M, Carpagnano GE, Pelaia C, Santus P, Maniscalco M, Masieri S, Corsico A, et al. Mepolizumab effectiveness on small airway obstruction, corticosteroid sparing and maintenance therapy step-down in real life. Pulm Pharmacol Therap. 2020;61:101899. doi:10.1016/j.pupt.2020.101899.
- Pelaia C, Busceti MT, Solinas S, Terracciano R, Pelaia G. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: results of a single-centre observational study. Pulm Pharmacol Ther. 2018;53:1–5. doi:10.1016/j.pupt.2018.09.006.
- Pelaia C, Crimi C, Pelaia G, Nolasco S, Campisi R, Heffler E, Valenti G, Crimi N. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020;50(7):780–788. doi:10.1111/cea.13613.
- Resta E, Carpagnano G, lisianaPelaia C, D’Amato a, Crimi N, Scichilone N, Calabrese C, Resta O, Scioscia G, Pelaia G, et al. Cost-effectiveness of switching from omalizumab to mepolizumab in uncontrolled severe eosinophilic asthma. European Respiratory Journal. 2020. 56 (suppl 64)(4809). doi:10.1183/13993003.congress-2020.4809.
- Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30(3):452–456. doi:10.1183/09031936.00165106.
- Matsunaga K, Hirano T, Oka A, Tanaka A, Kanai K, Kikuchi T, Hayata A, Akamatsu H, Akamatsu K, Koh Y, et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol Pract. 2015;3(5):759–764 e1. doi:10.1016/j.jaip.2015.05.005.
- Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, Barros M, Buhl R, Howarth P, Albers FC, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742–1751 e7. doi:10.1016/j.jaci.2018.09.033.
- Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, Kato M, Albers FC, Bradford ES, Gilson MJ, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041–2056 e5. doi:10.1016/j.clinthera.2019.07.007.
- Kallieri M, Zervas E, Katsoulis K, Fouka E, Porpodis K, Samitas K, Papaioannou AI, Kipourou M, Gaki E, Vittorakis S, et al. Mepolizumab in severe eosinophilic asthma: a 2-year follow-up in specialized asthma clinics in Greece: an interim analysis. Int Arch Allergy Immunol. 2020;181(8):613–617. doi:10.1159/000508559.
- Global Initiative For Asthma. Global strategy for asthma management and prevention 2021. [accessed July 09 2021]. Available from: https://ginasthma.org/reports/.
- Corren J, Silver J, Molfino NA, et al. A real-world study of inhaled corticosteroid use in patients with severe eosinophilic asthma treated with mepolizumab. Ann Allergy Asthma Immunol. 2022;128(2):184–192.e1.
- Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, Martinot JB, Sagara H, Albers FC, Bradford ES, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208.
- Pavord ID, Chapman KR, Bafadhel M, Sciurba FC, Bradford ES, Schweiker Harris S, Mayer B, Rubin DB, Yancey SW, Paggiaro P. Mepolizumab for eosinophil-associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis. 2021;16:1755–1770. doi:10.2147/COPD.S294333.
- Casale T, Molfino NA, Silver J, Bogart M, Packnett E, McMorrow D, Wu J, Hahn B. Real-world effectiveness of mepolizumab in patients with severe asthma and associated comorbidities. Ann Allergy Asthma Immunol. 2021;127(3):354–362 e2. doi:10.1016/j.anai.2021.05.021.
- Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774. doi:10.1007/s12325-018-0805-y.
- Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18(1):7–26. doi:10.1177/0962280208092342.