Abstract
Objective
The open-label extension TRAVERSE study (NCT02134028) assessed dupilumab long-term safety and efficacy in patients who completed Phase 2/3 dupilumab asthma studies. This post hoc analysis evaluated long-term efficacy in type 2 patients with and without evidence of allergic asthma who enrolled in TRAVERSE from Phase 3 QUEST (NCT02414854) and Phase 2b (NCT01854047) studies. Non–type 2 patients with evidence of allergic asthma were also assessed.
Methods
Unadjusted annualized exacerbation rates during parent study and TRAVERSE treatment period, and changes from parent study baseline in pre-bronchodilator forced expiratory volume in 1 s (FEV1) and in 5-item Asthma Control Questionnaire (ACQ-5) scores were assessed in patients from QUEST and Phase 2b; change from parent study baseline in total IgE level was assessed in patients enrolled from Phase 2b.
Results
2062 patients from Phase 2b and QUEST enrolled in TRAVERSE. Of these, 969 were type 2 with evidence of allergic asthma; 710 were type 2 without evidence of allergic asthma; and 194 were non–type 2 with evidence of allergic asthma at parent study baseline. In these populations, reductions in exacerbation rates observed during parent studies were sustained during TRAVERSE. Type 2 patients who switched from placebo arm to dupilumab in TRAVERSE experienced similar reductions in severe exacerbation rates, and improvements in lung function and asthma control to those patients who already received dupilumab during the parent study.
Conclusion
Dupilumab efficacy was sustained for up to 3 years in patients with uncontrolled, moderate-to-severe type 2 inflammatory asthma, with or without evidence of allergic asthma.
ClinicalTrials.gov identifier: NCT02134028
Introduction
Asthma affects an estimated 3.6% of individuals globally, with a prevalence of up to 10% in high-income regions and is a leading cause of disability and death globally (Citation1). Type 2 inflammatory asthma is the most prevalent type of persistent uncontrolled asthma (Citation2,Citation3). The type 2 inflammatory cytokines interleukin (IL)-4, IL-5, and IL-13 play crucial roles in its pathogenesis (Citation4,Citation5), and in recent years, they have become therapeutic targets in attempts to inhibit the underlying inflammatory pathway driving this disease. However, type 2 inflammation is not restricted to asthma and occurs in a number of other conditions such as atopic dermatitis (AD), chronic rhinosinusitis with nasal polyps (CRSwNP), allergic rhinitis, and food allergies – type 2 disorders that often present as comorbidities in patients with type 2 asthma (Citation6).
Dupilumab, a fully human monoclonal antibody (Citation7,Citation8), blocks the shared receptor component of two of these key cytokines, IL-4 and IL-13, inhibiting their signaling, and has demonstrated efficacy in a number of type 2 diseases including asthma, AD, CRSwNP, and eosinophilic esophagitis (Citation9–16).
In the Phase 3 LIBERTY ASTHMA QUEST study (NCT02414854), add-on dupilumab 200 mg and 300 mg every 2 weeks (Q2W), vs placebo, significantly reduced severe asthma exacerbations and improved pre-bronchodilator forced expiratory volume in 1 s (FEV1) in the overall population of patients with uncontrolled, moderate-to-severe asthma. Treatment effects were greater in patients with elevated type 2 biomarkers at baseline (blood eosinophils ≥150 cells/µL or fractional exhaled nitric oxide [FeNO] ≥ 25 parts per billion [ppb]) (Citation10). A post hoc analysis of the QUEST study demonstrated that dupilumab was efficacious in treating patients with or without evidence of allergic asthma (Citation17). In the Phase 2b study (NCT01854047), add-on dupilumab 300 mg Q2W vs placebo reduced annualized severe exacerbation rates (AER), improved lung function, and was generally well tolerated in patients with uncontrolled, persistent asthma (Citation9). A post hoc analysis of the Phase 2b study demonstrated that dupilumab treatment led to similar reductions in AER and improvement in lung function in addition to improved asthma control and health-related quality of life in patients with or without evidence of allergic asthma (Citation18).
The LIBERTY ASTHMA TRAVERSE study (NCT02134028) was designed as a single-arm, open-label extension (OLE) study to evaluate the long-term safety and tolerability of dupilumab, added to standard-of-care background controller therapy, in adult and adolescent patients with asthma who participated in a previous dupilumab asthma study. In the study, AER remained low (0.33) and pre-bronchodilator FEV1 improvements were sustained to Week 96 of TRAVERSE (change from parent study baseline: 0.29 L) in non–oral corticosteroid (OCS)-dependent patients, and rapid, sustained improvements were observed in the patients who previously received placebo in the parent studies. Further improvements in AER, asthma control, and health-related quality of life were observed in patients who continued to receive dupilumab. Safety findings were consistent with the known dupilumab safety profile (Citation19).
In this post hoc analysis of patients with asthma who had rolled over from QUEST and the Phase 2b study into the TRAVERSE OLE study, we assessed the long-term efficacy of dupilumab in type 2 patients with and without evidence of allergic asthma at parent study baseline.
Methods
Study design and patients
TRAVERSE was a multinational, multicenter, single-arm, OLE study evaluating subcutaneous dupilumab 300 mg Q2W up to 2 years in patients with moderate-to-severe or OCS-dependent severe asthma. Full details of the study design and methodology of TRAVERSE have been published previously (Citation18). In brief, patients who had previously completed one of four dupilumab studies were eligible for enrollment in TRAVERSE: Phase 2a EXPEDITION (NCT02573233), Phase 2b study, QUEST, or Phase 3 VENTURE (in OCS-dependent patients; NCT02528214) (Citation9–11).
This post hoc analysis focuses on non–OCS-dependent patients who completed Phase 2b or QUEST studies and subsequently rolled over into TRAVERSE.
QUEST was a 52-week, Phase 3 randomized clinical trial that evaluated safety and efficacy of dupilumab in patients aged ≥12 years with moderate-to-severe asthma (Citation10). Patients were treated with dupilumab 200 mg/300 mg Q2W or matched placebo for 52 weeks. Phase 2b was a 24-week, dose-ranging randomized clinical trial that evaluated the efficacy and safety of dupilumab vs placebo in adults with uncontrolled persistent asthma (Citation9). Patients were treated with dupilumab 200 mg/300 mg or placebo, Q2W or every 4 weeks (Q4W).
At parent study baseline of Phase 2b and QUEST, patients were receiving medium- or high-dose inhaled corticosteroids (ICS) and at least one other controller medication; OCS use was not permitted in the QUEST population. Patients from QUEST rolled over to TRAVERSE directly from the end-of-treatment visit, whereas patients from Phase 2b completed a post-treatment follow-up period and were off treatment for at least 16 weeks (and up to 52 weeks) before starting TRAVERSE.
Both parent studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guideline, and applicable regulatory requirements. Full study details, patient eligibility criteria, and results are reported in the primary publications (Citation9,Citation10).
In TRAVERSE, all patients received dupilumab 300 mg Q2W, regardless of their parent study dose (200 mg or 300 mg dupilumab Q2W/Q4W or placebo). Patients who received placebo in the parent study and were exposed to dupilumab in TRAVERSE are referred to as the placebo/dupilumab group, while those who received dupilumab in both parent study and TRAVERSE are referred to as the dupilumab/dupilumab group. Patients were treated with dupilumab for 48 to 96 weeks. Following a protocol amendment (October 31, 2016), the treatment period was shortened from 96 to 48 weeks in some patients.
Populations analyzed
The populations examined in this subanalysis of TRAVERSE included those with a type 2 inflammatory phenotype, defined as baseline blood eosinophils ≥150 cells/µL or FeNO ≥25 ppb, with or without evidence of allergic asthma at baseline. Evidence of allergic asthma was defined as a total serum IgE ≥30 IU/mL and ≥1 perennial aeroallergen-specific IgE ≥0.35 kU/L, and this was assessed at parent study baseline as described previously (Citation17). The subgroup of patients with evidence of allergic asthma but a non–type 2 phenotype (defined as baseline blood eosinophils <150 cells/µL AND FeNO <25 ppb at parent study baseline) was also assessed, as was the overall exposed population stratified by the presence or absence of evidence of allergic asthma at baseline. IgE specific to the following perennial aeroallergens was assessed: molds (Aspergillus fumigatus, Alternaria tenuis/alternata, or Cladosporium herbarum/hormodendrum), dust mites (Dermatophagoides farinae/pteronyssinus), cat dander, dog dander, Oriental cockroach, and German cockroach.
Endpoints
Endpoints analyzed were the unadjusted AER during the parent studies and during Weeks 0–48 and 48–96 of TRAVERSE; change from parent study baseline in pre-bronchodilator FEV1 over time during TRAVERSE; change from parent study baseline in asthma control (5-item Asthma Control Questionnaire [ACQ-5] score) over time during TRAVERSE; and change in total serum IgE from parent study baseline in Phase 2b (following a protocol amendment, serum IgE was no longer assayed in TRAVERSE except for patients from Phase 2b, so limited data were available for patients from QUEST).
Statistical analysis
Unadjusted AERs were calculated as the total number of severe exacerbation events that occurred during the observation period divided by the total patient-years attended during that period. Changes from parent study baseline in pre-bronchodilator FEV1 and ACQ-5 scores were summarized using means (standard error [SE]).
Results
Study patients
Overall, 2062 patients with non–OCS-dependent moderate-to-severe asthma from Phase 2b and QUEST enrolled in TRAVERSE. Of these, 969 (47%) met the criteria of having a type 2 phenotype and evidence of allergic asthma at baseline (710 from QUEST; 259 from Phase 2b); 710 (34%) had a type 2 asthma phenotype but no evidence of allergic asthma (517 from QUEST; 193 from Phase 2b); and 194 (9%; 155 from QUEST; 39 from Phase 2b) had evidence of allergic asthma at baseline but were non–type 2 (i.e. baseline eosinophils <150 cells/µL and FeNO <25 ppb). The baseline characteristics of these three subpopulations of patients are presented in Table S1 (Supplementary material) by treatment received in the parent study and then in TRAVERSE.
In both studies, patients who met the criteria for allergic asthma, irrespective of type 2 status, were generally younger, had earlier onset of asthma, were more likely to have comorbid atopic conditions, and had higher levels of total IgE than non-allergic patients. Patients with type 2 asthma at baseline, irrespective of allergic status, had higher levels of blood eosinophils and FeNO at baseline, as would be expected, and were generally more likely to be receiving high-dose ICS at baseline than non–type 2 allergic patients (Table S1).
Annualized severe asthma exacerbations rate
shows the mean (SD) number of exacerbations experienced by patients in the year prior to initiating either parent study, and the subsequent effect of dupilumab treatment on AER at completion of the parent study, and during Weeks 0–48 and 48–96 of TRAVERSE.
Figure 1. Mean (SD) number of exacerbations in the year prior to study baseline and unadjusted annualized severe exacerbation rate over treatment periods: type 2 patients enrolled from (A) QUEST and (B) Phase 2b, with or without evidence of an allergic phenotype and non–type 2 patients with evidence of an allergic phenotype at parent study baseline. SD, standard deviation.

In both studies, patients with evidence of allergic asthma at baseline irrespective of type 2 status had fewer severe asthma exacerbations in the year prior to parent study baseline than did those with a non-allergic type 2 phenotype ().
In the type 2 population, regardless of their allergic status, the reductions in AER observed during the parent studies were sustained during TRAVERSE and showed additional reductions during Weeks 0–48 and 48–96 of TRAVERSE. Between Week 0 and 48 of TRAVERSE, the unadjusted AER for placebo/dupilumab and dupilumab/dupilumab type 2 groups was 0.414 and 0.345 (with evidence of allergic asthma) and 0.321 and 0.285 (without evidence of allergic asthma) in QUEST, and 0.309 and 0.376 (with evidence of allergic asthma) and 0.324 and 0.191 (without evidence of allergic asthma) in Phase 2b. Between Week 48 and 96, unadjusted AER for placebo/dupilumab and dupilumab/dupilumab type 2 groups was 0.234 and 0.254 (with evidence of allergic asthma), and 0.265 and 0.204 (without evidence of allergic asthma) in QUEST, and 0.359 and 0.336 (with evidence of allergic asthma), and 0.331 and 0.250 (without evidence of allergic asthma) in Phase 2b ().
The unadjusted AER for non–type 2 patients with evidence of allergic asthma was similar between the placebo and dupilumab groups at the end of the parent study and reduced further between Week 0 and 48 of TRAVERSE. Between Week 48 and 96, the unadjusted AER varied in both studies, which may be due to the low sample sizes in either of the treatment groups ().
Similar findings were observed in the overall exposed populations of QUEST and Phase 2b stratified by allergic status, as well as in type 2 populations from QUEST with and without evidence of allergic asthma with ≥2 exacerbations in the year prior to QUEST study baseline (see Figures S1 and S2, Supplementary material, respectively). Patients with evidence of allergic asthma at baseline had fewer exacerbations in the year prior to parent study baseline than those without evidence of allergic asthma, and the improvements in AER during the parent studies continued and reduced further during TRAVERSE, irrespective of allergic status (see Figure S1A and S1B, Supplementary material).
Effect of treatment on pre-bronchodilator FEV1 during TRAVERSE
Patients with evidence of allergic asthma, irrespective of type 2 status, had a higher mean (SD) pre-bronchodilator FEV1 at parent study baseline than did those without evidence of allergic asthma (QUEST type 2 patients with evidence of allergic asthma: 1.87 L [0.62], QUEST non–type 2 patients with evidence of allergic asthma: 1.85 L [0.60], QUEST type 2 patients without evidence of allergic asthma 1.69 L [0.58]; Phase 2b type 2 patients with evidence of allergic asthma: 1.90 L [0.57], Phase 2b non–type 2 patients with evidence of allergic asthma: 1.80 L [0.61], Phase 2b type 2 patients without evidence of allergic asthma: 1.70 L [0.43]).
For each of the three populations examined, the improvements in pre-bronchodilator FEV1 observed in dupilumab-treated patients in the parent studies were sustained during TRAVERSE, although the magnitude of improvement was greater in type 2 patients irrespective of allergic status than in non–type 2 allergic patients (). In placebo-treated patients who initiated dupilumab in TRAVERSE, rapid improvements in mean change from parent baseline (SD) pre-bronchodilator FEV1 were observed by Week 2 in QUEST type 2 allergic/type 2 non-allergic (0.35 [0.45]/0.33 [0.42]) patients, and in Phase 2b type 2 allergic/type 2 non-allergic (0.26 [0.35]/0.26 [0.45]) patients; these were then sustained throughout the OLE (). In patients with non–type 2 allergic phenotype, improvements were not as pronounced as in the type 2 populations ().
Figure 2. Mean change from parent study baseline over the treatment period in pre-bronchodilator FEV1: type 2 patients enrolled from (A) QUEST and (B) Phase 2b, with or without evidence of allergic asthma and non–type 2 patients with evidence of allergic asthma at parent study baseline. DPL, dupilumab; FEV1, forced expiratory volume in 1 s; PBO, placebo; SE, standard error.

Consistent findings were observed in the overall exposed populations of the parent studies stratified by allergic status alone, as well as in type 2 populations from QUEST with and without evidence of allergic asthma with ≥2 exacerbations in the year prior to QUEST study baseline (see Figures S3 and S4, Supplementary material, respectively).
Effect of treatment on asthma control (ACQ-5 scores) during TRAVERSE
presents the effect of dupilumab on asthma control, as measured by the ACQ-5, during Weeks 0–48 of TRAVERSE for each of the three subpopulations examined. In each group, the improvements in asthma control observed during each parent study were sustained during TRAVERSE ().
Figure 3. Mean change from parent study baseline over the treatment period in ACQ-5 score: type 2 patients enrolled from (A) QUEST and (B) Phase 2b, with or without evidence of allergic asthma and non–type 2 patients with evidence of allergic asthma at parent study baseline. ACQ-5, 5-item Asthma Control Questionnaire; DPL, dupilumab; PBO, placebo; SE, standard error.
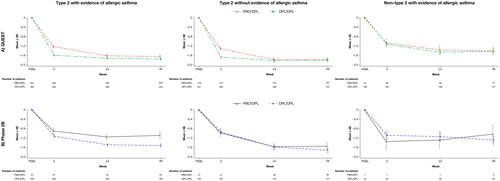
At Week 48 in QUEST, mean (SD) change from baseline in ACQ-5 scores for the placebo/dupilumab group and dupilumab/dupilumab group, respectively, was −1.64 (1.08) and −1.75 (1.05) for type 2 allergic patients, −1.77 (1.09) and −1.80 (1.09) for non-allergic type 2 patients, and −1.41 (1.04) and −1.42 (1.01) for non–type 2 allergic patients. In Phase 2b, the corresponding values were −1.09 (1.05) and −1.52 (1.05) for type 2 allergic patients, −1.54 (1.05) and −1.72 (1.16) for type 2 non-allergic patients, and −1.04 (0.85) and −1.30 (1.04) for non–type 2 allergic patients.
These improvements in asthma control relative to parent study baseline exceeded the minimal clinically important difference of ≥0.5 in the ACQ-5 score. As observed with the FEV1 endpoint, placebo-treated patients rolled over from QUEST and Phase 2b who initiated dupilumab treatment in TRAVERSE showed rapid improvements in asthma control, and these were then sustained through Week 48 ().
Consistent findings were observed in the overall exposed populations of the parent studies stratified by allergic status alone, as well as in type 2 populations from QUEST with and without evidence of allergic asthma with ≥2 exacerbations in the year prior to QUEST study baseline (see Figures S5 and S6, Supplementary material, respectively).
Effect of treatment on serum total IgE levels during TRAVERSE (Phase 2b only)
In Phase 2b, for each of the three populations assessed, mean serum total IgE levels decreased below the level observed at parent study baseline by Week 0 of TRAVERSE in the dupilumab/dupilumab group and, for both treatment groups, levels gradually decreased over time from Week 0 of TRAVERSE up to Week 96 (see Figure S7, Supplementary material).
In type 2 allergic patients, mean (SD) serum total IgE at Week 0 (i.e. TRAVERSE baseline after the break in treatment) was 610.3 IU/mL (915.8) in the placebo/dupilumab group and 480.5 IU/mL (795.6) in the dupilumab/dupilumab group. During TRAVERSE, these levels had decreased to 104.4 IU/mL (140.2) and 148.2 IU/mL (356.6), respectively, by Week 96 of TRAVERSE (equivalent to a mean percentage change from baseline of −78.4% and −79.8%, respectively). In non-allergic type 2 patients, mean (SD) levels at Week 0 were 273.1 IU/mL (367.9) and 129.4 IU/mL (255.1), respectively, dropping to 40.2 IU/mL (50.8) and 25.2 IU/mL (37.9) by Week 96 (mean percentage change from baseline: −77.1% and −80.8%). In non–type 2 allergic patients, mean (SD) levels at Week 0 were 395.6 IU/mL (331.7) and 302.5 IU/mL (603.9) in the placebo/dupilumab and dupilumab/dupilumab groups, respectively, dropping to 85.8 IU/mL (93.2) and 203.1 IU/mL (705.2) by Week 96 (mean percentage change from baseline: −70.3% and −72.1, respectively).
Similar findings were observed in the overall exposed population of Phase 2b stratified by allergic status alone (see Figure S8, Supplementary material).
Discussion
In this post hoc analysis of TRAVERSE, conducted in patients with uncontrolled, moderate-to-severe asthma who had rolled over into the OLE study from the Phase 2b and Phase 3 QUEST studies, dupilumab vs placebo reduced severe exacerbation rates and improved lung function (pre-bronchodilator FEV1) and asthma control (ACQ-5 scores) in patients with type 2 asthma, with or without evidence of allergic asthma at baseline. Dupilumab efficacy was sustained for up to 3 years (1 year in the parent study, 2 years in the OLE) in the three subpopulations examined, although the magnitude of improvement was less pronounced in non–type 2 patients.
In type 2 patients initiating dupilumab in the OLE, after having switched from the placebo arms of the parent studies, the improvements in lung function and asthma control were rapid, by the first assessment at Week 2, and were sustained throughout the respective assessment period (up to 96 weeks for FEV1 and 48 weeks for ACQ-5). These patients also experienced reductions in AER that were similar to those of patients who had received dupilumab during the parent study.
Dupilumab also reduced mean serum total IgE levels in patients from Phase 2b during TRAVERSE, with rapid decreases observed in the dupilumab/dupilumab group below parent study baseline, and by Week 96 both treatment groups were below the level observed at parent study baseline. Interestingly, the reductions observed in serum total IgE relative to parent study baseline that were achieved by the end of Phase 2b in the dupilumab group in the overall exposed population (Citation19) were still evident at baseline (Week 0) of TRAVERSE in the subgroups assessed in this analysis, despite an off-treatment period of at least 16 weeks, and these continued to decrease in all subpopulations during TRAVERSE.
A limitation of this study was the post hoc nature of the analyses, as the study was not powered specifically to investigate differences between patients with or without evidence of allergic asthma. In addition, percutaneous allergy skin testing was not performed, and evidence of allergic asthma was identified solely by levels of total serum IgE at baseline and sensitivity (specific IgE ≥0.35 kU/L) to at least one of a panel of perennial aeroallergens.
These are unique and important findings as allergic and eosinophilic phenotypes are often co-expressed in patients with type 2 inflammation (Citation20). While other biologics that target either the eosinophilic phenotype (anti–IL-5 or –IL-5R) (Citation21–23) or the allergic phenotype (anti-IgE) (Citation24) are available to patients with asthma, these and other data show that dupilumab is effective in patients with elevated blood eosinophils or FeNO, with or without an allergic phenotype. This has important therapeutic implications as biomarkers to identify patients most eligible for biologics have become increasingly important (Citation20) and are recommended by GINA guidelines (Citation25).
Conclusion
We have shown that the efficacy of dupilumab can be observed long-term in patients with moderate-to-severe asthma irrespective of the evidence of allergic asthma at baseline.
Supplemental Material
Download MS Word (1.9 MB)Supplemental Material
Download PDF (94.1 KB)Supplemental Material
Download PDF (108.4 KB)Supplemental Material
Download PDF (88 KB)Supplemental Material
Download PDF (125.6 KB)Supplemental Material
Download PDF (111.7 KB)Supplemental Material
Download PDF (177.7 KB)Supplemental Material
Download PDF (141.6 KB)Supplemental Material
Download PDF (149.9 KB)Supplemental Material
Download PDF (150.2 KB)Declaration of interest
LDS is an advisory board member for Aimmune Therapeutics, Optinose, Regeneron Pharmaceuticals Inc., and Sanofi; has received speaker fees from Regeneron Pharmaceuticals Inc., and Sanofi; and has been involved in clinical trials by Aimmune Therapeutics, Amgen, AstraZeneca, Circassia, DBV Technologies, Galderma, GSK, Lupin, Merck, Mylan, Novartis, Novo Nordisk, Optinose, Pearl Pharmaceuticals, Pfizer, Pulmagen, Roxane, Sanofi, Spirometrix, Teva, Vectura, and Watson Pharmaceuticals. JC reports research grants from and consultancy for AstraZeneca, Genentech, Novartis, Regeneron Pharmaceuticals Inc., and Sanofi; and has received speaker fees from AstraZeneca, Genentech, and Novartis.
IDP has received speaker fees from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi-Regeneron Pharmaceuticals Inc., and Teva; payments for organizing educational events from AstraZeneca, GSK, Sanofi-Regeneron Pharmaceuticals Inc., and Teva; consultant fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Genentech, GSK, Knopp Biosciences, Merck, Novartis, Sanofi-Regeneron Pharmaceuticals Inc, and Teva; reports international scientific meeting sponsorship from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, and Teva; a research grant from Chiesi; payments to support FDA approval meetings from GSK; payments for use of the Leicester Cough Questionnaire (of which he is a co-patent holder) in clinical trials from Bayer, Insmed, and Merck; and is an expert witness for a patent dispute involving AstraZeneca and Teva. ND is a former employee of Sanofi and may hold stock and/or stock options in the company. AA, MD, JAJ-N, and PJR are Sanofi employees and may hold stock and/or stock options in the company. XS, AR, and YD are employees and shareholders of Regeneron Pharmaceuticals Inc.
Data availability statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org
Additional information
Funding
References
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3.
- Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi:10.1038/nri3786.
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi:10.1038/nm.2678.
- Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334. doi:10.1067/mai.2001.118891.
- Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203(6):1435–1446. doi:10.1084/jem.20052448.
- Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi:10.1080/1744666X.2017.1298443.
- Macdonald LE, Karow M, Stevens S, Auerbach W, Poueymirou WT, Yasenchak J, Frendewey D, Valenzuela DM, Giallourakis CC, Alt FW, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111(14):5147–5152. doi:10.1073/pnas.1323896111.
- Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, Huang TT, Poueymirou WT, Esau L, Meola M, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153–5158. doi:10.1073/pnas.1324022111.
- Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, Pirozzi G, Sutherland ER, Evans RR, Joish VN, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi:10.1016/S0140-6736(16)30307-5.
- Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092.
- Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093.
- Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, Mullol J, Greos LS, Bosso JV, Laidlaw TM, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi:10.1016/S0140-6736(19)31881-1.
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, Simpson EL, Papp KA, Hong HC, Rubel D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi:10.1016/S0140-6736(17)31191-1.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, Silverberg JI, Deleuran M, Kataoka Y, Lacour JP, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi:10.1056/NEJMoa1610020.
- Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, Soong W, Worm M, Szepietowski JC, Sofen H, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52. doi:10.1016/S0140-6736(15)00388-8.
- Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, Schoepfer AM, Safroneeva E, Rothenberg ME, Falk GW, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158(1):111–122.e10. doi:10.1053/j.gastro.2019.09.042.
- Corren J, Castro M, O'Riordan T, Hanania NA, Pavord ID, Quirce S, Chipps BE, Wenzel SE, Thangavelu K, Rice MS, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–526. doi:10.1016/j.jaip.2019.08.050.
- Brusselle G, Quirce S, Papi A, Kuna P, Chipps BE, Hanania NA, Blaiss M, Msihid J, Jacob-Nara JA, Deniz Y, et al. Dupilumab efficacy in patients with uncontrolled or oral corticosteroid-dependent allergic and non-allergic asthma. J Allergy Clin Immunol Pract. 2023;11(3):873–884.e11. doi:10.1016/j.jaip.2022.11.044.
- Wechsler ME, Ford LB, Maspero JF, Pavord ID, Papi A, Bourdin A, Watz H, Castro M, Nenasheva NM, Tohda Y, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med. 2022;10(1):11–25. doi:10.1016/S2213-2600(21)00322-2.
- Canonica GW, Blasi F, Crimi N, Paggiaro P, Papi A, Fanelli F, Stassaldi A, Furneri G. Defining type 2 asthma and patients eligible for dupilumab in Italy: a biomarker-based analysis. Clin Mol Allergy. 2021;19(1):5. doi:10.1186/s12948-021-00146-9.
- NUCALA® (mepolizumab): Highlights of prescribing information. US Food and Drug Administration. [accessed 2023 Feb 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf.
- CINQAIR® (reslizumab): Highlights of prescribing information. US Food and Drug Administration. [accessed 2023 Feb 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf.
- FASENRA® (benralizumab): Highlights of prescribing information. US Food and Drug Administration. [accessed 2023 Feb 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf.
- XOLAIR® (omalizumab): Highlights of prescribing information. US Food and Drug Administration. [accessed 2023 Feb 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf.
- Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA); 2021. [accessed 2023 Feb 2]. https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
