Abstract
Objective
When selecting inhaled therapies, it is important to consider both the active molecules and the device. Extrafine formulation beclomethasone dipropionate plus formoterol fumarate (BDP/FF) has been available for some years delivered via pressurized metered-dose inhaler (pMDI). More recently, a breath-activated, multi-dose dry-powder inhaler (DPI), the NEXThaler, has been approved. The current study aimed to demonstrate the non-inferiority of BDP/FF delivered via the DPI vs. via the pMDI, in Chinese adults with asthma.
Methods
After a four-week run-in period, when all patients received BDP/FF pMDI 100/6 µg, two inhalations twice daily (BID), patients were randomized equally to BDP/FF pMDI or DPI, both 100/6 µg, two inhalations BID for 12 weeks. The primary objective was to demonstrate non-inferiority of BDP/FF DPI vs. BDP/FF pMDI in terms of average pre-dose morning peak expiratory flow (PEF) over the entire treatment period.
Results
Of 252 and 242 patients in the DPI and pMDI groups, respectively, 88.5% and 88.8% completed the study. The primary objective was met, with no statistically significant difference between the treatments in average pre-dose morning PEF, and with the lower limit of the 95% CI above the −15 L/min non-inferiority margin (adjusted mean difference: 5.25 L/min [95% CI: −0.56, 11.06]). Adverse events were reported by 48.4% and 49.6% patients in the DPI and pMDI groups, respectively, most mild or moderate.
Conclusions
The NEXThaler DPI is a similarly effective device to the pMDI for the administration of BDP/FF in adults, so extending the options available for the management of asthma.
Introduction
When selecting an inhaled therapy for asthma maintenance use, it is important to not only consider the active molecules but also the patient’s ability to use, and preference for, the device (Citation1). Advantages of pressurized metered-dose inhalers (pMDIs) include that they are familiar to most patients (especially since the most common rescue medications are delivered via pMDI), and instructions for use are consistent across products. However, their correct use requires coordination of hand actuation with inhalation (and continuing to inhale after actuation), which can be difficult for some patients (Citation2,Citation3). In addition, although the pMDI remains the most common inhaler overall, dry-powder inhalers (DPIs) are the most commonly prescribed inhalers for maintenance therapy of asthma and chronic obstructive pulmonary disease (COPD) (Citation4). However, no inhaler type is suitable for all patients, and so having the same molecules available in different devices can optimize care.
A pMDI extrafine formulation (i.e. with mass median aerodynamic diameter [MMAD] < 2 µm) of the inhaled corticosteroid (ICS) plus long-acting β2-agonist (LABA) combination beclomethasone dipropionate plus formoterol fumarate (BDP/FF) was first approved for the maintenance treatment of asthma in 2006. The NEXThaler is a breath-activated, multi-dose DPI that has been more recently approved in many countries for the delivery of extrafine formulation (MMAD <2 µm) BDP/FF in asthma and COPD. The NEXThaler can be used even by patients who can generate only a relatively low inspiratory force (Citation5), its delivery performance is independent of inhalation flow rate, and it incorporates a dose counter, indicating the number of doses remaining and that counts down only following successful activation. Furthermore, each inhaler contains sufficient drug to deliver a full month of therapy, eliminating the need to manually load individual capsules, and so removing one source of error with single-dose, capsule-based DPIs (Citation6). Additional advantages of DPIs compared to other delivery mechanisms include the ability to achieve deep lung deposition, and greenhouse gases are not emitted during actuation (Citation6).
A previous study, conducted in Caucasian adults with asthma, demonstrated the non-inferiority of BDP/FF delivered via the DPI vs. via the pMDI, in terms of peak expiratory flow (PEF) (Citation7). The current study aimed to extend these findings by comparing the DPI vs. pMDI formulations of BDP/FF, in terms of efficacy and safety, in Chinese adults with controlled asthma.
Methods
This was an active-controlled, double-dummy, parallel-group study. After a four-week run-in period, when all patients received BDP/FF pMDI 100/6 µg, two inhalations twice daily (BID), patients were randomized equally to either continue BDP/FF pMDI or switch to BDP/FF DPI, both 100/6 µg, two inhalations BID for 12 weeks. Patients were assigned to treatment via a balanced block randomization scheme stratified by site. Investigators, patients, and sponsor staff were blinded to treatment assignment by the double-dummy design: Patients randomized to BDP/FF DPI also used an identical placebo pMDI, and vice versa. BDP/FF pMDI and matching placebo contained 1,1,1,2-tetrafluoroethane propellant, ethanol anhydrous, and hydrochloric acid as excipients, whereas BDP/FF DPI and matching placebo contained lactose monohydrate.
Twice daily throughout the study (morning and evening), patients assessed their pre-dose PEF (using an electronic meter), and recorded study medication and rescue medication use, together with asthma symptoms in an electronic diary. Patients attended study visits every two weeks, when lung function (forced expiratory volume in 1 s [FEV1] and forced vital capacity [FVC]) was assessed pre-dose, and safety assessments were performed. The six-item Asthma Control Questionnaire (ACQ-6) was completed at baseline and Week 12.
Eligible patients were male or female adults of Chinese ethnicity who had been diagnosed with asthma ≥6 months prior to entry. Patients had pre-bronchodilator FEV1 >80% predicted, ACQ-6 < 0.75, and were on a stable dose for ≥1 month of either a medium- or high-dose ICS monotherapy, or a low- or medium-dose ICS plus LABA. Key exclusion criteria were: intermittent or near-fatal asthma, diagnosis of COPD, current or ex-smoker with total cumulative exposure ≥10 pack-years, or a severe asthma exacerbation or lower respiratory tract infection within one month prior to entry. All patients provided written informed consent prior to any study-related procedure. The full list of inclusion and exclusion criteria is in the supplement. Salbutamol was permitted as rescue medication, although not within 6 h prior to any in-clinic lung function assessment. ICSs and LABAs other than study medication and inhaled muscarinic antagonists were not permitted; systemic corticosteroids were only permitted in short courses.
The study was approved by the independent ethics committee at each institution (see supplement), and was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The study was registered at the China Center for Drug Evaluation (CTR20170917, on 27 November 2017) and ClinicalTrials.gov (NCT03453112, on 5 March 2018).
Outcomes
The primary objective was to demonstrate non-inferiority of BDP/FF DPI vs. BDP/FF pMDI in terms of change from baseline over the entire treatment period in average pre-dose morning PEF.
Secondary endpoints were change from baseline:
to each two-week treatment period in average pre-dose morning PEF
to each two-week treatment period and to the entire treatment period in: average pre-dose evening PEF; daily PEF variability; average use of rescue medication; percentage of rescue-use free days; average total morning asthma symptom scores; average total evening asthma symptom scores; percentage of asthma symptoms-free days; percentage of asthma control days
at each clinic visit in pre-dose morning FEV1 and FVC
at Week 12 in ACQ-6 total score.
Safety and tolerability were assessed throughout the study, with the relationship of adverse event to study medication being determined by the investigators.
Sample size and statistical methods
A total of 220 evaluable patients per group was estimated to provide approximately 85% power to demonstrate non-inferiority of BDP/FF DPI vs. BDP/FF pMDI for the primary endpoint, with a non-inferiority margin of −15 L/min and a one-sided significance level of 0.025, assuming a mean difference of 0 L/min between treatments and SD of 52 L/min. Assuming a non-evaluable rate of 10%, 490 patients (245 per group) would need to be randomized.
The primary endpoint was evaluated using an analysis of covariance (ANCOVA) model, including treatment, region, and sex as factors, and baseline value as covariate. Non-inferiority was to be demonstrated if the lower limit of the 95% CI of the adjusted mean difference between BDP/FF DPI and pMDI was ≥–15 L/min. ACQ-6 total score was evaluated using the same ANCOVA model as used for the primary endpoint. The other secondary efficacy endpoints were evaluated using a linear mixed model for repeated measures, including change from baseline at each inter-visit period/visit as dependent variable, treatment, visit/period, treatment by visit/period interaction, region, and sex as fixed effects, and baseline and baseline by visit/period interaction as fixed covariates.
The intention-to-treat (ITT) population was all randomized patients who received at least one dose of study medication and who had at least one post-baseline efficacy evaluation. The per-protocol (PP) population was all patients in the ITT population who did not have major protocol deviations. The primary efficacy variable was evaluated in both the ITT and the PP populations (with the two populations having equal importance); all other efficacy endpoints were evaluated in the ITT population only. Safety data were evaluated in the safety population, which was all randomized patients who received at least one dose of study medication.
Results
The study was conducted between October 2017 and December 2021 at 46 sites, all in China. Of 828 patients screened, 494 were randomized to treatment, 252 to BDP/FF DPI and 242 to BDP/FF pMDI, with 223 (88.5%) and 215 (88.8%) completing the study in the DPI and pMDI groups, respectively (). All patients received at least one dose of study medication, but one patient (in the DPI group) had no post-baseline efficacy data, and so was excluded from the ITT population. The characteristics of the ITT population at screening were similar in the two treatment groups, with age varying from 18 to 76 years, and most previously using an ICS/LABA combination (). Median exposure to study medication was 85.0 days in both groups, with 77.8% and 78.1% having ≥12 weeks’ exposure to BDP/FF DPI and pMDI, respectively.
Figure 1. Patient disposition. BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
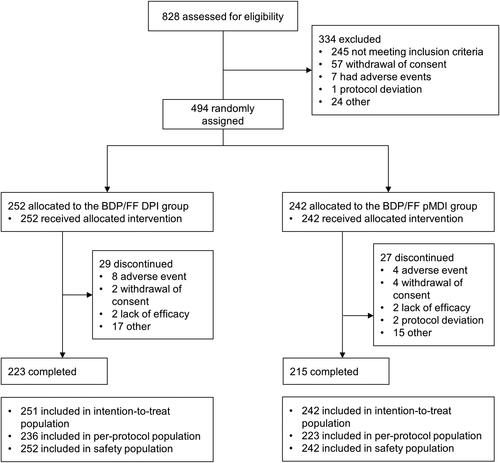
Table 1. Demographics and disease characteristics at screening (ITT population).
Efficacy
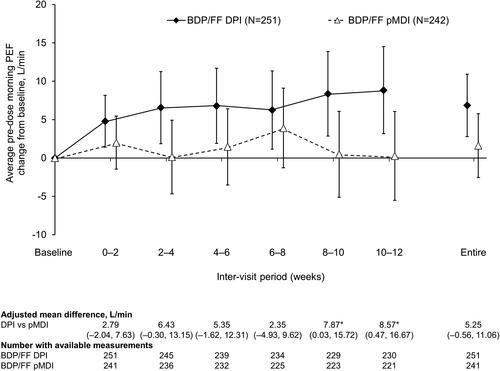
The primary objective was met, with the lower limit of the 95% CI of the adjusted difference between BDP/FF DPI and pMDI for average pre-dose morning PEF change from baseline assessed over the entire treatment period being above the non-inferiority margin of −15 L/min, in both the ITT (adjusted mean difference: 5.25 L/min [95% CI: −0.56, 11.06]; ) and PP populations (5.23 L/min [–0.84, 11.30]). When analyzed in the individual inter-visit periods, differences between treatments were not statistically significant, with the exception of Weeks 8–10 and 10–12, for which there were small, statistically significant differences in favor of BDP/FF DPI ().
Figure 2. Pre-dose morning PEF (ITT population). *p<.05 DPI vs. pMDI. Data are adjusted mean and 95% confidence interval. Entire treatment period analyzed using analysis of covariance; individual periods analyzed using mixed model for repeated measures. Mean baseline values were 383.6 and 384.1 L/min for BDP/FF DPI and pMDI, respectively. PEF: peak expiratory flow; BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
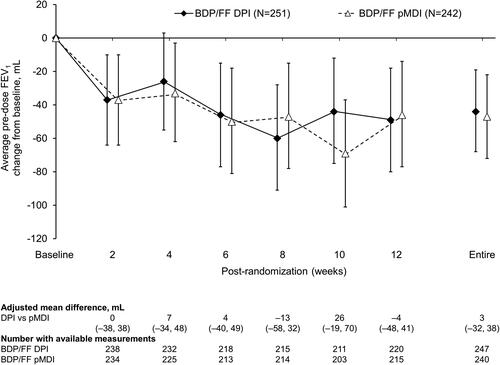
For average pre-dose evening PEF there were statistically significant differences in favor of BDP/FF DPI at the two later inter-visit timepoints, and over the entire treatment period (). There was a reduction in average PEF variability with both treatments, with no statistically significant differences between treatments (Supplementary Figure 1). Use of rescue medication was low throughout the study in both treatment groups, with no significant differences (Supplementary Figures 2 and 3). Furthermore, there was a decrease from baseline in asthma symptoms in both treatment groups, when analyzed over day-time, over nighttime, and as symptom-free days, with no significant differences between groups (Supplementary Figures 4–6). This improvement in symptoms was accompanied by an improvement from baseline in asthma control, although again with no difference between the two groups (). There were small decreases from baseline in pre-dose FEV1 and FVC in both treatment groups, but with no statistically significant differences between the groups ( and Supplementary Figure 7). Mean ACQ-6 scores at baseline were low in both groups (mean 0.117 and 0.138 for BDP/FF DPI and pMDI, respectively), with small changes over the follow-up period (adjusted mean changes from baseline at Week 12 − 0.010 [95% CI −0.035, 0.014] and −0.015 [–0.039, 0.010]) and with no difference between groups (adjusted mean difference 0.004 [–0.030; 0.039]).
Figure 3. Pre-dose evening PEF (ITT population). *p<.05 DPI vs. pMDI. Data are adjusted mean and 95% confidence interval, analyzed using mixed model for repeated measures. Mean baseline values were 388.8 and 390.5 L/min for BDP/FF DPI and pMDI, respectively. PEF: peak expiratory flow; BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
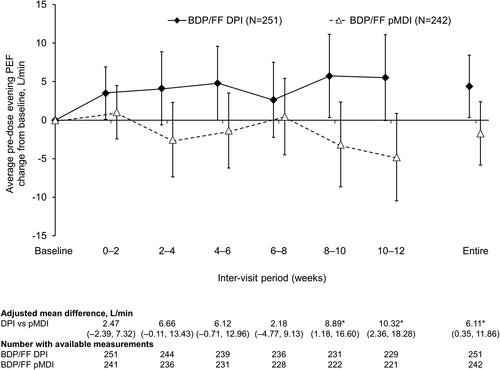
Figure 4. Percentage of asthma control days (ITT population). Data are adjusted mean and 95% confidence interval, analyzed using mixed model for repeated measures. Mean baseline values were 75.21 and 74.80% for BDP/FF DPI and pMDI, respectively. BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
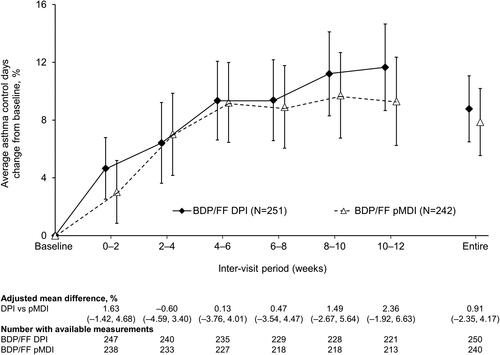
Figure 5. Pre-dose FEV1 (ITT population). Data are adjusted mean and 95% confidence interval, analyzed using mixed model for repeated measures. Mean baseline values were 2.81 and 2.85 L for BDP/FF DPI and pMDI, respectively. FEV1: forced expiratory volume in 1 s; BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
Safety
A similar proportion of patients reported adverse events in both treatment groups (48.4% vs. 49.6% with DPI and pMDI, respectively), most of which were mild or moderate in severity (). Few patients had adverse events that were considered by the investigators to be related to study treatment, none of which were serious, and no patients died during the study. Overall, changes in other safety parameters were minimal and did not differ between treatment groups.
Table 2. Treatment-emergent adverse events and serious adverse events (safety population).
Discussion
The overall aim of the study was met, with BDP/FF administered via the NEXThaler DPI meeting the non-inferiority efficacy criterion compared to administration via the pMDI, and with similar safety and tolerability profiles. Similar efficacy was demonstrated not only in terms of home-assessed morning and evening PEF, but also asthma symptoms and rescue medication use, with any differences between the two groups being small and not clinically important (Citation8). Interestingly, asthma control improved significantly from baseline over the duration of the study in both treatment groups, accompanied by improvements (i.e. decreases) in PEF variability and asthma symptoms. As would be expected given that patients were required to have stable asthma on entry to the study (with the baseline value assessed at the end of a four-week run-in on BDP/FF pMDI), there was no change from baseline in the home-assessed PEF with either device. There was a slight decrease from baseline in clinic-assessed lung function parameters with both devices, but this decrease was overall small and not clinically meaningful. The overall tolerability profile of BDP/FF delivered via the two devices was similar, with no new or unexpected adverse events. In particular, few events were considered treatment-related with either device.
The results of this study are broadly consistent with the previous study conducted in Caucasian patients with asthma, in which the difference in average pre-dose morning PEF over eight weeks between DPI and pMDI was −1.84 L/min, with the lower limit of the 95% CI being above the −15 L/min pre-defined margin, so demonstrating non-inferiority (Citation7). This previous study also included a third arm, in which patients received BDP monotherapy via the DPI – both of the BDP/FF groups demonstrated statistical superiority over the BDP monotherapy group for the morning PEF primary endpoint, so confirming assay sensitivity.
The main limitation of the current study is that all recruited patients were required to have stable, controlled asthma, with all but one patient receiving an ICS/LABA combination on entry. Although both study treatments were equally effective in providing improvements in asthma control, the lack of an ICS monotherapy arm means that we cannot say whether this improvement is due to the study treatments or is a study effect. However, the consistency of the results between this study and the prior study, which did include ICS monotherapy, is reassuring (Citation7). In addition, to be eligible, all patients were trained on, and had to demonstrate correct use of, the two devices, and so the results do not take account of any impact of incorrect use on efficacy. Finally, compliance (i.e. exposure) to study medication was based on patients’ self-reporting, although for the DPI this was also checked by the investigators using the dose counter.
Conclusions
The current study demonstrated that the NEXThaler DPI is a similarly effective device to the pMDI for the administration of BDP/FF, so extending the options available for the management of asthma in adults.
Supplemental Material
Download PDF (411.1 KB)Declaration of interest
In addition to the medical writing support declared above, the authors have the following conflicts of interest to declare: Jinping Zheng declares advisory board membership with Chiesi, GlaxoSmithKline and AstraZeneca, all outside the scope of the current manuscript. Jianyong Zhang has no other declarations. Xiuhua Fu has no other declarations. Changqing Lin has no other declarations. Xinri Zhang has no other declarations. Xiaodong Mei has no other declarations. Massimo Corradi received a research contract from Chiesi Farmaceutici SpA during the conduct of the study. Glauco Cappellini is an employee of Chiesi Farmaceutici SpA, the sponsor of the study. Emanuele Calabro is an employee of Chiesi Farmaceutici SpA, the sponsor of the study. Cissy Zhu is an employee of Chiesi Pharmaceutical Consulting (Shanghai) Co. Ltd. Eva Topole is an employee of Chiesi Farmaceutici SpA, the sponsor of the study.
Data availability statement
Chiesi commits to sharing with qualified scientific and medical researchers, conducting legitimate research, the anonymized patient-level and study-level data, the clinical protocol and the full clinical study report of Chiesi Farmaceutici SpA-sponsored interventional clinical trials in patients for medicines and indications approved by the European Medicines Agency and/or the US Food and Drug Administration after 1 January 2015, following the approval of any received research proposal and the signature of a Data Sharing Agreement. Chiesi provides access to clinical trial information consistently with the principle of safeguarding commercially confidential information and patient privacy. Other information on Chiesi’s data sharing commitment, access and research request’s approval process are available in the Clinical Trial Transparency section of http://www.chiesi.com/en/research-and-development/.
Additional information
Funding
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention [Internet]. Available from: https://ginasthma.org/gina-reports/ [2022; last accessed 11 January 2023].
- Vanoverschelde A, Van Der Wel P, Putman B, Lahousse L. Determinants of poor inhaler technique and poor therapy adherence in obstructive lung diseases: a cross-sectional study in community pharmacies. BMJ Open Respir Res. 2021;8(1):e000823. doi:10.1136/bmjresp-2020-000823.
- Van Beerendonk I, Mesters I, Mudde AN, Tan TD. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma. 1998;35(3):273–279. doi:10.3109/02770909809068218.
- Ding B, Small M, Scheffel G, Holmgren U. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma–COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927–936. doi:10.2147/COPD.S154525.
- Corradi M, Chrystyn H, Cosio BG, Pirozynski M, Loukides S, Louis R, Spinola M, Usmani OS. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclometasone and formoterol to treat large and small airways in asthma. Expert Opin Drug Deliv. 2014;11(9):1497–1506. doi:10.1517/17425247.2014.928282.
- Muralidharan P, Hayes D, Mansour HM. Dry powder inhalers in COPD, lung inflammation and pulmonary infections. Expert Opin Drug Deliv. 2015;12(6):947–962. doi:10.1517/17425247.2015.977783.
- Kanniess F, Scuri M, Vezzoli S, Francisco C, Petruzzelli S. Extrafine beclomethasone/formoterol combination via a dry powder inhaler (NEXThaler®) or pMDI and beclomethasone monotherapy for maintenance of asthma control in adult patients: a randomised, double-blind trial. Pulm Pharmacol Ther. 2015;30:121–127. doi:10.1016/j.pupt.2014.07.006.
- Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23–27. doi:10.1034/j.1399-3003.1999.14a06.x.

