Abstract
The King Abdullah Canal (KAC) is an artificial water conveyor serving as raw water source for drinking water supply of Amman and for irrigation purposes in the Jordan Valley. The main water sources for KAC originate from the Yarmouk River, the Mukheiba Wells, and the Peace Conveyor. Water samples were collected from December 2002 to May 2004 to investigate changes in physicochemical parameters and parameters related to eutrophication along KAC. The catchment area of KAC is highly heterogeneous in terms of topography, climate, geologic conditions, and land use, resulting in great variations in the physicochemical composition of the canal water. The Yarmouk River and the Mukheiba Wells reflect the water quality of precipitation without much dissolution of halides, whereas the Peace Conveyor shows a groundwater characteristic. Main dischargers of N and P compounds are the Yarmouk River, and dams and wadis along KAC. The concentrations in the canal are mostly above eutrophication level for N and P. Along KAC there is an increase in chlorophyll a concentration and plankton counts. The formation potentials for trihalomethanes (THMs) and for integrated organically bound halogens are determined by chlorination to evaluate the consequences of water disinfection. Due to relatively high bromide concentration mainly brominated THMs are formed.
Introduction
The King Abdullah Canal (KAC) is an artificial water conveyor of 65 km length, constructed in 1961 to serve irrigation purposes in the Jordan Valley area. Between 1969 and 1987 it was extended three times. Today the canal has a total length of 110 km with a head discharge capacity of 20 m3 s−1 (Jordan Valley Authority Citation2008). The coordinates of the canal are 175.000 N to 230.000 N and about 205.000 E to 207.000 E in the Palestine Grid, or 32°10′3.53″N to 32°39′48.70″N and 35°34′49.70″E to 35°36′13.72″E in the Universal Transverse Mercator (UTM) Grid.
KAC is the main municipal and agricultural water supply project for the Kingdom of Jordan. However, both the water quality and quantity highly depend on water sources from inside and outside Jordan, Israel, and Syria (Salameh Citation1987, Citation1996). KAC irrigates 23,000 ha (230,000 dunums) from the north to the south shores of the Dead Sea. In the early 1980s, it was decided that some of the KAC water should be used after proper treatment for the drinking water supply of Amman, the capital of Jordan (Jordan Valley Authority Citation2008). In 2004, about 45 × 106 m3 of water were pumped from KAC to Amman after being treated at the Zai treatment plant, located between the Deir Alla (the water intake site in the Jordan Valley) and Amman. Today, the amount has risen to about 60 × 106 to 70 × 106 m3 year−1. In the years 1987 and 1998, the water flowing in the KAC experienced incidents of severe taste and odor problems, and the treatment plant at Zai failed to cope with the water quality of the canal. Inadequately treated water was supplied to the inhabitants of Amman and other governorates, causing public unrest and protests. Taste and odor problems of KAC water were caused by excessive algal growth due to the high nutrient levels, and the incapability of the Zai treatment plant to treat that water properly for human consumption.
The problem of eutrophication in drinking water supply is mainly connected to a high load of organic carbon. This can be quantified based on the total organic carbon (TOC) or its dissolved fraction (dissolved organic carbon, DOC) by membrane filtration through 0.45 µm pore size filters. High organic loads are also the reason for higher counts of bacteria and an increased bacterial growth potential. Due to the hygiene requirements of drinking water regulation (WHO Citation1984, Citation2006; JISM Citation2001; TrinkwVO Citation2001), disinfection measures have to be taken.
The most common method for drinking water disinfection in public water supply is the use of chlorine. However, it includes the pitfall of the formation of disinfection by-products (DBP). Trihalomethane (THM) is a commonly used parameter for the control of DBP formation and there are maximum allowed concentrations for this parameter in drinking water legislation (0.05 mg L−1 total THM according to TrinkwVO Citation2001; 0.15 mg L−1 total THM according to JISM Citation2001; 0.3 mg L−1 chloroform, 0.1 mg L−1 bromoform, 0.1 mg L−1 dibromochloromethane, and 0.06 mg L−1 bromodichloromethane according to WHO Citation2006).
In regions like the water shed of KAC relatively high concentrations of bromine can occur. This leads to the formation of brominated or partly brominated THMs, which can be of toxicological relevance (Richardson Citation1998; Richardson et al. Citation2003; von Gunten Citation2003). Due to the toxicological importance of the rest of the halogenated compounds formed in chlorination reactions the integrated parameter of organically bound halogens absorbable on activated carbon (AOX) can be used for the safety of drinking water quality assessment (Frimmel et al. Citation2000; Zwiener Citation2006).
The aim of this work was to:
-
give the necessary background information on the water quality of the KAC as the main regional resource for drinking water supply,
-
characterize the important reasons for eutrophication and presence of algal species in the arid climate, and
-
determine the formation potential for THM and for the organic halogen compounds reflected as AOX as a consequence of water disinfection.
The importance of this work results from the fact that KAC is one of the main potable and agricultural water supplies in the country, so it is imperative to determine the reasons and the sources of eutrophication in it to find proper ways to reduce the overall eutrophication potentials.
Experimental section
Sampling
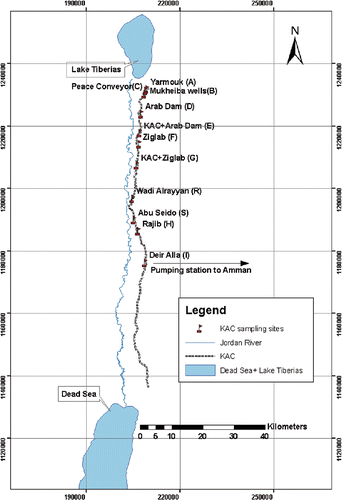
Sampling started from the intake of KAC, at the Yarmouk River (A) in Al Nafaq in the north, until the Deir Alla (I), the intake of Amman drinking water in the south at a distance of 65 km from Al Nafaq site ().
Water samples were collected in 1–1.5 monthly intervals during the period from December 2002 to May 2004 to investigate the changes in the concentrations of main parameters. Along the KAC, 14 times about 10 samples per each sampling campaign were taken. Temperature (T), pH, and electrical conductivity (EC) were measured on site. Water samples were taken in totally filled glass and polyethylene (PE) bottles, cooled immediately by using a cool box, and brought to the laboratory within a few hours. The analyses were done at the Department of Applied and Environmental Geology, Jordan University, Amman; at the Laboratories of the Ministry of Water and Irrigation, Amman; and at the Chair of Water Chemistry, Engler-Bunte-Institut, Universität Karlsruhe (TH), Germany.
Materials and methods
EC, T, and pH were measured using electronic field equipment. The chemical analyses of the main cations and anions were carried out according to American Standard Methods and German Standard Methods for Water Analysis (American Public Health Association 1997, 2000, Fachgruppe Wasserchemie in der GDCh 1982, 1985, 1995, 2002). shows the analytical methods used for the different parameters.
Table 1. Parameters, threshold values, and analytical methods used.
Chlorination
The chlorination was done in bottles with conical shoulder with a volume of 118 mL. Before the experiment, the bottles were treated with sodium hypochlorite for 24 h to ensure, that no chlorine was consumed by the bottles during the experiment. Before chlorination the DOC concentrations of the samples were measured. Most of the samples showed 2–4 mg L–1 DOC concentrations. The samples with higher DOC concentrations were diluted to 3 mg L–1 DOC concentration. All other samples were chlorinated without dilution. Sodium hypochlorite was used as chlorination agent, and the chlorine concentration of the original solution was determined by a rapid test. By dilution with demineralized water a stock solution with 3.33 g L–1 free chlorine was prepared. The necessary volume of the stock solution to reach a Cl/DOC mass ratio of 3 : 1 was calculated for every sample. The bottles were filled completely and four glass beads were added to each bottle for better intermixing. The calculated water volumes plus 1 mL for the addition of phosphate buffer were taken out of the sample bottles and replaced by the hypochlorite stock solution and the buffer to reach gas-free mixtures. The solutions were shaken for 1 min and then kept in the dark. After 48 h, 30 mL-vials (glass) with septum were filled completely (gas-free) for the THM measurements. Then the chlorination was stopped with a point of spatula of sodium sulphite and all volatile compounds were blown out with nitrogen for 30 min. Afterwards the samples were prepared for AOX-measurements.
AOX measurement
The samples were diluted with demineralized water to be in the optimum range for the measurement of the AOX. Fifty milligrams of powdered activated carbon (PAC) (F 300), five drops of 65% nitric acid, and 5 mL nitrate solution (1.4 mL HNO3 65% and 17 g NaNO3 per litre H2O) were added to 100 mL of the diluted samples. After 24 h of shaking the loaded PAC was filtered and the samples were measured with an ECS 1200 AOX analyzer from Euroglas Analytic Instruments, Delft, the Netherlands.
THM measurement
The THM concentration was determined using a gas chromatographic system CP 9000 from Chrompack, Berlin, Germany, an Autosampler 955 PTM, a purge and trap injector, and a CP-Sil CB column. The signal was measured by an electron capture detector (ECD).
Algae identification, chlorophyll a, and plankton counting
The algae genera were identified according to microscopic pictures and classified based on their water quality related characteristics according to Kumar and Singeh (Citation1982). Chlorophyll a and plankton counting was performed according to Standard Method 10200H13 (American Public Health Association Citation2000). The samples were analysed by the laboratories of the Ministry of Water and Irrigation, Amman.
Results and discussion
Physicochemical analyses
KAC extends from the upper part of the Valley at Adasieh down to the Dead Sea. The canal is fed mainly from the Yarmouk River, the Peace Conveyor water from Israel, and the Mukheiba Wells. In addition, the canal collects water from wadis and dams along the canal in Jordan (Wadi Al Arab, Wadi Ziglab, and King Talal Dam) (Brett and Eikum Citation2000; Brett, Palmer, and Ryu Citation2002).
The study area extends along KAC from its intake at the Yarmouk to the Deir Alla, at an elevation of 235 m below sea level to the south, along the left-hand side of the Jordan River terraces with a length of 65 km between the Yarmouk and the Deir Alla ().
shows the maximum, minimum, and average concentrations of some basic physicochemical parameters in the water samples of KAC and the tributaries, which were measured during December 2002 and May 2004. The physical parameters and the chemical composition of the collected samples showed great variations due to several factors.
Table 2. Maximum (Max.), minimum (Min.), and average (Av.) concentrations of physicochemical parameters in King Abdullah Canal and tributaries from December 2002 to May 2004.
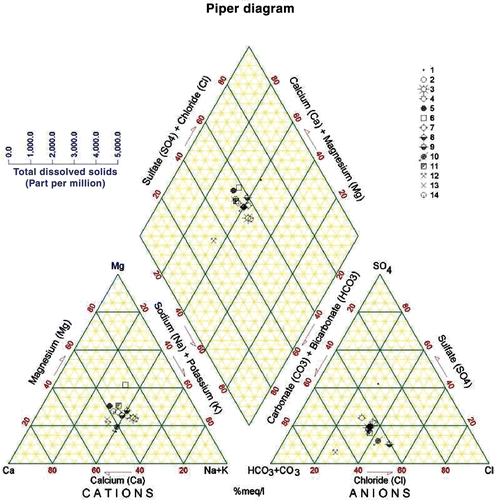
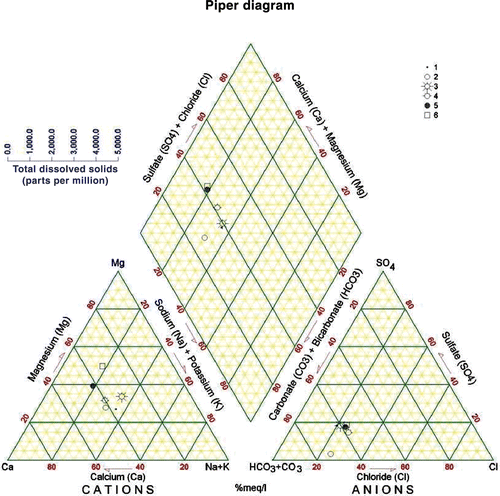
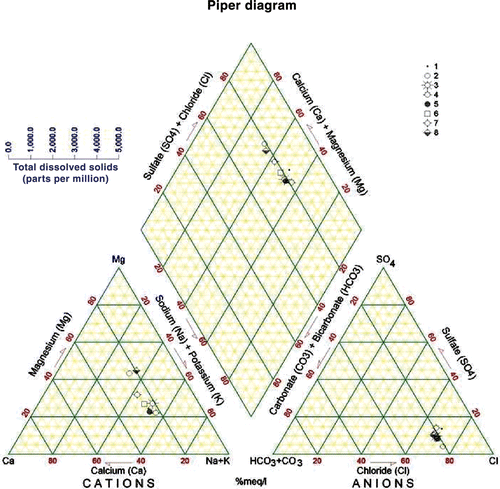
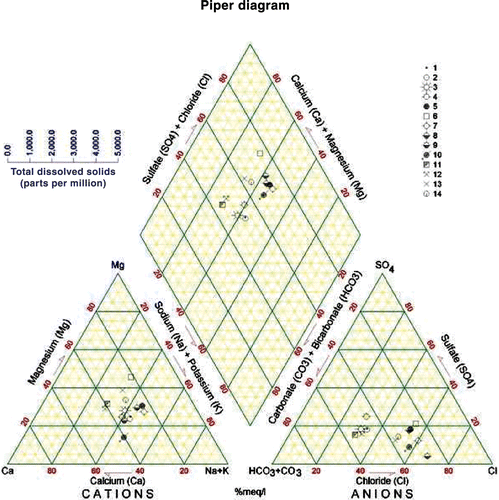
The water of KAC originates from a highly heterogeneous area in terms of topography, climate, geologic conditions, and land use. The equivalent ionic ratios generally reflect the information about the sources and genesis of the water composition (Matthess Citation1994) (). The Na+/Cl− ratios in the Yarmouk River (A) and the Mukheiba Wells (B) reflect that of precipitation of water without much dissolution of halides. This can be explained by the flow of water in the flushed carbonate rocks covering the catchment area. The water coming through the Peace Conveyor (C) shows a water source with deep circulation and dissolution of halides, and is similar to the water in the deep aquifers in Jordan or those affected by the Lisan Formation in the Jordan Valley area (Salameh Citation2001). This also applies for the /Cl− ratios (Rosenthal Citation1988; Salameh Citation2001) (). The water coming through the Peace Conveyor (C) has also higher Mg2+/Ca2+, Ca2+/
, Na+/Ca2+, and Ca2+/(
+
) ratios, which reflect the composition of groundwater in the surroundings of Lake Tiberias (Rosenthal Citation1988) and those in the deep aquifers along the eastern escarpment of the Jordan Valley affected by the Lisan Formation (Salameh Citation2001). Water of Mukheiba Wells (B) and Yarmouk River (A) is of alkaline-earth type with increasing portions of alkalis, whereas that of the Peace Conveyor (C) is of alkaline type with increased portions of chloride (Figures ).
Table 3. Ionic ratios (meq/meq) of the main water sources of the King Abdullah Canal.
The catchment area of the Yarmouk River (A) is agrarian, with small types of industries located in the main towns in Jordan and Syria. The effluents of two wastewater treatment plants reach the river during floods (Salameh Citation1996). In the Yarmouk River Ca2+ and Mg2+ concentrations ranged from 1.83 and 0.90 (Min.) to 4.99 and 5.03 (Max.) meq L−1, respectively, while Na+ and K+ ranged from 1.41 and 0.10 to 4.57 and 0.25 meq L−1, respectively. Cl− and concentrations ranged from 1.10 and 0.25 (Min.) to 4.88 and 4.90 (Max.) meq L−1respectively. Mukheiba Wells (B) is mainly fed by rain water (approximately 350 mm year–1) and represents the second important tributary to KAC. Compared to Yarmouk River (A) they show lower concentrations of Ca2+, Mg2+, Na+, K+, Cl− and
. The Peace Conveyor (C) supplies KAC with one-third to one-half of the flux compared to the Yarmouk River (A) and the Mukheiba Wells (B). It has higher concentrations of Mg2+, Na+, and Cl− compared to the two other sources. The variations in the concentration of the basic water constituents were lower for the water from the Mukheiba Wells (B) and the Peace Conveyor (C) compared to the Yarmouk River (A) composition throughout the sampling period. The Yarmouk River showed the highest variations as the river discharges flood and base flow water in winter time. Whereas, during summer the water is mainly composed of spring water and irrigation return flows with less than 1% of untreated domestic effluents (Salameh Citation1996).
There is a large variation in water flow as shown for the years 2000–2003 ( and ). The water inflow into the canal and the outflow vary between 99 × 106 and 129 × 106 m3 for the inflow, and between 86 × 106 and 114 × 106 m3 for the outflow during these years (Ministry of Water and Irrigation, Jordan Citation2003). With regard to the water inflow into the canal, the tributaries of the Arab Dam (D), the Ziglab Dam (F), and the Wadi Rajib (H) are of minor importance. However, the discharge of and P compounds from wadi and dam water is relatively high (), and the water quality of the KAC inflows is influenced to a great extent by these compounds.
Table 4a. Total flux related amount of water in the King Abdullah Canal for the years 2000–2003 in 106 m3 per month and per year.
Table 4b. Flux related amount of water in the King Abdullah Canal for the year 2002 for different sampling points in 106 m3 per month and per year.
Table 5. Chemical analysis of the main eutrophication parameters along the King Abdullah Canal and its water sources (sampling from December 2002 to May 2004).
It is important to note that the sources of the KAC water and their mixing ratio differ significantly in time and place, which has a great impact on the water quality. Furthermore, KAC does not have a constant water flux as the canal is used as a storage reservoir. Check gates (37 along the canal) are used as regulators. The average velocity of the KAC water is 0.9–1 m s−1.
Parameters influencing eutrophication
shows the maximum, minimum, and average concentrations of basic parameters influencing eutrophication in the tributaries and in KAC, which were measured during December 2002 and May 2004. One main discharge of N and P compounds is given by the Yarmouk River (A), showing the highest concentration values for and total P.
This can be explained by the agrarian catchment area of the Yarmouk River (A) and by the influence of wastewater effluents. High concentrations of also originate from the Arab Dam water (D) and the Ziglab Dam outlet (F). The discharge of P compounds into the canal is also highly influenced by the Arab Dam water (D). Even though the inflow of (D) and (F) was low during the years, the impact on eutrophication of the water along the KAC was significant. The concentrations along the canal were mostly above the eutrophication potential levels for N (0.65 mg L−1) and P (0.03 mg L−1) (Ryding and Rast Citation1989), and all data showed great variations.
High organic loads originated generally from the Arab Dam water (D), which exhibited the highest concentrations of DOC and TOC. DOC and TOC concentrations in samples of the Peace Conveyor were higher compared to the ones in the Yarmouk River (A). Along the flow of the canal (D to H), DOC concentrations did not change to a high extent. The average values showed concentrations around 2.5 mg L−1 DOC.
Biological analysis
The algal species in the four major sites the Yarmouk River (A), the Mukheiba Wells (B), the Peace Conveyor (C), and the Deir Alla (I) during the period of study are listed in . The algae genera found were identified according to microscopic pictures and classified into six main groups on the basis of their water-quality-related characteristics according to Kumar and Singeh (Citation1982): (a) taste and odor algae, (b) filter clogging algae, (c) polluted water algae, (d) clean water algae, (e) plankton and other surface water algae, (f) algae growing on reservoir walls. The limited number of samples investigated does not allow a thorough interpretation of the different identified species. However, it is interesting to note that the pragmatic classification of the algae found clearly shows the dominance of species, which are typical for polluted waters and those, which cause problems by clogging technical filters. This information underlines the need of measures to improve the water quality in KAC.
Table 6. Algal species found (#) in the main sites along King Abdullah Canal.
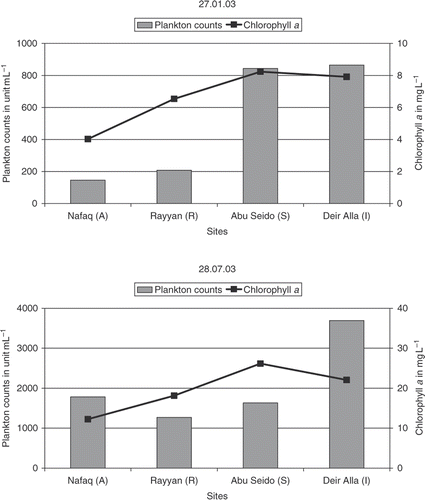
Along the canal, there was an increase in the chlorophyll a concentration and in the plankton counts, as shown for a winter and a summer sample (). During summer the two parameters show much higher values compared to the winter season with lower temperature and sun illumination. The downstream distance from sampling point A to the sampling sites Wadi Al Rayyan (R) and Abu Seid (S) is about 35 km and 50 km, respectively.
Formation potential for THM and AOX
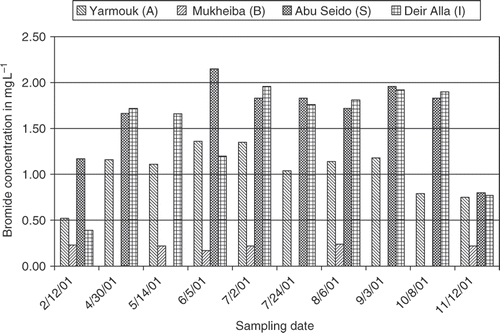
The bromide concentrations in KAC were relatively high and fairly constant over most of the year (). It is obvious that the high concentrations arose from the Yarmouk River (A), one of the main tributaries of the canal. Lower values were observed for the winter season (0.52–0.75 mg L−1) compared to higher values in the summer season (1.04–1.36 mg L−1, between May and September). The Mukheiba Wells (B), one of the three main discharge waters for KAC contributed only to a low extent. Here, the bromide concentrations ranged between 0.17 and 0.24 mg L−1 all over the year. Along the canal, the bromide concentration increased. In Abu Seido (S), about 35 km downstream, the bromide concentrations showed the same level as in the Deir Alla (I) where the KAC water is used as raw water source for drinking water supply of Amman. Due to the special geological and hydrogeological situation of the region and its climatic character, the concentrations ranged from 0.39 to 0.77 mg L−1 in the winter season, up to concentrations between 1.2 and 1.9 mg L−1 in the summer season. As a consequence, high concentration levels in brominated THM result after disinfection with chlorine during the water treatment process.
THM formation and AOX formation as well as the formation potentials for THM and AOX (THM FP and AOX FP, respectively) were studied by chlorination of different water samples from KAC and its main discharges (). The THM FP (in CHCl3 equivalents) ranged between 16 and 39 µg mg−1, whereas the AOX FP showed values between 32 and 82 µg mg−1. The organic matter of the main tributaries to the canal [Yarmouk site (A) and the Peace Conveyor (C)] contributed to the formation of DBP. Along the flow to Wadi Rajib (H), which is near the station pumping the raw water to the treatment plant of Amman, the data of the THM FP and AOX FP do not show any trend. Brominated THM were detected in each sample. The concentrations of CHBr3, and CHClBr2 turned out to be much higher compared to the ones of CHCl2Br and CHCl3. There is no correlation for the formation of brominated THM and the bromide content of the different water samples.
Table 7. Maximum THM and AOX concentrations and formation potential (FP) for THM and AOX in the water samples of King Abdullah Canal and tributaries.
Conclusions
The results of this study show that the Yarmouk River is the main source contributing nutrients and a wide range of algal species. High organic loads originate generally from the Arab Dam water (D), which exhibited the highest concentrations of DOC and TOC. The water quality data along KAC indicate that water quality expectedly decreases along the canal starting from the Yarmouk River (A) to the Deir Alla (I). The nutrient concentrations measured in KAC together with the climatic situation suggest that this water system is eutrophic and develops algal activities. This is supported by the increase in the concentration of chlorophyll a and of the plankton counts from the feeding sources of KAC up to those measured in the Abu Seido (S) and the Deir Alla (I). During summer time, these two parameters show significantly higher concentrations, which reflect the role of intensive sun illumination and elevated temperature.
The high nutrient concentrations are not the only factor causing eutrophication, but there are other critical and substantial conditions supporting it. The UV radiation, for example, which may restrict the algae activity, is low in the Jordan Valley (lying at 220–250 m below sea level) because of the thicker atmospheric layer compared to other places in the world. This atmospheric layer is more effectively absorbing UV radiation than the atmosphere at higher elevations. Furthermore, the high sun illumination and the low velocity of the water in the canal are in favor of enhancing algal activities. All these conditions supply an ideal environment for a greater diversity of algal species and for their increased production to form algal blooms. In such an environment, the control of algal growth becomes complicated and requires monitoring programs, highly effective and fast responses to cope with increase in algae numbers and species, and rapid changes in technical water treatment procedures. An attempt was made by the Center for Environmental Studies and Research Management (CESAR) in 2000 using random measurements to identify parameters as indicators for quality impairment, but no long-term systematic measurements and evaluations have been carried out so far. In addition, the Japan International Cooperation Agency (JICA), in cooperation with the Environment Monitoring and Research Central Unit (EMARCU), has installed a few stations on the canal in 2002. Some pollution parameters have been monitored, but these data are not thought to predict algal growth. Our investigations show clearly that nitrate and phosphorus concentrations are much higher for a limitation of algal bloom. All efforts should be taken to decrease the load of these two substances in the KAC.
Since the water of KAC is an essential source for drinking water supply, water quality and human health effects have become crucial. Disinfection with chlorine is widely applied to guarantee hygienic safety, but also leads to reaction products of toxicological relevance. The organic matter of the main tributaries to the canal [Yarmouk site (A) and the Peace Conveyor (C)] and the organic matter in KAC contribute to the formation of DBP. An additional challenge is the relatively high concentrations of bromide in the KAC, arising mostly from the Yarmouk River. As a consequence, high concentration levels of the highly toxic brominated THMs result after disinfection with chlorine as a part of the water treatment process. The harmfulness of brominated DBP, and of brominated THM in particular, have to be considered by running the treatment process. Right now there seems to be no feasible alternative for disinfection with chlorine. Therefore, the concentration of DOC in the raw water has to be decreased, in order to minimize the dose of chlorine needed for the disinfection process, leading to a decrease of the DBP. As a consequence this poses considerable concern on the water agencies using KAC water as a source for drinking water.
All this does not only ask for the application of efficient technical tools for a reliable and safe water treatment but also for a well planned sustainable water management. Countries where water does not belong to the abundant goods can teach us ways of caring for this non-substitutable basis for life and social development.
Acknowledgments
This work was funded by the German Research Foundation (DFG) within the trilateral research project no. FR536/31. We thank the Ministry of Water and Irrigation, Amman, for the support in chemical and biological lab work and Dr Margit Müller for fruitful discussions.
References
- American Public Health Association (APHA). 1997a. Standard methods for the examination of water and wastewater: Conductivity 2510. Washington, DC: APHA
- American Public Health Association (APHA). 1997b. Standard methods for the examination of water and wastewater: Potassium 3500, Washington, DC: APHA
- American Public Health Association (APHA). 1997c. Standard methods for the examination of water and wastewater: Sodium 3500. Washington, DC: APHA
- American Public Health Association (APHA). 2000. Standard methods for the examination of water and wastewater: Temperature 2550. Washington, DC: APHA
- Brett, M.T., and A.S. Eikum. 2000. King Abdullah Canal, Jordan/Water quality research program. Prepared to CESAR. Oslo, Norway.
- Brett, M.T., R.N. Palmer, and J.H. Ryu. 2002. Water quality simulation model for the King Abdullah Canal in Jordan, University of Washington, Department of Civil and Environmental Engineering. Prepared to CESAR. Seattle, Washington, USA.
- Environment Monitoring and Research Central Unit (EMARCU). Higher Council for Science and Technology (HCST), open files (Najeeb, Mohammad, pers. comm.)
- Fachgruppe Wasserchemie in der GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schlammuntersuchung (DEV). 1982. Bestimmung des Nitrat Ions DIN 38405 D9. Berlin: Beuth Verlag
- Fachgruppe Wasserchemie in der GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schlammuntersuchung (DEV). 1985a. Bestimmung der Chlorid Ionen DIN 38405 D1. Berlin: Beuth Verlag
- Fachgruppe Wasserchemie in der GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schiammurstersuchung (DEV). 1985b. Bestimmung der Sulfat Ionen DIN 38405 D5. Berlin: Beuth Verlag
- Fachgruppe Wasserchemie in cier GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schiammurstersuchung (DEV). 1995a. Bestimmung der gesamten und der zusammengesetztenAlkalinität DIN EN ISO 9963-1. Berlin: Beuth Verlag
- Fachgruppe Wasserchemie in der GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schlammuntersuchung (DEV). 1995b. Bestimmung der gelö sten Anionen mit Ionenchromatographie DIN EN ISO 10304-1. Berlin: Beuth Verlag
- Fachgruppe Wasserchemie in der GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schlammuntersuchung (DEV). 1999. Bestimmung des gesamten organisch gebundenen Kohlenstoffs (TOC) DIN; 1484H-3, ISO 8245. Berlin: Beuth Verlag
- Fachgruppe Wasserchemie in der GDCh. Deutsche Einheitsverfahren zur Wasser-, Abwasserund Schlammuntersuchung (DEV). 2002. Bestimmung von Calcium und Magnesium–komplexometrisches Verfahren DIN 38406 E3. Berlin: Beuth Verlag
- Frimmel , FH , Hesse , S and Kleiser , G . 2000 . “ Technology-related characterization of hydrophilic disinfection by-products in aqueous samples ” . In Natural organic matter and disinfection by-products–Characterization and control in drinking water , Edited by: Barrett , SE , Krasner , SW and Amy , GL . Washington, DC : ACS . ACS Symposium, Vol. 761, 84–95.
- International Organization of Standardization (ISO). 2004. Water Quality: Determination of adsorbable organically bound halogens (AOX), Deutsche Version EN 9562, ISO 9562
- International Organization of Standardization (ISO). 2008. Water-Quality: Determination of pH. ISO 105 23
- JISM, The Jordanian Institute of Standards & Metrology . 2001 . Technical regulation, Water–Drinking Water , 4th , 286 Hashemite Kingdom of Jordan : JISM .
- Jordan Valley Authority, Amman (pers. comm.)
- Kumar , HD and Singeh , HN . 1982 . A text book on algae , 3rd , Madras : Affiliated East West Press .
- Matthess , G . 1994 . Lehrbuch der Hydrologie. Vol. 2, Die Beschaffenheit des Grundwassers , Stuttgart : Gebr. Borntraeger Verlagsbuchhandlung .
- Ministry of Water and Irrigation, Jordan, 2003. Open files (pers. comm.)
- Richardson , SD . 1998 . “ Drinking water disinfection by-products ” . In Encyclopedia of environmental analysis and remediation , Edited by: Meyers , RA . 1398 – 421 . New York : John Wiley & Sons .
- Richardson , SD , Thruston , AD , Rav-Acha , C , Groisman , L , Popilevsky , I and Juraev , O . 2003 . Tribromopyrrole, brominated acids, and other disinfection byproducts produced by disinfection of drinking water rich in bromide . Environmental Science and Technology , 37 : 3782 – 93 .
- Rosenthal , E . 1988 . Ca2+-Chloride brines at common outlets of the Beat Shean Multiple aquifer system . Journal of Hydrology , 97 : 89 – 106 .
- Ryding , SO and Rast , W . 1989 . The control of eutrophication of lakes and reservoirs , Vol. 1 , Paris : UNESCO and The Partheron Publishing Group .
- Salameh , E . 1987 . The potential of surface water utilization for domestic purposes in Jordan . International Journal of Environmental Studies , 28 : 291 – 300 .
- Salameh , E . 1996 . Water quality degradation in Jordan–Impacts on environment, economy and future generations resources base , 13 – 19 . Amman, , Jordan : The Higher Council of Science and Technology .
- Salameh , E . 2001 . Sources of water salinities in the Jordan Valley Area/Jordan . Acta Hydrochimica et Hydrobiologica , 29 : 329 – 62 .
- TrinkwVO, Trinkwasserverordnung 2001. Verordnung über Trinkwasser und über Wasser für Lebensmittelbetriebe
- von Gunten , U . 2003 . Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodine or chlorine . Water Research , 37 : 1469 – 87 .
- WHO, World Health Organization. 1984. Guidelines for Drinking-Water Quality, Vol 1–3. Geneva
- WHO, World Health Organization. 2006. Guidelines for Drinking-Water Quality, 3rd Edition, incorporating first Addendum
- Zwiener , C . 2006 . “ THMs, HAAs, and Emerging Disinfection By-Products in Drinking Water ” . In Organic pollutants in the water cycle , Edited by: Reemtsma , T and Jekel , M . 251 – 286 . Weinheim : Wiley-VCH .