Abstract
Previous studies have attempted to explain forces holding particles together in densified biomass pellets using theories of forces of attraction between solid particles, forces of adhesion and cohesion, solid bridges and mechanical interlocking bonds including interfacial forces and capillary pressure. This study investigated the bonding mechanism of primary biomass components in densified pellets through the use of advanced analytical instruments able to go beyond what is visible to the naked eye. Data obtained were used to predict how primary biomass components combine to form pellets based on the theory of functional groups and the understanding of structural chemistry. Results showed that hydroxyl and carbonyl functional groups played key roles in helping to identify the type of forces acting between individual particles, at a molecular level. At a microscopic level, morphological examination of the pellet clearly showed solid bridges caused by intermolecular bonding from highly electronegative polar functional groups linked to cellulose and hemicellulose.
INTRODUCTION
The main goal of increasing the use of biomass as a source of energy is often driven by the desire to reduce CO2 emissions and to mitigate dependency on fossil fuels. Typical sources of biomass include wood and agricultural residues, industrial residues and waste products. However, an effective way to utilize biomass as an energy source is in the form of pellets, which drastically reduces the technical limitations of using the biomass in its natural form.[Citation1,Citation2] Biomass pellets such as wood pellets, as a fuel source, offers safe and convenient ways to bridge the energy gap in the transition away from the use of fossil fuels. Advantages of using biomass pellets include preservation of the biomaterial to ensure value as a fuel, less handling and storage issues due to its high density (ca. 650 kg−1 m−3), low moisture content (ca. 5–12%), standardization of the pellets which allows for easier internal and global market transportation, more cost-effective due to uniform shape and size; which makes the pellets suitable for small household heating systems such as stoves and boilers. The pellets are also used in large scale power plants where up to 300,000 tons of wood pellets are used annually.[Citation2] Biomass pellets are often made from dry, untreated sawdust, wood chips or shavings and its production are relatively cheap, safe and the industrial development of pellet producing plants is combined with low risk of failure when compared to other technologies that promote the use of biofuels.[Citation2–4]
Biomass pelleting is performed by continuously pressing finely ground biomass material through die holes of the pellet press that are about 6 to 8 mm in size. This creates elongated pellets with round cross-sections that are cut to the desired sizes. Under the right pelleting conditions, primary components (such as cellulose, hemicellulose and lignin) are pressed together by strong forces at elevated temperatures to initiate binding. In addition, strong bonds are generated between the compressing components and, the exact nature of the created bonds remains completely vague. In fact, how primary biomass components combine to form densified pellets under optimum pelleting process conditions have not been clearly explained from a structural chemistry perspective. Several studies[Citation3,Citation5–8] have attempted to explain the binding forces acting between individual particles in biomass pellets using the theories of attraction forces between solid particles, adhesion and cohesion forces, interfacial forces and capillary pressure, solid bridges including mechanical interlocking bonds. While this may be a well-conceived idea, the correlation between biomass characteristics and these physical/mechanical events as well as pelleting process conditions relevant to the production of good quality biomass pellets have never been clearly established from a chemistry perspective, where the pellets are structurally diagnosed such that the functional groups attached to their carbon backbone are identified and their role in bonding tacitly explained. A full understanding of the structure and properties of biomass is a prerequisite to complete understanding of the bonding mechanism of primary components of the biomass in pelleting processes.[Citation9–13]
The bonding mechanism of primary components in densified biomass pellets is such a complex event to comprehend, and previous studies[Citation14–16] have alluded to insufficient knowledge about the interrelationship between strong bonding mechanisms and biomass characteristics. Biomass is diverse in nature and this accounts for why there is still uncertainty about how particles of the biomass combine to form densified pellets. A way to explain biomass diversity, perceived as a good starting point, is to briefly describe biomass in general. Biomass is composed primarily of cellulose, hemicellulose and lignin, with varied structural characteristics that are bound by functional groups.[Citation17,Citation18] These primary components, due to their polymeric nature, have molecular structures with a carbon backbone and functional groups attached to the carbon chains. The entire carbon chain and its attached functional groups are affected by pelleting processing conditions such as temperature and compression force; for example, increased temperatures initiate chemical modifications that are significant to pellet properties.[Citation3] The functional groups are a collection of elements that make up the biomass and confer specific chemical characteristics.[Citation17,Citation19] For instance, hemicellulose has the ability to flow at elevated temperatures due to the presence of groups such as carbon-oxygen (C–O) in its molecular structure; these groups have low bond dissociation energies because of their single bonds. Lignin changes its characteristics to become glassified at high temperatures for the same reason given for hemicellulose; cellulose, due to its stiff nature and its many glucose units with multiple hydroxyl groups (–OH), softens at certain temperatures and pressure to become amorphous.[Citation18,Citation20–22] Studies have also shown that even within the same individual component of biomass, there are differences in functional groups; for example, xylan has carbon chains that are much more flexible than glucomannans, both of which are monomers of hemicellulose; xylan and mannan exhibit widely different behaviors during compression.[Citation23–25] It is vital to be able to identify functional groups and the physical and chemical properties that they afford organic materials.[Citation26] This creates a good starting point to understanding how primary components of biomass combine to form densified pellets under optimum pelleting conditions. In this regard, knowledge and understanding of structural chemistry are required for detailed explanation, which must be based on the theory of functional groups and how to measure and locate them through the use of advanced analytical instruments.
Biomass pelleting is regularly met with a lack of understanding of how primary components combine to form densified biomass pellets under optimum pelleting conditions. It is anticipated that considerable quantities of biomass utilization for future production of energy will rely on fuel pellets made from biomass. To achieve this goal, critical evaluation of both the chemical relationships and the specific structural modifications taking place as biomass undergoes pelleting are required, which of course requires the use of advanced analytical instruments able to provide information beyond what meets the eye. Therefore, the aim of this study was to investigate, from a structural chemistry perspective, the bonding mechanism of primary components of biomass in densified pellets using advanced analytical instruments able to provide information beyond what is visible to the human eye and use data obtained to predict how components combine to form pellets based on the theory of functional groups and the understanding of structural chemistry. What brings about adhesion and particle to particle bonding is believed to be concealed in the structural characteristics of biomass hence knowledge and understanding of structural chemistry are required to offer detailed explanation.
MATERIAL AND METHODS
Sample Preparation
The biomass pellets used in this study were industrially produced Norway spruce pellets, otherwise referred to as industrial spruce pellet throughout this article, obtained from a local pellet plant (Stora Enso Gruvön, Grums) in Karlstad, Sweden. Wood remains one of the most widely used raw materials for the production of fuel pellets hence industrial pellets made from Norway spruce wood was chosen as reference sample for this study. The pellets were produced under industrial conditions hence no pretreatment measures were undertaken. However, prior to analysis, the pellets were stored in air-tight vials to protect them from contamination and their average sizes were 8 mm in diameter and 20 mm in width. The conditions of pelleting are presented in .
TABLE 1. Pelleting conditions of industrial spruce pellets and some physical properties before and after pelleting.
The strength and durability of densified biomass pellets are dependent upon the bonding attributes of major components of the raw material.[Citation6] As previously mentioned, the bonding mechanism of primary biomass components in densified pellet involves the inter-play of many processes that cannot be felt by hand or visualized by the naked eye and justifies the need for the use of advanced analytical instruments for diagnosis of the pellets such that quintessential bonding properties and pellet-forming abilities of each component may be determined with certainty. The instruments used for diagnosis were Energy Dispersive X-Ray Spectroscopy (EDX), Fourier-Transform Infrared Spectroscopy (FT-IR), Thermogravimetric Analyzer (TGA), Scanning Electron Microscope (SEM), and Confocal Raman Microscopy (CRM). Restricted by the sample holders of the instruments used for analyses, industrial spruce pellets were manually cut into small sizes of < 2 mg using a razor blade. Because of the complex and anisotropic nature of the sample, different parts of the sample were manually cut into small sizes and three measurements were undertaken on each part with average results presented. The procedures for analysis, as well as motives for choice of analytical instruments, are explicitly described.
Compositional Analysis
Assessment of the use of biomass in any conversion process requires a good understanding of its basic composition, characteristics, and performance.[Citation17,Citation27,Citation28] The primary components of industrial spruce pellet (cellulose, hemicellulose and lignin) were quantified from the TGA plot presented in a subsequent section and were measured in accordance with the known decomposition temperatures of each component,[Citation29] after the evaporation of moisture. Due to the structural differences between the major constituents of biomass, they are commonly distinguished and identified by the use of TGA, and the temperature ranges for the thermal decomposition of these constituents had been previously reported.[Citation29–32] EDX was used to determine elemental components in terms of the weight percentages of C, O and S, while the weight fraction of H was determined by the carbon, hydrogen, nitrogen and sulfur (CHNS) analyzer. Experiments were repeated in triplicates and average values reported.
FT-IR Analysis
In FT-IR analysis, a sample is subjected to infrared radiation (IR) such that specific parts of the IR are absorbed by the sample in terms of its chemical composition. The stretch and deformation vibrations of specific molecular bonds in the sample correspond to absorption bands at specific wavenumbers of the IR spectrum, and the traditional unit for this analysis is expressed in cm−1.[Citation33]
FT-IR analysis was undertaken to identify the functional groups in the carbon backbone of the primary components of industrial spruce pellets and to establish the role of each group in the bonding properties of these components. As such, spectra were recorded using a Varian 680-IR FT-IR spectrometer equipped with a DTGS detector. The system was operated in an attenuated total reflectance (ATR) mode. An ATR crystal of diamond, having a contact area of Ø2 mm and a penetration depth of 2 µm, was used. According to Perkin Elmer,[Citation34] for FT-IR in an ATR mode to be successful, samples must be in direct contact with the ATR crystal, because evanescent wave only extends beyond the crystal at a penetration depth of 0.5 µm to a maximum of 5 µm, and diamond is the best ATR crystal material because of its durability. Background and sample spectra were scanned at a spectral resolution of 4 cm−1 and a spectral range of 4 000 cm−1 to 650 cm−1; 32 scans were collected. Spectra were ATR and baseline-corrected using Varian Resolution Pro software. Spectra were normalized at a signal of 1 160 cm−1.
TGA Analysis
When biomass is heated at elevated temperatures, changes that will affect its chemical structure and performance will occur, and the extent of the changes will depend on the temperature level and the duration of exposure conditions.[Citation29,Citation35] Therefore, thermo-analytical techniques such as TGA can provide information about chemical modifications in a relatively simple and straightforward manner without tedious sample preparation steps. Thus, TGA is a technique used to describe changes associated with a sample as a function of temperature as the sample is heated in an inert atmosphere of nitrogen or argon gas. It has been widely used to obtain information, not just about the thermal behavior of materials, but also to quantify the composition of biomass; it is a faster and less expensive technique to determine the composition of biomass than the wet chemical technique.[Citation36,Citation37] In this study, TGA was used for two major reasons: 1) to quantify primary components of industrial spruce pellets according to degradation temperature; 2) to determine chemical modification/transition temperature and its role in the bonding abilities of primary components. Knowledge of the chemical modification/transition temperature of biomass has fundamental importance in estimating bonding attributes of major components of the biomass.[Citation6]
A Mettler Toledo TGA/DSC1 Star system was used to evaluate changes occurring in the sample as a function of temperature and the procedure for analysis was such that the sample was first placed in the combustion chamber of the instrument and ignited through software programing. The experiment was conducted in an inert atmosphere of nitrogen gas in such a way that sample weight loss was monitored as it was combusted, cooled and isothermally held. The nitrogen gas flow rate was at 100 ml−1 min at a heating rate of 10 °C min−1. The experiment began at room temperature (25–30 °C) to a maximum temperature of ca. 1 000 °C.
CRM Analysis
To provide a quick and easy method of visualizing internal and surface structure of industrial spruce pellet in thick hand-cut sections and to understand how primary components are distributed, a confocal microscope was used. The CRM was invaluable in helping to achieve this goal and was basically used to study particle distribution and orientation as well as to uncover particle to particle interactions initiated through changes caused by pellet press conditions such as temperature and compression force.
A Leica SP8 confocal with a DMI 6 000 microscope was used to visualize hand-cut samples of industrial spruce pellets. The Leica TCS SP8 X was mounted on the Leica DMI 6 000 inverted microscope. Samples of the pellets were placed (without mounting medium) on a Petri dish with a coverslip bottom (cell view from greiner). The pellets were excited with a 405 laser, for ultra-violet (UV) fluorescence. Samples were illuminated at three different wavelengths, which were most suitable for the type of sample under study; a white light laser at 488 nm for green fluorescence, at 579 nm for a red fluorescence and at 633 nm for far-red fluorescence. Images were obtained under experimental conditions able to preserve the sample in its natural state.
SEM Analysis
A single imaging technique for visualizing lignocellulosic biomass samples cannot provide all information required concurrently with optimal temporal and spatial resolution, hence complementary techniques are often needed to achieve a full understanding of the structural changes that occur in biomass materials as they undergo physical, chemical or biochemical processing. Nonetheless, it is worthy to mention that each type of analytical instrument has its merits and demerits in their application for structural diagnosis of samples.[Citation38] While the CRM has the ability to track multiple biomolecular species in high-resolution images over a large surface area, SEM technique is able to image samples at sub-nanometre resolutions and allow good passage of light through the sample to make visualization less complicated; the limitations of the SEM technique include its inability to simultaneously track multiple biomolecular species, its inability to image large surface areas, and the fact that the sample has to be in close proximity with the analysis chamber.[Citation17,Citation38,Citation39] In spite of these limitations, however, the SEM remains a high-resolution analytical technique able to offer internal and surface visualization of samples at higher magnifications of up to 2 million times. It is also worthy to mention that the limitations of one technique are often compensated for by the advantages of the other.
Cross-sections of manually cut industrial spruce pellets were visualized under a JEOL (JSM-6390LV) model SEM instrument fitted with an EDX analyzer, which was used to quantify elemental components. Prior to examination, samples were prepared by mounting them on a stub with a carbon double-sided tape and sputter-coated with gold (Au) in order to increase conductivity and to make microscopic viewing less complex. Sputter-coating with Au was also necessary to reduce the possibilities of sample contamination. The sputter coater used was an Eiko IB3 Ion Coater, which uses argon gas and a small electric field. After sputter-coating, samples were loaded in the sample chamber of the instrument for morphological examination.
RESULTS AND DISCUSSION
Content and Elemental Analysis
Biomass materials are composed of varying proportions of bio-polymers and differing fractions of elements with significant properties that are relevant to the pellet-forming abilities of the biomass.[Citation17,Citation39,Citation40] Content analysis of industrial spruce pellet was undertaken to determine primary components and establish bonding characteristics as well as pellet-forming abilities of each components relevant to the production of good quality pellet. It is worthy to mention that the elemental constituents of industrial spruce pellet are off-shoots of its primary organic components. The role of the components in bonding was established in accordance with the properties of their polymeric constituents. The primary organic and elemental constituents of industrial spruce pellet are presented in .
TABLE 2. Primary organic and elemental constituents of industrial spruce pellet.
According to the data presented in , the primary organic components of industrial spruce pellet make up about 96.1% of its total composition. This means that other components such as extractives, proteins, ash, etc., account for only about 3.9%, which was considered insignificant hence they were not reported in this study. The same applies to elemental constituents, which shows carbon as the major chemical element and supports the fact that organic materials comprise mainly of a carbon backbone with functional groups attached to the chain, which help create micro and macroscopic bonds with other molecules.[Citation26] The functional group analysis data is presented in section ‘Determination and Location of Functional Groups from FT-IR Analysis’ and its role in the bonding of biomass pellets explicitly described. However, the goal of this compositional analysis once more was to establish the role of primary components in bonding and in pellet formation. The skeletal structures of the primary components of biomass, from previous studies,[Citation1,Citation18,Citation41] indicate a carbon backbone in these structures. Therefore, carbon will form a major part of results interpretation. What makes organic materials ubiquitous is mainly attributed to the chemistry of their carbon backbone.[Citation42] Carbon instigates the sharing of electrons through particle to particle interaction and linkages to create an electrostatic force of attraction that results into binding in the process and cause particles to stick together upon the application of compression force. As particles interact during pelleting and at points of contact, solid bridges are formed by molecular diffusion due to the application of high temperatures and compression force.[Citation6,Citation43] Furthermore, as pellet press temperature increases, melting of components occur and solidifies upon cooling, creating chemical modifications that confer important properties to the pellets.[Citation3] Thus, due to the high oxygen content of industrial spruce pellets (), components of the pellets on solidification are held together by hydrogen bonding as a result of oxygen-hydrogen (O–H) bonds. The FT-IR analysis data presented in a preceding section corroborates the presence of these bonds in terms of functional groups. A statement of fact in chemistry which supports this theory is that hydrogen bonding occurs in materials containing highly electronegative elements (such as oxygen) that are directly bound to hydrogen. The quality of biomass pellets is measured in terms of density (strength, durability and hardness), which is largely dependent upon the type of material pelletized and the nature of the bonds holding individual components of the material together as the material undergoes pelletization under optimum conditions such as those presented in a previous section.[Citation3,Citation4]
Determination and Location of Functional Groups from FT-IR Analysis
FT-IR is an invaluable tool used in the determination and verification of the structure of organic materials because the energy that corresponds to the specific value of the wavenumber allows identification of various functional groups contained in the structure of the material. The IR region is generally divided into three regions: near (13 300–4 000 cm−1), middle (4 000–200 cm−1), and far (200–3.3 cm−1) IR regions. However, the widely used region is the mid-IR region because organic materials have fundamental vibration bands around this region of the IR spectroscopy.[Citation37] presents the FT-IR spectra of industrial spruce pellet. Numbers are assigned to each peak, which represents absorption bands of specific functional groups.
Figure 1 FT-IR spectrum of industrial spruce pellet showing absorption bands that qualitatively identifies primary functional groups.
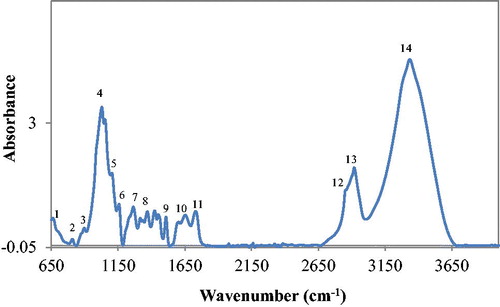
The chemical composition of biomass plays an active role in the pelleting process of the biomass and its bonding attributes.[Citation44] For clarity sake and to avoid spectra congestion in assigning functional groups at points of absorption, specific molecular bonds in the sample and their corresponding absorption bands at specific wavenumbers of the IR spectrum are presented in . Spectral interpretation from FT-IR analysis of certain biomass samples such as wood is such that one chemical component may absorb at several frequencies and, on the other hand, two or more components may contribute to the same absorption band.[Citation45]
TABLE 3. FT-IR absorption bands of industrial spruce pellet and assignment of functional groups.
ATR mode absorption bands in FT-IR analysis of biomass usually relates to the contents of cellulose, hemicellulose and lignin.[Citation46] A host of functional groups linked to the three primary components of industrial spruce pellet were identified according to the data in and . To avoid ambiguity, the role played by a few important functional groups in the bonding mechanism of primary components of industrial spruce pellet is discussed according to the presented data. A strong wide absorption band at 3 335 is assigned to hydroxyl functional groups (–OH) stretching vibrations caused by the presence of phenolic hydroxyl groups linked to certain carbohydrate and polymeric constituents of cellulose and hemicellulose as well as lignin. –OH groups create cleavages of β-O-aryl ether interunit bonds.[Citation47] This implies that intermolecular forces associated with covalent bonding, hydrogen bonding, and dipole-dipole interactions were triggered as particles combined under elevated temperatures and pressure during production of the pellet. This is because –OH group is polar hydrophilic in nature and typically charge-polarized with the ability to form a variety of intermolecular bonding when it interacts with other polar substances like water (H2O) in the form of moisture. –OH groups also form active binding surfaces that consist of residues capable of forming bonds. However, for the –OH group to be able to form active binding surfaces, it must be able to do so at specific material softening/transition temperature; all three primary components of industrial spruce pellet contain free –OH groups capable of hydrogen bonding with itself and with H2O; for cellulose, its polymer chains are densely packed with –OH groups (with three in each glucose unit) that are able to form both intra and intermolecular linkages to increase pellet stiffness,[Citation2] which may imply that the pellet used in this study as a reference sample is of good quality in terms of density and corroborates the bulk density data of the pellet presented in a previous section. The amount of hydrogen bonding between macromolecules and H2O is so large that a gel capable of increasing the gluing effect of components is formed.[Citation2] It is worthy to mention that the presence of –OH could also be related to moisture content of the sample because moisture absorbs energy by O–H stretching vibrations in the IR region between 3 000 and 3 700 cm−1.[Citation48] The C = O group, also linked to the primary components of industrial spruce pellet, is a carbon atom double-bonded to an oxygen atom and equally a polar group responsible for increasing material melting point. Cellulose and lignin have several absorption bands within the IR region, which can be used to image their distribution in sections of the biomass; the range of absorption of these primary constituents of biomass in the FT-IR is between 1 530 cm−1 for cellulose, and 1 490 cm−1 for lignin, respectively.[Citation49] The C–O–C and C–C groups are associated with lignin, a three-dimensional polymer with phenyl-propane precursor monomer units as its basic units, which are linked by C–O–C and C–C bonds; because lignin is covalently bonded to hemicellulose, it is able to crosslink a variety of polysaccharides and confers strength to the cell wall of plant biomass.[Citation50]
The binding forces acting between individual particles in densified spruce pellets are described in accordance with the theory of intermolecular bonding (covalent bonding) because the structures of its three primary components (cellulose, hemicellulose, and lignin) are linked by carbon–carbon (C–C), carbon–hydrogen (C–H), and carbon–oxygen (C–O) bonds, which forms part of the functional groups that confer specific properties and take part in bonding as a single unit.[Citation40]
Determination of Modification Temperature Relevant to Bonding from TGA Analysis
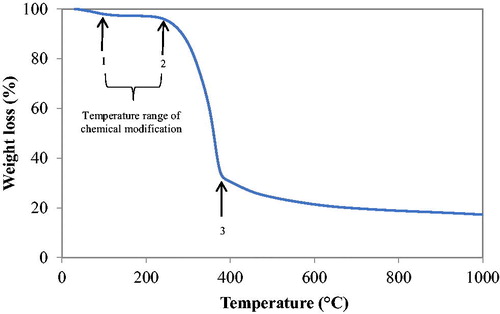
Knowledge of the thermal events that occur when biomass is heated at elevated or moderate temperatures create better ways to identify any chemical modifications that may lead to better understanding of how particles combine to form pellets during pelleting of biomass. As such, chemical modifications initiated by temperature and its relevance to bonding were of interest in this analysis. According to Back,[Citation7] sufficient bonding areas are created when wood components are plasticized above their glass transition temperatures. , therefore, shows typical thermogram of industrial spruce pellet tested under the experimental conditions described in section ‘TGA Analysis’.
The movement of gases as biomass undergoes combustion is often determined by biomass structure such as the development of cracks, surface regression and primary fragmentation.[Citation51] Additionally, the weight loss process of biomass as a result of combustion is typically controlled by its primary constituents (cellulose, hemicellulose and lignin), which as previously mentioned, degrades at different temperature ranges under thermal treatment.[Citation29–32,Citation52] From , the degradation process of industrial spruce pellet under thermal analysis is characterized by three weight loss stages: numbered 1, 2, and 3, respectively. These weight loss stages are indicative of the stages of chemical modifications that the raw material undergoes when temperatures soar during pelleting. The first stage of modification (stage 1) occurred typically at temperatures below 100 °C and indicates the evaporation of moisture. At the second stage (stage 2), a rapid weight loss is noticeable at ca. 245 °C, a condition attributed to the release of an increased amount of volatile gases emanating from polymeric constituents linked to cellulose and hemicellulose as well as partly to lignin. The third and final stage of the degradation process occurred at ca. 275 °C, a stage characterized by degradation of lignin components and which continues up to temperatures above 900 °C, leaving residual ash (stage 3). Lignin is a major contributor to the generation of residual char and ash; hence it degrades at a much wider temperature range, from 400 to 1 000 °C.[Citation29,Citation53] As previously indicated however, the interest in this analysis remains the temperature range in which chemical modifications relevant to bonding occur, which relates to the temperatures between the first stage of weight loss and the beginning of the second stage (between 85 °C and below 100 °C). Beyond 200 °C, rapid degradation occurs due to the release of volatiles, leaving the raw material too flaky for a particle to particle bonding.[Citation2,Citation39] The modification stage, also known as the transition stage, which literally began at temperatures around 85 °C to < 100 °C, ushered in cleavages of α– and β– bonds from cellulose with splitting of aliphatic and aromatic side chains to create cleaved carbon–carbon (C–C), carbon-oxygen (C–O) as well as carbon-hydrogen (C–H) linkages between structural units of primary components, particularly lignin. Transition temperatures of biomass under thermal analysis create an increase in the potential for bonding.[Citation54,Citation55] The cleavages and linkages between these polymer chains constitute active particle to particle bonding areas where a variety of primary intermolecular bonds such as covalent and hydrogen bonds are formed as a result of compression force and elevated temperatures. Particle to particle bonding surface areas were also predominated by secondary intermolecular bonds initiated by pelleting process conditions; the modification stage temperatures ensure chain mobility at the expense of intermolecular bonds, which are considerably reduced to an extent that chain ends and segments revolve around their axis; at this point, polymer viscosity drastically drops to show noticeable flow characteristics relevant to bonding.[Citation2,Citation7,Citation21] This facilitates the diffusion of polymer chains and chain ends to stimulate bonding surface areas, particularly when a compression force is applied and temperature increases a little above the modification temperature. All bonds formed are therefore consolidated on cooling.[Citation7]
Sample Visualization Using CRM
For bonding to occur during biomass pelleting, components must be randomly distributed across surface areas to create maximal surface molecular and particle to particle interactions, particularly as temperature soar.[Citation56] presents the confocal images of industrial spruce pellet showing distribution and orientation of primary components (cellulose, hemicellulose and lignin) depicted by the various color reflections in the images. The images were obtained at a magnification of ×100.
Figure 3 Confocal images of industrial spruce pellet showing: (a) internal structure from a section of the pellet; (b) surface structure from the same section as the previous; (c) internal structure from a different part of the pellet; (d) surface structure from same part as c. The color interpretation of the images is such that deep blue indicates the presence of cellulose, yellowish-green depicts hemicellulose, and reddish coloration implies the presence of lignin.
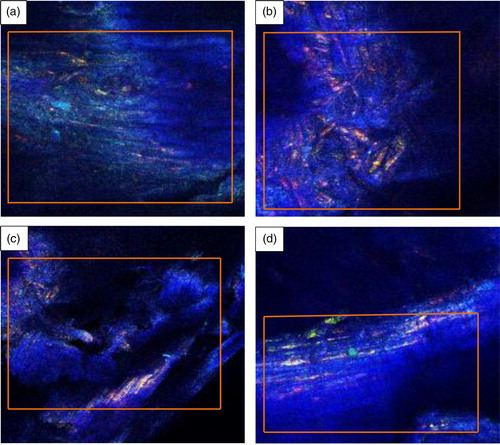
The images in were taken from different parts of the pellet and generally show that components are tightly connected to each other and distributed in a somewhat irregular manner. The images show fewer distinguishing features; however, traces of how primary components (cellulose, hemicellulose and lignin) are distributed can still be seen. The highlighted sections in the images are points of components distribution according to the defined imaging parts of the pellet. The components appear as deformed and shapeless particles reflected in bright colors that are randomly distributed. The color interpretation in the images are such that deep blue reflection depicts cellulose distribution, while hemicellulose and lignin can be seen as yellowish-green and reddish color particles that are superimposed in the deep blue color reflection of cellulose with glossy surfaces that are prone to intermolecular bonding due to the presence of polar functional groups (such as –OH and C=O) activated by hot pressing.[Citation2] Imaging, chemical and thermal techniques can be used to detect lignin through a brilliant red color development that indicates the presence of coniferaldehyde groups, which are components associated with lignin.[Citation57] The red color distribution could also imply the presence of phenolic components, which are equally polymeric constituents connected to lignin; these components have binding properties that can be activated by moisture and temperature.[Citation58] Furthermore, from the images (), primary components are distributed according to their percentage composition, which corroborates the percentage content data presented in . Cellulose chains are straight elongated rods that easily form organized fibers in hierarchical microfibrils structure, which are bundles of elementary fibers that stick together in highly ordered crystalline regions of woody biomass.[Citation2] The distribution of the primary components in the images is evidence of particle to particle interaction. For bonding to occur during pelleting of biomass, components must be randomly distributed in order to create maximal surface interaction at the application of heat.[Citation56] However, judging by the color components that are reflected in red, lignin is slightly less distributed in than it is in b, which supports the fact that lignin is heterogeneously distributed in cell walls of woody biomass, according to Henriksen et al.[Citation2] and c also show a multi-color reflection and superimposition of components from internal and surface imaging of industrial spruce pellet. Because sample examination under the CRM can be very subjective, the analysis did not offer any definitive conclusions on active binding sites and how particles are connected to each other through bonding. The noticeable dark parts in the images, mostly at the corners of the images, could not be accounted for and were assumed to result from emission/absorption of light from the instrument.
Morphological Diagnosis with SEM
For a more comprehensive visualization and understanding of sample composition and bonding characteristics as well as for studies of developmental changes caused by process conditions of the pellet press, a correlative microscopic technique that involves SEM was used. Internal and surface morphology of the pellet was undertaken and images obtained at different magnifications for better interpretation of results. Because sample morphology was highly magnified, and thus covers only a small area, it is vital that the area selected for visualization is typical of bonded surface areas. The SEM images of industrial spruce pellet are presented in . Included in each one of the images were specific examination conditions.
Figure 4 SEM images of industrial spruce pellet obtained at different magnifications: (a) ×100; (b) ×200; (c) ×500; (d) ×1000. The images reflect internal and surface morphology taken from different parts of the pellet.
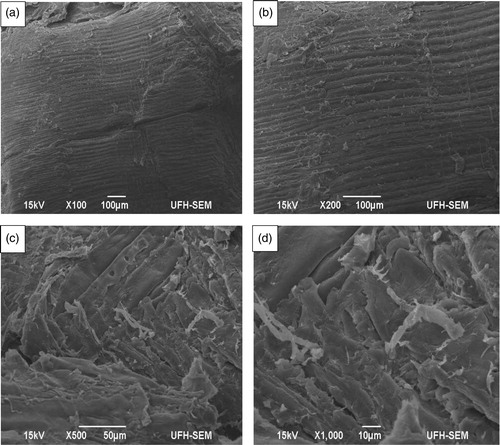
Generally, from the SEM images in , the first observable feature is the way particles are densely packed, a proof of high density and durability that defines good quality pellets. The images also show how severely distorted and deformed particles are, which is a direct consequence of the application of compression force. The compact nature of the pellet seen under the SEM also show surface structures that are entangled and linked to each other, which is equally a direct evidence of how bio-polymers migrate to create active surfaces relevant to bonding at the application of temperature and compression force. However, parallel lines could be noticed in , which becomes quite conspicuous at higher magnifications of ×200 (. This is an indication of the formation of solid bridges created by molecular diffusion as a result of particle interaction and attraction to one another during pelleting. This feature is usually made prominent at the application of heat and compression force during pelleting.[Citation59–61] One of the notable features of biomass pelleting is the formation of solid bridges stimulated by rising temperatures and compression force of the pellet press, a condition also facilitated by chemical reactions, crystallization of dissolved substances and solidification of melted components.[Citation59–61] The solid bridges are also an indication of the presence of strong intermolecular bonding linked to covalent bonding, dipole-dipole interaction and hydrogen bonding resulting from the highly electronegative O–H bonds in the cellulose and hemicellulose structure; these two components of biomass have more –OH groups in their molecular structure than their lignin counterparts.[Citation2] According to the adhesion theory, chemical bonding is established when maximum binding force reaches minimum potential energy. Compression force, temperature and moisture content facilitate adhesion with solid bridges formed between combining particles as biomass undergoes pelleting.[Citation62] The internal microstructure of the pellet, revealed at higher magnification (×500) shows how tightly connected particles are to each other (), which is an indication of adhesion and intertwining fiber tracheid caused by crystallization and solidification of softened components that were a direct consequence of pelleting process compaction and/or compression force. Higher magnification () shows how smaller particles bind to larger ones by entanglement, exposing adhesive surfaces that are relevant to bonding. Again, these were as a result of compression force from the pellet press, and the presence of highly electronegative polar functional groups such as the –OH groups. Particle entanglement and exposure of adhesive surfaces stimulate particle to particle bonding during pelleting of biomass.[Citation63,Citation64]
The stunning extent of organized and compact structure seen in the SEM images is a reflection of the carbon backbone that characterizes organic materials and accounts for the high density that constitutes one of the features of pelletized biomass; the degree of organized structure preserved in compacted biomass in a pelleting process account for the total density of the biomass pellet.[Citation2] In addition, the microscopic bonding of particles revealed by the images was facilitated by the presence of functional groups that are attached to the carbon chain.
Active binding sites were created by the presence of polar functional groups ( and ) and these groups are affected by pellet press temperature and compression force, which stimulated variations in surface properties and morphology of the pellet sample. According to Liu et al.,[Citation65] active binding surfaces are created by the presence of polar functional groups, pelleting temperature and compression force, which in turn create variations in surface properties and morphology of biomass. This was further supported by the studies undertaken by Johansson et al. and Bryne et al.[Citation66,Citation67] Nonetheless, the morphological features of the industrial Norway spruce pellet used in this study can be compared with literature data on its SEM image before pelleting, which shows heterogeneity and variability of particles as well as higher amounts of smaller particles and fragments.[Citation68–71]
CONCLUSIONS
This study investigated the bonding mechanism of primary biomass components of industrial spruce pellet using advanced analytical instruments whose data provided information at a molecular and microscopic level based on the theory of functional groups and the understanding of structural chemistry. Much of the experimental procedures undertaken in the study focused on factors that could not be observed by examination with the naked eye, an indication that factors contributing to bonding in biomass pellets span several microscopic, molecular and even nanoscopic levels. Data obtained were used to predict how primary components combined to form pellet as a way to contribute to the development of the fundamental understanding of how biomass is transformed from powder to pellet during pelleting. However, the study conclusively established that certain analytical instruments are more favorable than others in predicting how bonding occurs in biomass pellets. Thus, the following conclusions were drawn from the study:
At molecular level, satisfactory information were obtained on the type of binding forces acting between particles of industrial spruce pellet according to the FT-IR data, which revealed most important functional groups that were used to predict forces holding particles together. These forces included both intra and intermolecular attraction forces.
The TGA gave a clear indication of chemical modification range temperatures where primary structural polymeric constituents soften to create flow characteristics that were considered relevant to particle bonding. During biomass pelleting, chemical modifications of bio-polymers, which can be of significance to the features of biomass pellets, are often initiated at elevated temperatures.[Citation3,Citation6]
The CRM allowed visualization of industrial spruce pellet over a large area, but proved quite challenging in creating internal and surface images clear enough for conclusive information to be drawn on particle bonding mechanism and active bonding sites.
The SEM instrument used in this study provided a clearer morphological view of internal and surface structures of industrial spruce pellet and how particles are connected to each other than the CRM, which did not offer much information on binding and bonding. In addition, the parallel lines that were evidence of the formation of solid bridges were also distinctly seen under the SEM, which were impossible to visualize with the CRM.
Even though the SEM instrument provided a clearer view of how particles were bound to each other through noticeable entanglements, there were no direct visual evidences of how powder was transformed to pellet at nanoscopic or even microscopic levels.
In view of the above conclusions therefore, further studies are required on the use of complimentary advanced imaging techniques at both microscopic and nanoscopic levels for comprehensive visualization and elucidation of how biomass is transformed from powder to pellet. The study must incorporate the use of a variety of industrial pellets made from different biomass materials and those made from a single pellet press machine (as reference samples for examination) for the purpose of comparison in order to conclusively establish how components combine to form pellets under optimum conditions of pelleting such as those presented in .
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical support of the Department of Engineering and Chemical Sciences of Karlstad University; and the administrative assistance of Birgitta Naumburg, who is the Secretary (Forest Section) of the Royal Swedish Academy of Agriculture and Forestry (KSLA).
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
FUNDING
This work was supported by the Department of Engineering and Chemical Sciences of Karlstad University under the FOSBE project; and the Royal Swedish Academy of Agriculture and Forestry (KSLA) under grant number UFK 62833.
References
- Anukam, A. Gasification characteristics of sugarcane bagasse. Masters dissertation, University of Fort Hare, Alice, South Africa, 2013.
- Henriksen, U. B.; Holm, J. K.; Simonsen, P.; Berg, M.; Posselt, D.; Nikolaisen, L.; Plackett, D.; Møller, J. D. Fundamental understanding of pelletization. Technical report EFP-2005 project (33031-037), 2008.
- Frodeson, S.; Henriksson, G.; Berghel, J. Pelletizing pure biomass substances to investigate the mechanical properties and bonding mechanisms. BioResources 2018, 13, 1202–1222. DOI: 10.15376/biores.13.1.1202-1222.
- Ståhl, M. Improving wood fuel pellets for household use: perspectives on quality, efficiency and environment. PhD thesis, Karlstad University, Karlstad, Sweden, 2008.
- Mobarak, F.; Fahmy, Y.; Augustin, H. Binderless lignocellulose composite from bagasse and mechanism of self-bonding. Holzforschung 1982, 36, 131–135. DOI: 10.1515/hfsg.1982.36.3.131.
- Kaliyan, N. Binding mechanism of corn stover and switchgrass in briquettes and pellets. Presented at the ASABE 2008 Annual International Meeting, Providence, RI, June 29–July 2, 2008; Paper 084282.
- Back, E. L. The bonding mechanism in hardboard manufacture. Holzforschung 1987, 41, 247–258. DOI: 10.1515/hfsg.1987.41.4.247.
- Mani, S.; Tabil, L. G.; Sokhansanj, S. An overview of compaction of biomass grinds. Powder Handling and Processing 2003, 15, 160–168.
- Philipson, W. R.; Ward, J. M.; Butterfield, B. G. The Vascular Cambium: Its Development and Activity; Chapman & Hall: London, UK, 1971.
- Barnett, J. R. Secondary xylem cell development. In Xylem Cell Development; Barnett, J. R., Ed.; Castle House Publications: Royal Tunbridge Wells, UK, 1981, 47–95.
- Iqbal, M.; Ghouse, A. K. M. Cambial concept and organization. In The Vascular Cambium; Iqbal, M., Ed.; Research Studies Press: Taunton, Somerset, 1990, 1–36.
- Catesson, A. M. Cambial ultrastructure and biochemistry: changes in relation to vascular tissue differentiation and the seasonal cycle. International Journal of Plant Sciences 1994, 155, 251–261. DOI: 10.1086/297165.
- Larson, P. R. The Vascular Cambium: Development and Structure; Springer-Verlag: Berlin, Germany, 1994.
- Mani, S.; Tabil, L. G.; Sokhansanj, S. Effects of compressive force, particle size and moisture content on mechanical properties of biomass pellets from grasses. Biomass and Bioenergy 2006, 30, 648–654. DOI: 10.1016/j.biombioe.2005.01.004.
- Ramírez-Gómez, Á. Research Needs on Biomass Characterization to Prevent Handling Problems and Hazards in Industry. Particulate Science and Technology 2016, 34, 432–441. DOI: 10.1080/02726351.2016.1138262.
- Stelte, W.; Sanadi, A. R.; Shang, L.; Holm, J. K.; Ahrenfeldt, J.; Henriksen, U. B. Recent developments in biomass pelletization – a review. BioResources 2012, 7, 4451–4490.
- Anukam, A. I.; Mamphweli, S. N.; Reddy, P.; Okoh, O. O. Characterization and the effect of lignocellulosic biomass value addition on gasification efficiency. Energy Exploration and Exploitation 2016, 34, 865–880. DOI: 10.1177/0144598716665010.
- Felhofer, M. Raman imaging to reveal in-situ molecular changes of wood during heartwood formation and drying. Masters dissertation, University of Natural Resources and Life Sciences, Vienna, Austria, 2016.
- Neil, E. Organic Chemistry Structure and Function, 6th ed.; W. H. Freeman: New York, NY, 2011.
- Whittaker, C.; Shield, I. Factors affecting wood, energy grass and straw pellet durability – a review. Renewable and Sustainable Energy Reviews 2017, 71, 1–11. DOI: 10.1016/j.rser.2016.12.119.
- Irvine, G. M. The glass transitions of lignin and hemicellulose and their measurement by differential thermal analysis. TAPPI Journal 1984, 67, 118–121.
- Cottrell, T. L. The Strengths of Chemical Bonds, 2nd ed.; Butterworth: London, UK, 1958.
- Berglund, J.; Angles d'Ortoli, T.; Vilaplana, F.; Widmalm, G.; Bergenstråhle-Wohlert, M.; Lawoko, M.; Henriksson, G.; Lindström, M.; Wohlert, J. A molecular dynamics study of the effect glycosidic linkage type in the hemicellulose backbone on the molecular chain flexibility. The Plant Journal 2016, 88, 56–70. DOI: 10.1111/tpj.13259.
- Frodeson, S.; Henriksson, G.; Berghel, J. Effects of moisture content during densification of biomass pellets, focusing on polysaccharide substances. Biomass and Bioenergy 2019, 122, 322–330. DOI: 10.1016/j.biombioe.2019.01.048.
- Kilpeläinen, A.; Peltola, H.; Ryyppö, A.; Sauvala, K.; Laitinen, K.; Kellomäki, S. Wood properties of scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiology 2003, 23, 889–897. DOI: 10.1093/treephys/23.13.889.
- Lumen boundless chemistry. 2019. Organic chemistry: functional group names, properties and reactions. https://courses.lumenlearning.com/boundless-chemistry/chapter/functional-group-names-properties-and-reactions/ (accessed March 19, 2019).
- Anukam, A.; Okoh, O.; Mamphweli, S.; Berghel, J. A comparative analysis of the gasification performances of torrefied and untorrefied bagasse: influence of feed size, gasifier design and operating variables on gasification efficiency. International Journal of Engineering and Technology 2018, 7, 859–867. DOI: 10.14419/ijet.v7i2.8489.
- Anukam, A.; Mamphweli, S.; Meyer, E.; Okoh, O. Computer simulation of the mass and energy balance during gasification of sugarcane bagasse. Journal of Energy 2014, 2014, 1–9. DOI: 10.1155/2014/713054.
- Anukam, A.; Mamphweli, S.; Okoh, O.; Reddy, P. Influence of torrefaction on the conversion efficiency of the gasification process of sugarcane bagasse. Bioengineering 2017, 4, 22–23. DOI: 10.3390/bioengineering4010022.
- Chen, W. H.; Kuo, P. C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. DOI: 10.1016/j.energy.2010.02.054.
- Chen, W. H.; Kuo, P. C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. DOI: 10.1016/j.energy.2010.12.036.
- Yang, H.; Yan, R.; Chen, H.; Lee, D. H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. DOI: 10.1016/j.fuel.2006.12.013.
- Günzler, G.; Gremlich, H.-U. IR Spectroscopy; Wiley-VCH Verlag: Weinheim, Germany, 2002.
- Perkin Elmer Life and Analytical Sciences. FT-IR spectroscopy: attenuated total reflectance (ATR). Technical note, 2005. https://shop.perkinelmer.com/content/TechnicalInfo/TCH_FTIRATR.pdf (accessed April 13, 2019).
- LeVan, S. L. Thermal degradation. In Concise Encyclopedia of Wood & Wood-Based Materials, 1st ed.; Schniewind, A. P., Ed.; Pergamon Press: Elmsford, NY, 1989; 271–273.
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass and Bioenergy 2011, 35, 298–307. DOI: 10.1016/j.biombioe.2010.08.067.
- Ghaffar, S. H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass and Bioenergy 2013, 57, 264–279. DOI: 10.1016/j.biombioe.2013.07.015.
- Moran-Mirabal, J. M. Advanced-microscopy techniques for the characterization of cellulose structure and cellulose-cellulase interactions. In Cellulose: Fundamental Aspects; Van De Ven, T. G. M., Ed.; InTech: London, UK, 2013.
- Anukam, A.; Mamphweli, S.; Reddy, P.; Okoh, O.; Meyer, E. An investigation into the impact of reaction temperature on various parameters during torrefaction of sugarcane bagasse relevant to gasification. Journal of Chemistry 2015, 2015, 1–12. DOI: 10.1155/2015/235163.
- Barmina, I.; Lickrastina, A.; Zake, M.; Arshanitsa, A.; Solodovnik, V.; Telysheva, G. Experimental study of thermal decomposition and combustion of lignocellulosic biomass pellets. Latvian Journal of Physics and Technical Sciences 2013, 50, 35–48. DOI: 10.2478/lpts-2013-0018.
- Sugar Milling Research Institute. Sugarcane bagasse. Proceedings of South African Sugar Technologists Association 2008, 81, 266–273.
- BC campus Open Education. 2019. Anatomy and physiology: the chemical level of organization. https://opentextbc.ca/anatomyandphysiology/chapter/2-5-organic-compounds-essential-to-human-functioning/ (accessed March 14, 2019).
- Rumpf, H. The strength of granules and agglomerates. In Agglomeration, Knepper, W. A., Ed.; John Wiley and Sons: New York, NY: 1962, 379–418.
- Tumuluru, J. S. Effect of pellet die diameter on density and durability of pellets made from high moisture woody and herbaceous biomass. Carbon Resources Conversion 2018, 1, 44–54. DOI: 10.1016/j.crcon.2018.06.002.
- Kotilainen, R. A.; Toivanen, T.-J.; Alén, R. J. FTIR monitoring of chemical changes in softwood during heating. Journal of Wood Chemistry and Technology 2000, 20, 307–320. DOI: 10.1080/02773810009349638.
- Keskar, S. S.; Edye, L. A.; Christopher, M.; Fellows, C. M.; Doherty, W. O. S. ATR-FTIR measurement of biomass components in phosphonium ionic liquids. Journal of Wood Chemistry and Technology 2012, 32, 175–186. DOI: 10.1080/02773813.2011.631718.
- Nuopponen, M.; Vuorinen, T.; Jämsä, S.; Viitaniemi, P. Thermal modifications in softwood studied by FT‐IR and UV resonance Raman spectroscopies. Journal of Wood Chemistry and Technology 2005, 24, 13–26. DOI: 10.1081/WCT-120035941.
- Brys, A.; Brys, J.; Ostrowska-Ligeza, E.; Kaleta, A.; Gornicki, K.; Głowacki, S.; Koczon, P. Wood biomass characterization by DSC or FT-IR spectroscopy. Journal of Thermal Analysis and Calorimetry 2016, 126, 27–35. DOI: 10.1007/s10973-016-5713-2.
- Faix, O. Classifcation of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. DOI: 10.1515/hfsg.1991.45.s1.21.
- Su, Y.; Luo, Y.; Wu, W.; Zhang, Y.; Zhao, S. Characteristics of pine wood oxidative pyrolysis: degradation behaviour, carbon oxide production and head properties. Journal of Analytical and Applied Pyrolysis 2012, 98, 137–143. DOI: 10.1016/j.jaap.2012.07.005.
- Biswas, A. Effect of chemical and physical properties on combustion of biomass particle. PhD thesis, Luleå University of Technology, Luleå, Sweden, 2015.
- Prins, M. J.; Ptasinski, K. J.; Janssen, F. J. J. G. Torrefaction of wood: part 1. Weight loss kinetics. Journal of Analytical and Applied Pyrolysis 2006, 77, 28–34. DOI: 10.1016/j.jaap.2006.01.002.
- Shen, D. K.; Gu, S.; Luo, K. H.; Bridgwater, A. V.; Fang, M. X. Kinetic study on thermal decomposition of woods in oxidative environment. Fuel 2009, 88, 1024–1030. DOI: 10.1016/j.fuel.2008.10.034.
- Schniewind, A. P. Concise Encyclopedia of Wood and Wood-Based Materials, 1st ed.; Pergamon Press: Elmsford, NY, 1989, 271–273.
- Brackley, A. M.; Parrent, D. J. Production of wood pellets from Alaska-grown white spruce and hemlock. United States Department of Agriculture, Forest Service General Technical Report PNW-GTR-845, Pacific Northwest Research Station, 2011.
- Mittal, K. L. The role of the interface in adhesion phenomena. Polymer Engineering and Science 1977, 17, 467–473. DOI: 10.1002/pen.760170709.
- Harkin, J. M. Lignin production and detection in wood. U.S. Forest Service Research Note FPL-0148, 1966.
- Kaliyan, N.; Morey, R. V. Factors affecting strength and durability of densified products. Presented at the 2006 ASABE Annual International Meeting, Portland, Oregon, July 9–12, 2006; Paper 066077.
- Tabil, L. G.; Sokhansanj, S.; Tyler, R. T. Performance of different binders during alfalfa pelleting. Canadian Agricultural Engineering 1997, 39, 17–23.
- Tabil, L. G.; Sokhansanj, S. Process conditions affecting the physical quality of alfalfa. American Society of Agricultural Engineers 1996, 12, 345–350.
- Sokhansanj, S.; Tabil, L.; Wang, W. Characteristics of plant tissue to form pellet. Powder Handling and Processing: The International Journal of Strong, Handling and Processing Powder 1999, 11, 149–159.
- Harun, N. Y.; Parvez, A. M.; Afzal, M. T. Process and energy analysis of pelleting agricultural and woody biomass blends. Sustainability 2018, 10, 1–9. DOI: 10.3390/su10061770.
- Gardner, D. J. Adhesion mechanism of durable wood adhesive bonds. In Characterization of the Cellulose Cell Wall; John Wiley & Sons, Inc.: Hoboken, NJ, 2006, 254–265.
- Mani, S.; Sokhansanj, S.; Bi, X.; Turhollow, A. Economics of producing fuel pellets from biomass. Applied Engineering in Agriculture 2006, 22, 421–426.
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Applied Energy 2014, 113, 1315–1322. DOI: 10.1016/j.apenergy.2013.08.087.
- Johansson, L. S.; Campbell, J. M.; Hänninen, T.; Ganne-Chédeville, C.; Vuorinen, T.; Hughes, M.; Laine, J. XPS and the medium-dependent surface adaption of cellulose in wood. Surface and Interface Analysis 2012, 44, 899–903. DOI: 10.1002/sia.4839.
- Bryne, L. E.; Lausmaa, J.; Ernstsson, M.; Englund, F.; Wålinder, M. E. P. Ageing of modified wood. Part 2: determination of surface composition of acetylated, furfurylated, and thermally modified wood by XPS and ToF-SIMS. Holzforschung 2010, 64, 305–313. DOI: 10.1515/hf.2010.062.
- Wang, Z.; Winestrand, S.; Gillgren, T.; Jönsson, L. J. Chemical and structural factors influencing enzymatic saccharification of wood from aspen, birch and spruce. Biomass and Bioenergy 2018, 109, 125–134. DOI: 10.1016/j.biombioe.2017.12.020.
- Rodriguez, Y. P.; Puhakka-Tarvainen, H.; Pastinen, O.; Siika-Aho, M.; Alvila, L.; Turunen, O.; Morales, L.; Pappinen, A. Susceptibility of pretreated wood sections of Norway spruce (Picea abies) clones to enzymatic hydrolysis. Canadian Journal of Forest Research 2012, 42, 38–46. DOI: 10.1139/x11-154.
- Azhar, S. Extraction of polymeric hemicelluloses from spruce wood. PhD thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2015.
- Källbom, S. Surface characterisation of thermally modified spruce wood and influence of water vapour sorption. Licentiate thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2015.