Source identification is critical for the effective management of air pollution. The ratio of levoglucosan (1,6-anhydro- β -D-glucose) to organic carbon has been used to identify and quantify the contribution of biomass combustion to the organic carbon content in aerosols. Therefore, accurate levoglucosan measurements in particulate matter are important. This study determined levoglucosan concentrations in urban dust Standard Reference Materials (SRMs) 1649a and 1648 available from the National Institute of Standards and Technology (NIST) as well as two particulate matter samples with particle sizes nominally 2.5 μ m and smaller collected in Baltimore, MD. Levoglucosan was extracted using both pressurized fluid and Soxhlet techniques. Quantification was performed with gas chromatography/mass spectrometry (GC/MS) using a deuterated levoglucosan internal standard and two different GC columns. Levoglucosan concentrations were 81.1 μ g g−1 with a standard deviation of 9.4 μ g g−1 (n = 9) for SRM 1649a, 107 μ g g−1 with a standard deviation of 18 μ g g −1 (n = 8) for SRM 1648, 225 μ g g−1 (standard deviation of 41 μ g g−1 n = 4) and 138 μ g g−1 (standard deviation of 39 μ g g − 1 n = 5) for the two fine particulate matter samples collected in Baltimore, MD.
INTRODUCTION
The importance of aerosols on our society has been recognized for decades due to the direct effects associated with respiratory diseases along with the indirect effects associated with visibility and the earth's albedo. The origins of these aerosols encompass a broad range of natural and anthropogenic causes. One of the most predominant and globally occurring sources of aerosols to the atmosphere is biomass combustion CitationSimoneit (2002). Reports on the fraction of organic carbon in ambient atmospheric aerosols due to biomass combustion range from 4% to 35% (CitationGraham et al. 2004; CitationZdrahal et al. 2002). Researchers use individual chemical markers or chemical ratios to attribute the ambient aerosol concentrations to specific sources such as biomass combustion, vehicle emissions, and industrial sources. Ideally, these markers should be unique to the source, stable in the atmosphere, have fixed emission ratios, and be present in measurable quantities. Previous investigations reveal several potential candidates for the general source of biomass combustion as well as candidates specific to a particular biomass fuel. Levoglucosan (1,6 anhydro-β -D-glucose) shows great promise as a biomass marker (CitationSimoneit et al. 1999). It is formed by the combustion of cellulose at temperatures over 300°C (CitationSimoneit 2002). Levoglucosan is emitted in relatively high concentrations 40 mg kg− 1 to 1200 mg/kg− 1 of wood burned and shows no decay over an eight-hour exposure to ambient atmospheric conditions CitationLocker (1988). Potential acid-catalyzed hydrolysis of levoglucosan in atmospheric droplets was investigated as one potential loss mechanism. Results using simulated rainwater indicate no degradation over a 10-day period (CitationFraser and Lakshmanan 2000). The thermal alteration of foods containing carbohydrates and starches can also generate levoglucosan (CitationLakshmanan and Hoelscher 1970). However, typical cooking techniques do not reach the temperatures needed to generate levoglucosan. Several studies characterizing the chemicals in wood smoke or in the ambient atmosphere report detectable levoglucosan concentrations. Levoglucosan has been measured in the ambient atmosphere in Asia, Europe, the Middle East, and North and South America. Recently levoglucosan has been found in aerosols collected from: New Hampshire (CitationLocker 1988), California (CitationFine et al. 2004; CitationNolte et al. 2001), Texas (CitationFraser and Lakshmanan 2000), Belgium (CitationPashynska et al. 2002; CitationZdrahal et al. 2002), Brazil (CitationDos Santos et al. 2004; CitationGraham et al. 2003; CitationZdrahal et al. 2002) Malaysia (CitationAbas et al. 2004), and Tel Aviv, Israel (CitationGraham et al. 2004). In summary, levoglucosan is unique to the combustion of cellulose, relatively stable under atmospheric conditions, and is emitted in sufficient quantities that it poses an ideal marker candidate for general biomass combustion.
As levoglucosan concentrations are more routinely quantified in aerosols, it becomes increasingly important to be able to validate the analytical methods using reference materials such as the National Institute of Standards and Technology's (NIST) urban dust Standard Reference Materials (SRMs). This need is of even greater importance for levoglucosan and other chemical markers whose concentrations translate into source identification and the source's partial responsibility for ambient aerosol loads. Currently, NIST does not have values assigned for levoglucosan in any of their SRMs. NIST has traditionally used multiple independent methods of analysis to certify the concentrations of selected analytes in SRMs. The goal of this study was to develop at least two methods of analysis for levoglucosan in particulate matter samples. This paper describes the method development and quantification of levoglucosan in NIST SRM 1649a, SRM 1648, and two fine particulate matter materials (with particle size nominally less than 2.5 μ m) (PM2.5) that were collected at different times in Baltimore, MD. These data will be used in conjunction with data from interlaboratory studies to value assign concentrations of levoglucosan in these samples.
Experimental Section
Chemicals
Levoglucosan (1,6-anhydro-β -D-gluocose) was obtained from Sigma Aldrich Fine Chemicals (St. Louis, MO) with a stated purity of 99%. Levoglucosan-d7 and levoglucosan-13C6 were obtained from Cambridge Isotope Laboratory (Andover, MA). N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA), N-methyl-N-trimethylsilyl-trifuoroacetamide (MSTFA), and BSTFA catalyzed with 1% trimethylchlorosilane (TMCS) were obtained from Pierce Biotechnology (Rockford, IL). Anhydrous pyridine (spectro grade) was obtained from Eastman Kodak (Rochester, NY). Methylene chloride (HPLC grade), hexane (HPLC grade), ethyl acetate (A.C.S. grade), acetonitrile (HPLC grade), acetone (HPLC grade) and methanol (HPLC grade) were obtained from J. T. Baker (Phillipsburg, NJ). Hydromatrix (diatomaceous earth) was obtained from Isco, Inc. (Lincoln, NE).
Particulate Matter Samples
During the mid to late 1970s, NIST collected urban particulate matter SRM 1649a from the Washington, DC area and SRM 1648 from the St. Louis, MO area. SRM 1649a consists of particles with diameters ≤ 123 μ m, and SRM 1648 consists of particles with diameters ≤53 μ m. With the interest in smaller sized aerosols, NIST has collected particulate matter ≤2.5 μ m in Baltimore, MD during two time periods, the first between 1998 and 2001 (PM 2.5-I) and the second at the same location in the fall of 2002 (PM 2.5-II).
Sample Preparation
Labware
Laboratory glassware was cleaned in soapy water, rinsed with deionized water and heated to 450°C for 8 h to remove organic contaminants. Metal labware was cleaned with soapy water, rinsed with deionized water, and sequentially sonicated in methanol, methylene chloride, and hexane. Hydromatrix is used to fill the void space in the pressurized fluid extraction vessels. It is cleaned prior to use by sequential sonication in methanol, hexane, and methylene chloride.
Extraction
This study used two extraction techniques each with different solvents to provide an internal confirmation of results. The first method employed pressurized fluid extraction (PFE) using a Dionex (Salt Lake City, UT) Accelerated Solvent Extractor (ASE) with ethyl acetate as the solvent. Samples varying in size from 20 mg to 100 mg were mixed with Hydromatrix in 11 mL ASE vessels along with 1 mL of the deuterated levoglucosan standard (4,100 ng levoglucosan-d 7) as an internal standard. The ASE parameters were 0 min preheat, 5 min heat, 5 min static, 90% volume flush, 90 s purge, 3 cycles, 2000 psi maximum pressure, and 100°C temperature. With these settings, ∼ 90% to 95% of the levoglucosan was removed after the first three cycle extraction. Each subsequent three cycle extraction removed ∼ 90–95% of the remaining levoglucosan. Many of the samples received multiple sequential three cycle extractions. The sequential extracts were combined and reduced to ∼ 0.5 mL under nitrogen using an automated evaporation system. Samples were subsequently transferred to 2 mL amber sample vials for further subsampling and derivatization.
Soxhlet extraction was also used in this study. Particulate matter samples ranging between 9 mg and 100 mg were combined with ∼ 4 g of Hydromatrix and 1 mL of deuterated levoglucosan standard (4,100 ng levoglucosan-d 7) in glass Soxhlet thimbles. Two cycle volumes of 80:20 methylene chloride:acetone were added to the thimble containing Soxhlet apparatus. The condensers were cooled by a water supply maintained at 12°C. Following a 24 h extraction, the samples were reduced to ∼ 0.5 mL using an automated evaporation system, solvent transferred to ethyl acetate and filtered using a 0.45 μ m glass fiber syringeless filter (Xydex, Bedford, MA) and placed in 2 mL amber sample vials.
Derivatization
Aliquots of 100 μ L were combined with 45 μ L of pyridine and 10 μ L of the derivatization agent in coned gas chromatography (GC) autosampler vials. The vials were purged with nitrogen gas and sealed with a Teflon-lined crimp top. Initial investigations were made comparing the effectiveness of BSTFA, MSTFA, and BSTFA with 1% TMCS as well as derivatization time (1 h, 2 h, and 3 h) and temperature (50°C, 60°C, and 70°C). Comparable results were obtained using each of the derivatizing agents, times and temperatures. Therefore, all samples were processed using BSTFA with 1%TMCS for 1 h at 60°C.
Analysis
For analysis, 1 μ L of the derivatized sample was automatically injected into an Agilent 6890/5973 gas chromatograph/ quadrapole mass spectrometer (GC/MS) operated in select ion monitoring mode. Either a moderately polar 50% phenyl phase (DB-17MS) or a relatively non-polar 5% phenyl phase (DB-5MS) column was used to separate the levoglucosan from other compounds in the sample. Both capillary columns were 30 m × 250 μ m id with a 0.25 μ m film thickness (J&W Scientific, Folsom, CA). Helium was used as the carrier gas at a constant flow 1.0 mL min− 1. The GC/MS parameters using either column were as follows. The inlet was in the splitless mode with an injection temperature of 260°C, 84 kPa pressure, and 1.5 min purge time. The GC oven followed a heating program consisting of: initial isothermal heating at 100°C for 10 min, then ramped 5°C min−1 to 188°C, then isothermal for 1 min, finally 30°C min−1 to 325°C. The MS was operated in an electron impact mode with 70 eV ion source energy. Levoglucosan, levoglucosan-d 7, and levoglucosan-13C6 were identified by GC retention time, comparison with authentic standards and full mass spectra scan (50–500 m/z). Quantification of unlabeled and deuterated levoglucosan was based on the 217 and 220 m/z fragments, respectively. Confirmation ions were 73 and 204 for levoglucosan and 73 and 206 for the levoglucosan-d7. Chemstation software version B.02.05 was used to identify and integrate peaks.
For quantification, six gravimetrically prepared solutions of levoglucosan in ethyl acetate (initial mass of levoglucosan ranged from 3 μ g to 50 μ g) were used to construct a linear response curve (r2 = 0.999).
Blanks were run along side samples with each extraction procedure. Blanks consisted of Hydromatrix spiked with the deuterated internal standard. The mean mass of levoglucosan in the blanks was 130 ng (with a standard deviation of 38 ng). The mass of levoglucosan found in actual samples ranged from 940 ng in a 12 mg Baltimore PM2.5 II sample to 29,810 ng levoglucosan in a 112 mg Baltimore PM2.5 I sample. The mass of levoglucosan found in 31 of 32 samples analyzed exceeded 10 times the mean blank. Therefore, the concentrations reported in this study are not blank corrected. The one sample analyzed, a 12 mg Baltimore PM2.5 II sample had a levoglucosan mass less than 10 times the mean blank. If contamination was a significant source of levoglucosan to that sample, then it would be expected to have a higher than average levoglucosan concentration. The 12 mg Baltimore PM2.5 II sample in question actually had a levoglucosan concentration less than the average indicating that blank contamination did not affect the outcome.
RESULTS AND DISCUSSION
Choice of Internal Standard
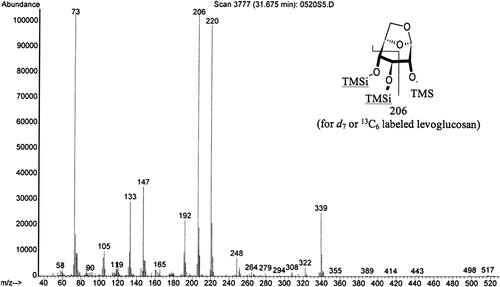
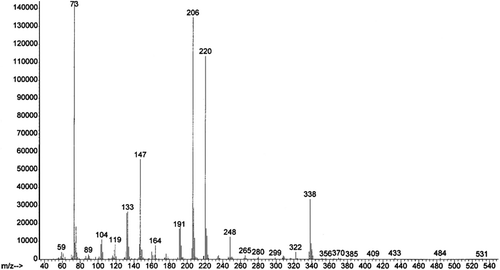
Initially, the GC/MS separation of derivatized levoglucosan from the derivatized levoglucosan-13C6 and derivatized levoglucosan-d 7 was examined to determine which labeled levoglucosan to use for quantification. The mass spectra of the derivatized levoglucosan exhibits key ion fragments 73, 204 and 217. The derivatized levoglucosan-d 7 and the levoglucosan-13C6 have nearly identical mass spectra with key ion fragments 73, 206 and 220 m/z ( and ). It appears that the 204/206 fragment consists of C2H2(OSi(CH3)3)2, while the 217/220 fragment is C3H3(OSi(CH3)3)2. There is also a relatively minor ion fragment at m/z 333 for the unlabeled levoglucosan and m/z 338 for both the deuterated and carbon-13 labeled levoglucosan. The 217/220 pair was selected for quantification over the 204/206 pair due to the presence of 206 in the unlabeled levoglucosan which was not baseline resolved from the 206 in the internal standard. The chromatographic separation between levoglucosan-d 7 and unlabeled levoglucosan was better than the chromatographic separation between levoglucosan-13C6 and unlabeled levoglucosan on both the non-polar and moderately polar GC columns. Therefore levoglucosan-d 7 was used for quantification of the reference material samples to maximize baseline resolution between the analyte and the internal standard.
SRM 1649a and 1648
SRM 1649a Urban Dust was collected in Washington, DC during the mid to late 1970s, and SRM 1648 was collected in St. Louis, MO during the same period. The collected PM was sieved and the fraction passing through a 123 μ m sieve for SRM 1649a or a 53 μ m sieve for SRM 1648 was bottled. SRM 1649a and SRM 1648 have been stored at room temperature since collection except for a small sample of bulk sieved SRM 1649a that has been stored frozen since the original preparation.
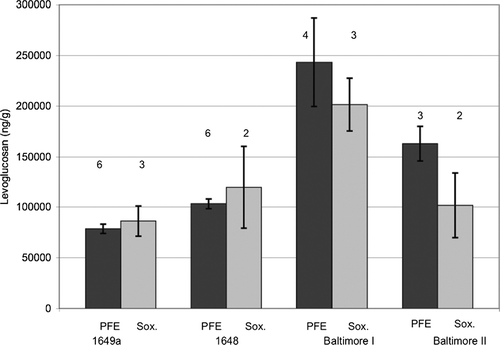
SRM 1649a was used to examine the extraction of levoglucosan from PM as summarized in . Three samples of SRM 1649a were extracted using PFE with ethyl acetate and a single three cycle extraction while an additional three samples of SRM 1649a were extracted using PFE with ethyl acetate and four sequential three cycle extractions. The results from the sequential PF extraction (80.3 μ g g−1 with a standard deviation of 6.1 μ g g−1) are higher than those from the single PF extraction (76.9 μ g g−1 with a standard deviation of 2.6 μ g g−1). A Student t test at the 99% confidence interval indicates that these values are not significantly different. The shelf stored SRM 1649a was also Soxhlet extracted (n = 3) using methylene chloride and acetone (80:20 v:v) for 24 h. The mean levoglucosan concentration from this method was 86.2 μ g g−1 with a standard deviation of 14.9 μ g g−1. While there is a larger standard deviation from the Soxhlet extraction method, the mean is not significantly different from either of the PFE trials at the 99% confidence interval. The overall mean concentration and pooled standard deviation of the nine samples is 81.1 (9.4) μ g g−1.
TABLE 1 Concentrations, μ g/g,Footnote a of Levoglucosan in SRM 1649a using various extraction methods
Eight samples of SRM 1648 (varying in mass between 69 mg and 129 mg) had a mean levoglucosan concentration of 107 (18) μ g g−1 (parentheses indicate standard deviation). The concentration of levoglucosan in SRM 1648 (collected in St. Louis, MO) is significantly higher (p < 0.05) than in SRM 1649a (collected in Washington, DC) as shown in .
FIG. 3 Summary of Levoglucosan in shelf stored urban particulate matter reference material. Numbers above columns represent number of replicates. Soxhlet extraction represented by Sox. Pressurized fluid extraction indicated by PFE.
Information concerning the long-term stability of levoglucosan is important to determining the appropriate frequency for stability testing. As mentioned above, a small batch of 1649a has been stored in a 4°C freezer since 1980. Triplicate aliquots from this frozen batch were extracted by PFE with ethyl acetate as the solvent. The mean levoglucosan concentration in the freezer stored 1649a is 162 μ g g−1 with a standard deviation of 8 μ g g−1. This concentration is almost 2× higher than the levoglucosan in the shelf stored SRM 1649a (81.1 μ g g−1). This difference may be due to acid hydrolysis of the levoglucosan to β -D glucose at room temperature storage for 25 years. The acid hydrolysis is a documented industrial reaction which allows the use of levoglucosan as a feedstock for the production of polymers (CitationKops and Spanggaard 1972; CitationPenczek et al. 1985).
Baltimore PM 2.5 Materials
Two fine particulate matter (nominally < 2.5 μ m particle size) materials were collected in Baltimore, MD, the first between 1998 and 2001 and the second in the fall of 2002. The first one (designated as Baltimore PM2.5 I in ) had a mean (standard deviation) levoglucosan concentration of 225 μ g g−1 (41 μ g g−1). In comparison, urban particulate matter collected at the same location during the fall of 2002, Baltimore PM2.5 II material, had a mean (standard deviation) levoglucosan concentration of 138 μ g g− 1 (39 μ g g−1), n = 5. Since combustion products are known to be found predominantly in the fine particulate matter fraction, it was not surprising to find that the PM 2.5 samples had higher levoglucosan concentrations than the SRM 1649a and SRM 1648 which both had larger particle size. Additionally, if degradation at room temperature storage is removing levoglucosan, then the Baltimore samples which are over two decades newer than SRMs 1648 and 1649a, should have higher concentrations. The difference between the two samples collected in Baltimore may indicate differences in source profiles during the two sampling periods. This hypothesis is supported by a recent study, which reported significant seasonal variations in source apportionment of polycyclic aromatic hydrocarbons in Baltimore (CitationLarsen and Baker 2003).
CONCLUSIONS
As a marker of biomass combustion, levoglucosan can provide important information for many types of environmental and geochemical research. Levoglucosan is present in all of the NIST particulate matter reference materials. Careful cleaning and handling of materials, such as labware and hydromatrix, is essential to minimize levoglucosan in blanks. Sample sizes as small as 20 mg are sufficient to quantify levoglucosan at levels 10 to 100 times greater than blank values. Soxhlet extraction and PFE give comparable results for the extraction of levoglucosan; however, the precision in the Soxhlet extracts tended to be poorer. This study provides values for levoglucosan in two SRMs using a GC/MS method.
RL gratefully acknowledges financial support from the National Institute of Standards and Technology (contract PWS#04-839-8990).
Disclaimer: Certain commercial equipment, instruments, or materials are identified in this report to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are the best available for the purpose.
Notes
a Concentration is the mean and one standard deviation of the number of samples indicated.
b SRM 1649a is stored at room temperature.
c This bulk sample of SRM 1649a has been stored frozen at 4°C.
d RM8785 is a PM2.5 fraction of SRM 1649a that has been collected on Teflon filters.
e Baltimore I fine particulate matter was collected in Baltimore between 1998 and 2001.
f Baltimore II fine particulate matter was collected in Baltimore fall of 2002.
REFERENCES
- Abas , M. R. B. , Oros , D. R. and Simoneit , B. R. T. 2004 . Biomass Burning as the Main Source of Organic Aerosol Particulate Matter in Malaysia During Haze Episodes . Chemosphere. , 55 : 1089 – 1095 . [CSA]
- Abas , M. R. B. , Simoneit , B. R. T. , Elias , V. , Cabral , J. A. and Cardoso , J. N. 1995 . Composition of Higher Molecular Weight Organic Matter in Smoke Aerosol from Biomass Combustion in Amazonia . Chemosphere. , 30 : 995 – 1015 . [CROSSREF] [CSA]
- Dos Santos , C. Y. M. , Azevedo , D. U. and Neto , F. R. U. 2004 . Atmospheric Distribution of Organic Compounds from Urban Areas Near a Coal-Fired Power Station . Atmos. Environ. , 38 : 1247 – 1257 . [CROSSREF] [CSA]
- Echalar , F. , Gaudichet , A. , Cachier , H. and Artaxo , P. 1995 . Aerosol Emissions by Tropical Forest and Savanna Biomass Burning: Characteristic Trace Elements and Fluxes . Geophys. Res. Lett. , 22 : 3039 – 3043 . [CROSSREF] [CSA]
- Fine , P. M. , Chakrabarti , B. , Krudysz , M. , Schauer , J. J. and Sioutas , C. 2004 . Diurnal Variations of Individual Organic Compound Constituents of Ultrafine and Accumulation Mode Particulate Matter in the Los Angeles Basin . Environ. Sci. Technol. , 38 : 1296 – 1304 . [INFOTRIEVE] [CROSSREF] [CSA]
- Fraser , M. P. and Lakshmanan , K. 2000 . Using Levoglucosan as a Molecular Marker for the Long-Range Transport of Biomass Combustion Aerosols . Environ. Sci. Technol. , 34 : 4560 – 4564 . [CROSSREF] [CSA]
- Graham , B. , Falkovich , A. H. , Rudich , Y. , Maenhaut , W. , Guyon , P. and Andrea , M. O. 2004 . Local and Regional Contributions to the Atmospheric Aerosol Over Tel Aviv, Israel: A Case Study Using Elemental, Ionic and Organic Tracers . Atmos. Environ. , 38 : 1593 – 1604 . [CROSSREF] [CSA]
- Graham , B. , Guyon , P. , Taylor , P. E. , Artaxo , P. , Maenhaut , W. , Glovsky , M. M. , Flagan , R. C. and Andreae , M. O. 2003 . Organic Compounds Present in the Natural Amazonian Aerosol: Characterization by Gas Chromatography-Mass Spectrometry . J. Geophys. Res. Atmos. , 108 : D24 [CSA]
- Kops , J. and Spanggaard , H. 1972 . Low Temperature Polymerization of Endo- and Exo-2-methyl-7-oxabicyclo-[2.2.1]-heptane . Polymer Preprints. , 13 : 90 – 95 . [CSA]
- Lakshmanan , C. M. and Hoelscher , H. E. 1970 . Production of Levoglucosan by Pyrolysis of Carbohydrates . Die Starke. , 22 : 261 – 264 . [CSA]
- Larsen , R. K. and Baker , J. E. 2003 . Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods . Environ. Sci. Technol. , 37 : 1873 – 1881 . [INFOTRIEVE] [CROSSREF] [CSA]
- Locker , H. B. 1988 . Ph.D. Thesis Hanover, N.H. : Dartmouth College .
- Nolte , C. G. , Schauer , J. J. , Cass , G. R. and Simoneit , B. R. T. 2001 . Highly Polar Organic Compounds Present in Wood Smoke and in the Ambient Atmosphere . Environ. Sci. Technol. , 35 : 1912 – 1919 . [INFOTRIEVE] [CROSSREF] [CSA]
- Olmez , I. , Sheffield , A. E. , Gordon , G. E. , Houck , J. E. , Pritchett , L. C. , Cooper , J. A. , Dzubay , T. G. and Bennett , R. L. 1988 . Compositions of Particles from Selected Sources in Philadelphia for Receptor Modeling Applications . JAPCA. , 38 : 1392 – 1402 . [INFOTRIEVE] [CSA]
- Pashynska , V. , Vermeylen , R. , Vas , G. , Maenhaut , W. and Claeys , M. 2002 . Development of a Gas Chromatographic/Ion Trap Mass Spectrometric Method for the Determination of Levoglucosan and Saccharidic Compounds in Atmospheric Aerosols. Application to urban aerosols . J. Mass Spectrom. , 37 : 1249 – 1257 . [INFOTRIEVE] [CROSSREF] [CSA]
- Penczek , S. , Kubisa , P. and Matyjaszewski , K. 1985 . Cationic Ring-Opening Polymerization. Part II: Synthetic Applications , 317 Berlin : Springer .
- Schauer , J. J. , Kleeman , M. J. , Cass , G. R. and Simoneit , B. R. T. 1999 . Measurement of Emissions from Air Pollution Sources. 1. C1 through C29 Organic Compounds from Meat Charbroiling . Environ. Sci. Technol. , 33 : 1566 – 1577 . [CROSSREF] [CSA]
- Simoneit , B. R. T. 2002 . Biomass Burning—A Review of Organic Tracers for Smoke from Incomplete Combustion . Appl. Geochem. , 17 : 129 – 162 . [CROSSREF] [CSA]
- Simoneit , B. R. T. , Cox , R. E. and Standley , L. J. 1988 . Organic Matter of the Troposphere-IV. Lipids in Harmattan Aerosols of Nigeria . Atmos. Environ. , 22 : 983 – 1004 . [CROSSREF] [CSA]
- Simoneit , B. R. T. , Schauer , J. J. , Nolte , C. G. , Oros , D. R. , Elias , V. O. , Fraser , M. P. , Rogge , W. F. and Cass , G. R. 1999 . Levoglucosan, A Tracer for Cellulose in Biomass Burning and Atmospheric Particles . Atmos. Environ. , 33 : 173 – 182 . [CROSSREF] [CSA]
- Simoneit , B. R. T. , Sheng , G. Y. , Chen , X. J. , Fu , J. M. , Zhang , J. and Xu , Y. P. 1991 . Molecular Marker Study of Extractable Organic-Matter in Aerosols From Urban Areas of China . Atmos. Environ. , 25 : 2111 – 2129 . [CSA]
- Zdrahal , Z. , Oliveira , J. , Vermeylen , R. , Claeys , M. and Maenhaut , W. 2002 . Improved Method for Quantifying Levoglucosan and Related Monosaccharide Anhydrides in Atmospheric Aerosols and Application to Samples from Urban and Tropical Locations . Environ. Sci. Technol. , 36 : 747 – 753 . [INFOTRIEVE] [CROSSREF] [CSA]