The masses and diameters of monodisperse polystyrene latex particles ranging from 100 nm to 1 μ m in average diameter were determined using the electro-gravitational aerosol balance, a method that we proposed in a previous paper. Charged latex particles were introduced between parallel plate electrodes similar to a Millikan cell, and the particle survival rate after a certain holding time had elapsed was measured as a function of the applied voltage. The number average diameter and the corresponding particle mass were determined by fitting a theoretical survival rate spectrum to the experimentally observed spectrum using the least squares method. In this fitting, the value of the particle density obtained in a separate experiment was used. Detailed uncertainty analysis of the number average diameters thus determined was carried out. The expanded uncertainty with a coverage factor of 2, which corresponds approximately to a 95% confidence interval, was 0.66 nm, or 0.66%, for 100 nm particles and 1.8 nm, or 0.18%, for 1 μ m particles. The diameter values of 200 nm particles obtained under different operating conditions were found to agree within 0.3%. These results demonstrate that the present method is appropriate for use as the primary sizing method to develop particle size standards in the size range of 100 nm to 1 μ m.
INTRODUCTION
In a previous article, we proposed a method for accurate mass and size measurement of monodisperse particles (CitationEhara et al. 2006). In this method, charged particles are introduced between parallel plate electrodes similar to a Millikan cell, and the temporal decrease in the number of particles is used to detect the balance between the electrostatic and gravitational forces exerted on the particles. The performance of this method is characterized by the survival function, which is defined as the ratio of the number of particles left suspended after a certain holding time has elapsed, to the initial number of particles as a function of the particle mass. The expressions of the survival function for nondiffusing particles and diffusing particles were derived in the previous paper.
For particles that have a dispersion in size, the particle survival rate is represented by an integral of the size distribution function with the survival function as the integral kernel. The number average diameter can be determined by fitting a theoretically predicted survival rate spectrum to experimentally observed data. It was demonstrated in the previous paper that the present method is sensitive to the location of the number average diameter, although it is insensitive to the shape of the size distribution function. In the present study, we apply this method, called the electro-gravitational aerosol balance (EAB), to determination of the mass and size of polystyrene latex (PSL) particles with average diameters ranging from 100 nm to 1 μ m.
EXPERIMENT
Experimental Apparatus
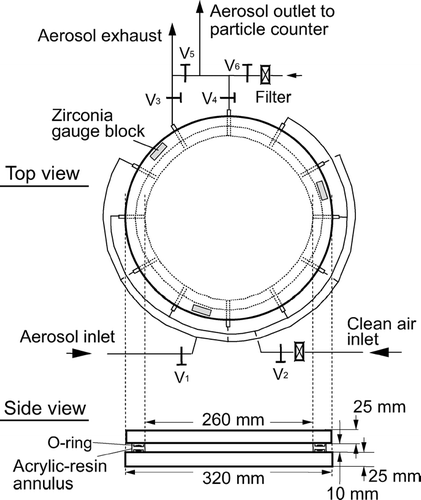
shows the top and side views of the electrodes used in this study. Two flat plate electrodes made of brass, 320 mm in diameter and 25 mm in thickness, are separated by three gauge blocks made of zirconia (Cera Block, Mitutoyo Corp.), each having a nominal height of 10 mm. The error in the nominal height is smaller than 0.06 μ m. The flatness of the electrode surfaces was evaluated with a three-dimensional coordinate measuring machine, from which the root mean square deviation of the electrode gap, H, from its design value of 10 mm was found to be 2.9 μ m (see Appendix A). The space between the electrodes is sealed by an acrylic-resin annulus with silicon-gel O-rings on the top and bottom of the annulus. The inside diameter of the annulus is 260 mm, and hence the volume of the sealed space amounts to 530.9 cm3. There are 12 holes bored on the side of the annulus, five of which serve as aerosol inlets, five as clean air inlets, and the remaining two as aerosol outlets. One of the aerosol outlets functions to exhaust the aerosol before the holding time has started, and the other to feed the aerosol to a particle counter after the holding time has elapsed. The voltage of the upper electrode relative to the lower one was chosen to be negative, and hence positively charged particles were the target of our measurement. The applied voltage was monitored with a digital multimeter (VOAC7522, Iwatsu Electric Co., Ltd.).
In order to suppress thermophoresis of particles, the temperature needs to be uniform over the two electrode surfaces. For this purpose, the electrodes are held in a cylindrical container having aluminum walls of 20 mm in thickness, and this container is placed in a heat-insulating vessel. During the holding time, the temperature on the back face of each electrode was monitored with a digital thermistor thermometer having a least significant digit of 0.01°C (D642, Technol Seven Corp.). It was confirmed that throughout the measurements the temperature difference between the upper and lower electrodes did not exceed the resolution limit of the thermometer. The temperature was within the range of 18 to 22°C, and its variation during the holding time was smaller than 0.5°C.
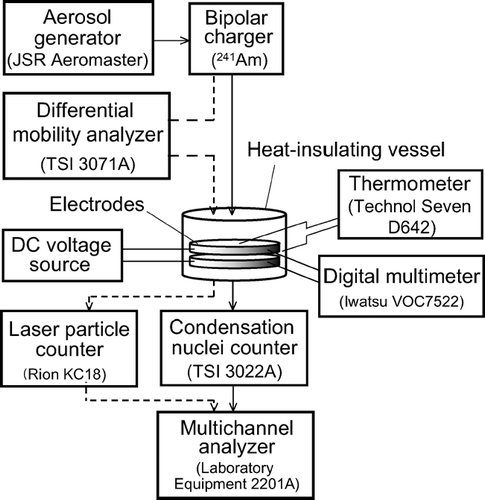
The entire experimental setup is shown schematically in . An aerosol suspending monodisperse PSL particles is generated from an aerosol generator (Aeromaster, JSR Corp.). The aerosol is passed through a 241Am bipolar charger, and introduced into the space between the electrodes. The number of particles exiting the electrodes is counted by either a condensation nuclei counter (CNC) (Model 3022A, TSI Inc.) or a laser particle counter having a detection limit of 100 nm (KC18, Rion Co., Ltd.). The output pulses from the counters are counted by a multichannel analyzer (Model 2201A, Laboratory Equipment Corp.).
When a relatively short holding time is chosen, the survival function can be accordingly broad, and hence a substructure due to multiply charged particles may appear in the survival rate spectrum. In some of the measurements, we installed a differential mobility analyzer (DMA) (Model 3071A, TSI Inc.) upstream of the electrodes in order to avoid the substructure. In this case, all particles introduced to the electrodes are singly charged. The flow rates and voltage of the DMA were chosen so that it had a trapezoidal transfer function (CitationKnutson and Whitby 1975), and thus the size distribution of PSL particles was not modified by the DMA. It was confirmed by use of another DMA in combination with the CNC that the size distributions upstream and downstream of the DMA were, in fact, identical.
Measurement Procedure
Prior to each measurement, the sample aerosol was passed through the electrodes for more than 10 minutes, so that the electrode space would be uniformly filled with the aerosol. During this period, the concentration of particles exiting the electrodes was measured. From the concentration and the volume of the electrode space, the initial particle number was calculated. Valves V1 to V4 in were then closed, and the particles were held for a certain time t h. When time t h had elapsed, valves V2 and V4 were opened, and the number of particles that remained suspended was counted. During this process, particle-free air fed in through V2 replaced the aerosol in the electrodes. The survival rate was calculated from the particle number thus obtained and the initial particle number. For each PSL species, the survival rate was obtained at 8–15 values of applied voltage.
Particle Density Measurement
The densities of PSL particles were determined by a standard method described in JIS Z8901:1995. First, several sodium chloride solutions of different concentrations whose densities differed incrementally by approximately 0.001 g/cm3 were prepared.Footnote 1 The densities of the solutions were determined by a hydrometer. A small amount of paste-like PSL particles that had been prepared by centrifugation was put into each solution, and the solution was mixed by stirring. The solutions were left to stand until coagulated particles that had either floated to the surface, settled at the bottom, or remained suspended in the solution could be clearly seen. The ambient temperature during this process was within 20 ± 2°C. For 100 nm particles, about 10 days were required for this process, while for larger particles, less time was needed. In this way, the particle density was determined with a resolution of 0.001 g/cm3.
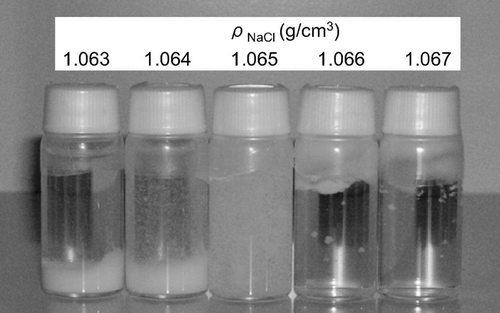
A photograph of PSL particles of about 100 nm in diameter immersed in density reference liquids after being left to stand for 30 days is shown in . This PSL species was provided by JSR Corp., and had been taken from the same batch as the 100 nm particle size standard (SRM 1963) distributed by the National Institute of Standards and Technology (NIST). In , it is observed that particles are distributed throughout the 1.065 g/cm3 solution, whereas a large portion of particles is seen precipitated at the bottom of the 1.064 g/cm3 solution. From this observation, the density of this PSL species was determined to be 1.065 g/cm3. The exact value of the density is expected to lie between 1.064 g/cm3 and 1.065 g/cm3, and is likely to be closer to 1.065 g/cm3.
FIG. 3 Photograph of 100 nm PSL particles immersed in density reference liquids after settling for 30 days.
Using this method, we determined the densities of two PSL species manufactured by JSR Corp. Because the results in both cases were found to be identical with the densities given in the specifications of the particles, we adopted the values in the specifications for the remainder of the particle species.
Strictly speaking, the density determined in this way is an average value, and the particle density can have a dispersion around this value. It is seen in that except for minor outliers, the density distribution extends at most from 1.063 g/cm3 to 1.066 g/cm3. If we crudely assume a uniform density distribution within this interval, its relative standard deviation is estimated to be 0.081%. On the other hand, if we apply the moment method based on electrical mobility analysis (CitationEhara et al. 2000) to this particle species, a relative standard deviation in terms of size of about 1.8% is obtained. This corresponds to a relative standard deviation in terms of mass of about 5.4%. This estimate indicates that the mass dispersion originates almost solely from the size dispersion, and that the density can be assumed to be uniform. Accordingly, with the density being known, any function of the particle mass can be regarded as a unique function of the particle size, and vice versa. In the derivation below, we employ this property without further mention of this point.
Data Analysis
First, we consider the case in which no DMA is installed upstream of the electrodes. We examine the survival rate of singly charged particles, and let q(D p) denote the probability that particles with diameter D p carry a single positive charge in the equilibrium charge state of the aerosol. The survival rate is then given by
We assume that f(D p) can be approximated by a Gaussian distribution,
We regard three parameters, D pa, σ, and c, as adjustment parameters, and fit S(V) to a measured spectrum by the least squares method. Because the fitting is nonlinear, it was carried out numerically by an iteration procedure. It should be noted that, in principle, the factor n 0/n′0 n 0 n 0 in c ought to be unity from the definition of n 0. However, this factor can be smaller than unity, in practice, due to particle loss processes that were not considered in deriving the survival function. Possible candidates for such a process include an excess particle loss due to aerosol convection at the initial stage of the holding period, the effect of space charge, and particle coagulation during the holding time. It is expected that such a process, if it exists, will not noticeably affect the measured D pa , provided that it depends moderately on D p in the narrow range of f(D p).
It should also be noted that if we use g(m)dm instead of f(D p)dD p in Equation (Equation1), where g(m) is the mass distribution function, we can also determine the number average particle mass. This may be slightly larger than the mass corresponding to D pa defined by
As an expression of the slip correction factor needed to calculate the survival function, we used the expression presented by CitationAllen and Raabe (1985).
RESULTS
EAB measurement was carried out for seven PSL particle species. The result for each species is described below. summarizes the results of all the measurements.
TABLE 1 Summary of mass and diameter measurements of PSL particles
(a) NIST SRM 1963
The survival rate spectrum obtained for NIST SRM 1963, which has a certified diameter of 100.7 nm, is shown in . The holding time, t h, was set at 14 hours, and a DMA was installed upstream of the electrodes. The best fit survival rate spectrum was obtained for D pa = 100.8 nm, which is in good agreement with the certified diameter.
FIG. 4 Survival rate spectrum obtained (a) for NIST SRM 1963; (b) for JSR SC-011-S. The data indicated by the empty diamonds were not included in the least squares fitting; (c) for JSR SC-021-S under two different conditions: t h = 6 h and t h = 3 h. Note that the two data sets are plotted on different scales; (d) for NIST SRM 1691. The data indicated by the empty diamonds were not included in the least squares fitting; (e) for JSR SC-048-S; (f) for NIST SRM 1690; (g) for JSR SC-101-S.
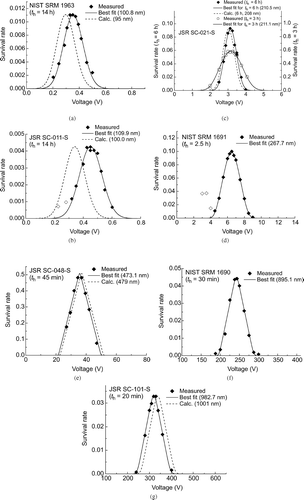
As previously mentioned, the density measurement of this particle species had been performed for paste-like particles taken from the same batch as this species. According to the specifications of the particles provided by the manufacturer, its nominal diameter determined by transmission electron microscope (TEM) is 95 nm. The theoretical spectrum calculated for D pa = 95 nm is shown by the dashed curve in . This value is obviously too small to account for the observed spectrum.
(b) JSR SC-011-S
The survival rate spectrum for JSR SC-011-S is shown in . Because no DMA was used upstream of the electrodes, a substructure due to multiply charged particles appears in the spectrum. Accordingly, the two data marked by empty diamonds in were excluded from data analysis. The best fit spectrum was obtained for D pa = 109.9 nm.
The nominal diameter of SC-011-S, determined by TEM, is 100 nm. The theoretical spectrum calculated for D pa = 100 nm in indicates that this nominal diameter is too small to account for the observed spectrum.
(c) JSR SC-021-S
For JSR SC-021-S, two measurements were performed to examine the reproducibility: one with t h = 6 h and the other with t h = 3 h. The results are shown in . In the measurement with t h = 6 h, no DMA was used upstream of the electrodes, and hence a substructure due to multiply charged particles is recognized in the spectrum. The data denoted by the empty diamond was therefore excluded from data analysis.
The best fit spectra for t h = 6 h and t h = 3 h were obtained for D pa = 210.5 nm and D pa = 211.1 nm, respectively. The nominal diameter of SC-021-S is 208 nm, and agrees with our results within 1.5%. The theoretical spectrum calculated assuming the nominal diameter is also shown in .
(d) NIST SRM 1691
The survival rate spectrum obtained for NIST SRM 1692 is shown in . The holding time was 2.5 h, and no DMA was used. The secondary peak in the spectrum is located at half the main peak voltage, and should be due to doubly charged particles. The data denoted by the empty diamonds were therefore excluded from data analysis.
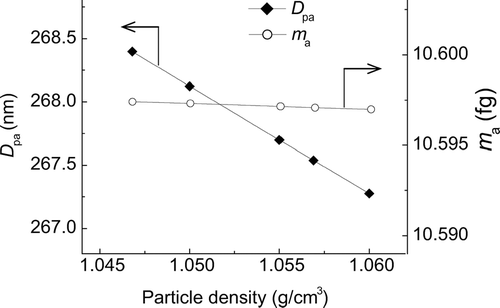
Because we could not perform a density measurement for this particle species due to the nonavailability of paste-like particles, we chose the value 1.055 g/cm3 as a rough estimate of its density. How the choice of particle density affects D pa will be discussed later. Assuming ρp = 1.055 g/cm3, we obtained D pa = 267.7 nm. The certified diameter of SRM 1691 is 269 nm, which is in good agreement with our result.
(e) JSR SC-048-S
The survival rate spectrum measured for JSR SC-048-S with t h = 45 min is shown in . In the data analysis, the survival function for nondiffusing particles was used. The root-mean-square displacement due to Brownian diffusion of a particle with diameter of about 470 nm for 45 min is approximately 0.60 mm. The smallness of this value compared with H justifies the use of the nondiffusing survival function. The best fit spectrum was achieved for D pa = 473.1 nm. The nominal diameter provided by the manufacturer is 479 nm, which agrees with our result within 1.5%. In , the survival rate spectrum predicted for D pa = 479 nm is also shown.
(f) NIST SRM 1690
The survival rate spectrum measured for NIST SRM 1690 with t h = 30 min is shown in . The survival function for this holding time is so narrow that multiply charged particles do not contribute to the spectrum, even though no DMA was used upstream of the electrodes. Because, similarly to SRM 1692, no information on the particle density of this particle species was available, a tentative value of 1.055 g/cm3 was used in the data analysis. With this density, D pa = 895.1 nm was obtained. The NIST certified diameter is 895 nm, which coincides almost exactly with our result.
(g) JSR SC-101-S
The survival rate spectrum obtained for JSR SC-101-S with t h = 20 min is shown in . The best fit spectrum was obtained for D pa = 982.7 nm. The nominal diameter provided by the manufacturer is 1001 nm, and the spectrum calculated by assuming this diameter is also shown in . The discrepancy with our result is small but distinct.
UNCERTAINTY ANALYSIS
In this section, we carry out the uncertainty analysis for D pa , based on the ISO Guide to the expression of uncertainty in measurement (ISO 1993). We begin with the following equation derived from Equation (Equation4),
The uncertainty of m a consists of a systematic component u s(m a) and a random component u r(m a); the former corresponds to a shift in the survival rate spectrum as a whole, and the latter to the residual fitting errors in the survival rate spectrum. The systematic component can be derived from
For the random component u r(m a), we have to examine the fitting procedure of the survival rate spectrum. Let u(S i ) denote the uncertainty associated with the residual fitting error of the i-th survival rate S i . Then the random component can be evaluated by
Because the least squares fitting here is nonlinear and ∂ m a/∂ S i cannot be calculated analytically, we make the calculation by a numerical method. Assuming two datasets, {S 1, S 2, …, S i − 1, S i , S i + 1, …,S n } and {S 1, S 2, …, S i − 1, S i +Δ S i , S i + 1, …, S n }, where the first dataset represents the actual data, while in the second dataset, Δ S i is a small arbitrary increment, we recalculated m a for each dataset by the least squares method, and calculated their difference Δ m a. The partial derivative can now be approximated as
The uncertainty analysis for NIST SRM 1963 is summarized in . It is noted that the overall uncertainty is determined almost solely by the residual fitting errors, u(S i ). This is also the case for the other particle species. The combined standard uncertainty for each particle species is listed in .
TABLE 2 Summary of uncertainty analysis of D pa for NIST SRM 1963
Because EAB is primarily a mass measurement method, thermal expansion of PSL particles does not have to be considered in the evaluation of u(m a).Footnote 3 However, it can have some impacts on the particle density measurement, which are discussed in Appendix A. If the temperature is not specified when using the value of D pa, its deviation from the reference temperature at which the particle density was measured can be a source of error in D pa. Let Δ T r and αp denote the temperature deviation and the volume thermal expansion coefficient of PSL particles, respectively. The relative error in D pa is then
There are possible sources of uncertainty other than those considered in the above analysis that might affect the measurements. We list these possible sources in .
TABLE 3 List of possible uncertainty sources not included in the uncertainty analysis
Nonvolatile impurities that may be contained in the PSL particle suspensions can increase the apparent mass and size of the particles when they are aerosolized (CitationKinney et al. 1991). As discussed in Appendix B, we consider this effect to be negligible in our experiment.
The electrostatic field may be distorted due to the presence of the electrode edges, as well as due to the presence of the acrylic resin annulus, which has a relative dielectric constant of about 3. Accordingly, the field strength near the acrylic resin annulus may be slightly weaker than that at the center of the electrodes. Also, the work functions of the two electrode surfaces might be different due, for example, to deterioration of the surfaces. If this is the case, the field strength will be affected by the work function difference.
Air convection, particle coagulation, and the effect of space charge will all affect the particle survival rates. As long as the size distribution is narrow enough, these effects are not considered to contribute to a bias in m a and D pa. It is therefore expected that these effects, if they exist, will mainly contribute to apparently random variations in the survival rates, which are already taken into account in u(S i ) in Equation (Equation11).
If the temperature difference of 0.01°C between the upper and lower electrodes persists for 14 hours, the migration distance of 100 nm particles due to thermophoresis will amount to approximately 1 mm (see, for example, CitationHinds 1998), which will cause a nonnegligible impact on the particle survival rate. If the temperature gradient in one direction is maintained for all the data points that constitute one survival rate spectrum, this will result in a bias in determining m a and D pa. However, as described in Appendix C, no systematic temperature difference that exceeded the least significant digit of 0.01°C of the digital thermometer was observed. Therefore, we consider that the major effect of thermophoresis, if it exists, is to cause apparently random variations in the particle survival rates (see Appendix C). It is to be noted that the random variations are already included in u(S i ) given in Equation (Equation11).
In the analyses of survival rate spectra, it was assumed that the observed spectra were associated with singly charged singlets (i.e., singly charged single PSL particles). We were able to identify spectrum structures due to multiply charged singlets, and exclude them from analysis. However, spectrum structures due to multiply charged multiplets (multiply charged clusters of multiple PSL particles) may overlap and distort the main spectra. We have assumed that the concentration of multiply charged multiplets was much lower that that of singly charged singlets, and neglected their effect in the data analyses.
Finally, we have assumed that the particle size distributions and densities are uniform within a batch, and within a bottle of PSL particles. If this is not the case, the uncertainty associated with the nonuniformity will have to be included in the total uncertainty.
We believe that the effects of the uncertainty sources listed in can be neglected. Other than the impurity effect, however, quantitative estimates of them have not been made in the present study, but left for future investigation.
DISCUSSION
The densities of NIST SRM 1691 and SRM 1690 are not known exactly, and a tentative value of 1.055 g/cm3 was used in the data analysis. CitationAllen and Raabe (1985) made density measurements on 0.79 μ m and 1.18 μ m PSL particles (supplied by then Dow Diagnostics), and PSL particles cross-linked with 8% divinylbenzene (Duke Scientific Corp.) using density gradient ultracentrifugation, and obtained a value of 1.0468 g/cm3. They state that the particle density does not appreciably depend on particle size. CitationPugh and Heller (1957), using differential pycnometry, obtained a value of 1.0569 g/cm3for the density of PSL particles. We again performed least squares fitting to the EAB data for SRM 1691, assuming ρp = 1.0468, 1.05, 1.055, 1.0569, and 1.06 g/cm3. The resultant values of D pa and m a are shown in . The values of D pa corresponding to ρp suggested by CitationAllen and Raabe (1985), and to ρ p suggested by CitationPugh and Heller (1957) differ only by 0.32%. This result demonstrates that the effect of the choice of ρp on D pa is not significant. It is seen in that the effect on m a is almost negligible, as expected.Footnote 4
A confidence interval for a measurement result is often expressed in terms of an expanded uncertainty U (ISO Guide 1993), which is the standard uncertainty multiplied by a coverage factor k. Roughly speaking, ± U with k = 2 corresponds to a 95% confidence interval. From , the expanded uncertainty with k = 2 for D pa = 100 nm to 300 nm in the EAB measurements is from 0.40 nm to 0.66 nm. In the size range exceeding 300 nm, the uncertainty is larger, but still consistently smaller than 2.0 nm for D pa smaller than 1 μ m.
Because the EAB measurement is, in general, time-consuming, repetition of measurement under exactly the same condition was conducted only for limited data points; namely, the pair of data at approximately 0.45 V for JSR SC-011-S in , and the pair at approximately 3.5 V in the spectrum with t h = 6 h for JSR-021-S in . It should be noted, however, that u(S i ) given in Equation (Equation11) includes the effect of random variations that would occur in measurement repetition. The measurement period needed to obtain a complete survival rate spectrum of relatively small particles was quite long, extending to about three weeks for 200 nm particles and about four weeks for 100 nm particles, including irregular intervals between measurements. For these particles, the experimental apparatus except the Millikan-type cell was powered off during the holding time, and the whole apparatus was powered off during measurement intervals. Therefore, if unidentified error sources, such as those associated with day-to-day variations in ambient temperature, pressure, or humidity, or with restarting of the experimental apparatus, should affect the measurements, these effects would be expected to manifest themselves in random variations of the particle survival rates.
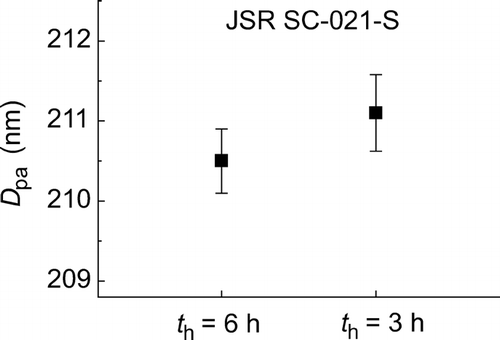
In order to confirm the reproducibility of the measurements, the survival rate spectra were obtained under two different holding times, 3 h and 6 h, for JSR SC-021-S particles (). The diameter obtained in each measurement was 210.5 ± 0.40 nm and 211.1 ± 0.48 nm, respectively, where the confidence intervals indicate the expanded uncertainties with k = 2. The results are summarized in . The difference in D pa between the two measurements is 0.6 nm, or 0.28%, and is consistent with the evaluated uncertainties as seen in .
FIG. 6 Reproducibility of D pa obtained for JSR SC-021-S. The confidence interval for each of the data indicates the expanded uncertainty with a coverage factor of 2.
For NIST SRM 1963, particle size measurement has been performed by several groups using different methods, and the results have been summarized by CitationMulholland et al. (1999) and CitationGermer et al. (2002). CitationMulholland et al. (1999) performed a careful measurement using a DMA and obtained a mean diameter of 100.7 nm with an expanded uncertainty (k = 2.03) of 0.95 nm, or 0.94%. In their measurement, NIST SRM 1963 having a certified diameter of 0.895 μ m was used to calibrate the DMA. CitationGermer et al. (2002) developed a method employing angle-resolved laser-light scattering from particles deposited on a silicon wafer, and obtained a diameter of 99.7 nm with an expanded uncertainty (k = 2) of 1.73 nm, or 1.7%. CitationStover and Scheer (2001) used a method based on the same principle but with a different apparatus and data analysis procedure, and obtained a diameter of 100.6 nm with an expanded uncertainty (k = 2) of 0.7 nm, or 0.70%. All these results agree fairly well with our result of D pa = 100.8 nm.
CitationMulholland et al. (1999) also cited measurement results for NIST SRM 1963 obtained by other groups: The number average diameter obtained by TEM analysis carried out by Duke Scientific was 100.2 nm with an expanded uncertainty (k = 2) of 2.6 nm, or 2.6%; a measurement based on field-flow separation in combination with multiangle laser-light scattering was made by Wyatt Technology in which a number average diameter of 99.2 nm was obtained; and an AFM measurement was carried out by Park Scientific in which a number average diameter of 101.5 nm was obtained.
JSR SC-011-S particles have also been measured by several groups. CitationMulholland et al. (1999) used both a DMA and a TEM to compare the size of SC-011-S with that of NIST SRM 1963. They found that SC-011-S particles were larger than SRM 1963 particles by 11.2% in the DMA measurement and by 9.6% in the TEM measurement. If the certified size of 100.7 nm is assumed for NIST SRM 1963, the average diameter of SC-011-S particles based on the DMA and TEM measurements is estimated to be 112 nm and 110 nm, respectively. CitationJung et al. (2002) carried out a careful measurement of SC-011-S particles by TEM, and obtained an average diameter of 109.2 nm with an expanded uncertainty (k = 2) of 2.3 nm, or 2.1%. CitationKousaka et al. (1988) performed a relative measurement using a DMA, and obtained an average diameter of 100 nm. In their measurement, PSL particles of about 1 μ m whose size was determined using a Millikan-type cell was used to calibrate the DMA. CitationYokochi (1994) used both a DMA and an optical particle counter (OPC), obtaining an average diameter of 110 nm by the DMA and 108 nm by the OPC.
According to the specifications of JSR SC-011-S, its nominal size, determined by TEM, is 100 nm. CitationKitajima et al. (1998) reexamined the TEM analysis performed by JSR Corp. and found that the shrinkage of PSL particles due to electron beam exposure, especially that occurring within the initial 15 s of exposure, might be a nonlinear function of the exposure time. They suggested that for particles smaller than 150 nm, TEM analysis might result in an apparently smaller size if the effect of nonlinear shrinkage were not taken into consideration. As stated above, most of the recent measurements of SC-011-S indicate that its actual size is larger than the nominal size by about 8% to 12%, and this is consistent with our result.Footnote 5
At present, the smallest size for which EAB measurement can be carried out with a satisfactorily small uncertainty is approximately 100 nm. For smaller particles, the residual fitting error as defined in Equation (Equation11) tends to increase significantly. As shown in our previous article (CitationEhara et al. 2006), the long holding time necessary to maintain a high resolution for relatively small particles can lead to a serious decrease in the height of the survival rate spectrum due to significant Brownian motion. In this situation, even a slight deviation in the number of particles that are lost during the holding time can bring about a large deviation in the survival rate. This effect might be the reason for the observed large residual fitting errors for particles smaller than 100 nm, although further studies will be required to establish the lower size limit of the present method.
CONCLUSION
The number average diameters and the corresponding masses of seven species of polystyrene latex particles ranging from 100 nm to 1 μ m in average diameter were determined by the electro-gravitational aerosol balance method. A detailed uncertainty analysis was performed, which showed that the expanded uncertainty (k = 2) ranges from 0.40 nm to 2.0 nm. The reproducibility of measurement evaluated for 200 nm particles was better than 0.3%. The results of some carefully performed measurements by other groups based on different principles were found to agree fairly well with our results. These results show that the present method can be used as the primary sizing method to develop particle size standards in the size range from 100 nm to 1 μ m.
APPENDIX A: EVALUATION OF THE UNCERTAINTIES OF V 0, H, g, and, ρp
The uncertainties associated with the values of V 0, H, g, and ρp are evaluated here. Because the uncertainty of V 0 depends on the magnitude of V 0 itself, its evaluation process is shown explicitly only for NIST SRM 1963 particles.
(1) u(V 0)
The uncertainty of V 0 consists of two components: the uncertainty due to measurement errors of the digital multimeter, and that due to voltage variation during the holding time. According to the calibration certificate of the digital multimeter, the measurement accuracy in the voltage range relevant to SRM 1963 particles is 8.0× 10−5 V. The a priori distribution (ISO Guide, 1993) of the true value of V 0 may therefore be assumed to be a uniform distribution with a half width of 8.0× 10−5 V. The corresponding standard uncertainty is then given by
It is expected that the voltage variation partly contributes to a random variation of survival rates, and hence a part of the effects of u VS(V 0) is already accounted for in u(S i ). For safety, however, we include the entire u VS (V 0) in u(V 0). Combining u VM(V 0) and u VS(V 0), we obtain
(2) u(H)
According to the calibration certificates of the gauge blocks used as the electrode spacers, the standard uncertainty of the nominal height of the gauge blocks at 20°C is 0.03 μ m. We may assume that the a priori distribution of the temperature of the gauge blocks during the EAB measurements is a uniform distribution within 20.0 ± 2.0°C. Since the linear thermal expansion coefficient of the gauge blocks is 9.3× 10− 6 K−1, as given in their specifications, the uncertainty associated with the thermal expansion of H is 9.3× 10− 6× 2.0/√3 × H ≅ 0.11 μm. The combined standard uncertainty of the gauge block height including these two components is then
(3) u(g)
It is reported that the gravitational acceleration, g, at the campus in which our experiments were conducted is 9.799492550 m/s2 with a standard uncertainty of 4.6× 10− 8 m/s2 (Mizushima et al. 2002). We may assume that the elevation of our laboratory is within ± 10 m, at most, from that at which the value of g was obtained. Using −2.5 μ Gal/cm (CitationMizushima et al. 2003) as the gradient of g, we evaluate the total uncertainty of the gravitational acceleration at our experiment site as
(4) u(ρ p )
The density of a sodium chloride solution used as a density reference liquid, ρ ref, has an uncertainty associated with the hydrometer that was used to determine ρ ref. From the calibration certificate of the hydrometer, this is given by
The effects of temperature on the density reference liquids and the hydrometer are not corrected, but treated as uncertainty components. The calibration of the hydrometer was performed at temperature T 0 = 20°C. The a priori distribution of the temperature T 1 at which we measured ρref may be assumed to be a uniform distribution within 20 ± 2°C. The uncertainty of ρ ref associated with the temperature difference (T 1− T 0) is then
The resolution of our density measurement is determined by the increment in density of the sodium chloride solutions, which is 0.001 g/cm3. The a priori distribution of ρ p associated with the resolution may be regarded as a uniform distribution with a half width of 0.001 g/cm3, and hence the uncertainty is given by
APPENDIX B: EFFECT OF IMPURITIES IN PSL PARTICLE SUSPENSIONS
In generating an aerosol of PSL particles, first a concentrated PSL particle suspension is diluted with deionized clean water, and then the diluted suspension is atomized to produce particle-carrying droplets. Upon being mixed with clean dry air, the droplets evaporate, and we are left with an aerosol suspending PSL particles. If nonvolatile impurities are contained in the PSL particle suspension, they can increase the apparent mass and size of PSL particles when aerosolized. Let the volumetric concentration of the impurities in the suspension be denoted by C i, and the diameter of the droplets before evaporation by D d. The increase in the diameter D p of the PSL particles after being aerosolized is given by (CitationKinney et al. 1991)
The effect of impurities was investigated in detail by CitationKinney et al. (1991). They examined the changes in diameter of 0.269 μ m PSL particles (NIST SRM 1691) and 0.1 μ m PSL particles that would be expected to occur when the dilution rate of the suspension was varied, but found no systematic changes in the diameters. They state that this result would suggest that impurities are not influencing the measured diameters of the PSL particles.
As an alternative estimate of the impurity effect, CitationKinney et al. (1991) also conducted experiments to evaluate C i and D d, and obtained C i = 6 ppm and D d = 0.9 μm. Substituting these values into Equation (EquationA16), they estimated Δ D p to be 0.15 nm for 100 nm PSL particles. The geometric mean diameter of particle-carrying droplets produced by the same type of aerosol generator as we used in the present study was estimated by CitationKousaka et al. (1989) to be 0.38 μ m. If we adopt D d = 0.38 μ m, and use C i = 6 ppm as given by CitationKinney et al. (1991), Equation (EquationA16) leads to Δ D p = 0.01 nm, or 0.01%, for 100 nm particles, which we can safely neglect.
Another way of examining the increase in particle diameter due to impurities is to examine the effect of the size of particle-carrying droplets that are produced by aerosol generators. The aerosol generator used in the present study is of a pneumatic type, and the typical diameter of droplets it produces is expected to be about 0.38 μ m, as stated above. On the other hand, the TSI model 3480 aerosol generator is of an electrospray type, and according to the specifications of this generator, it produces droplets whose typical size before evaporation is 0.15 μ m. From Equation (EquationA16), Δ D p for 100 nm particles that are aerosolized using these two aerosol generators is expected to differ by a factor of approximately 16. An experiment was conducted to compare D p of PSL particles aerosolized using these two aerosol generators (CitationTakahata and Ehara 2004), but no significant difference was detected.
Based on these investigations, we consider that the impurities in the PSL particle suspensions do not noticeably increase the apparent diameter of the particles after being aerosolized.
APPENDIX C: EFFECT OF THERMOPHORESIS
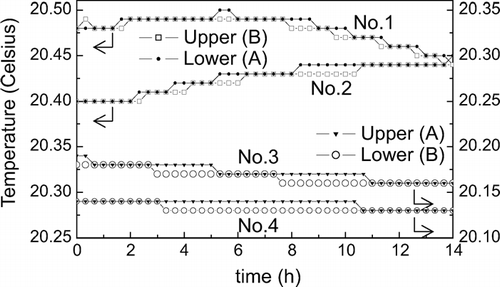
shows examples of the drifts in the temperatures of the electrodes. Thermistors were attached to the back faces of the electrode plates, and the temperatures were recorded every 20 minutes for 14 hours. When thermistors A and B were attached to the lower and upper electrodes, respectively (the curves denoted as No. 1 and No. 2), the temperatures of the two electrodes coincided for roughly half the period, and the upper electrode temperature was apparently higher by 0.01°C, which is the least significant digit of the thermometer used, for the rest of the period. However, when thermistors A and B were exchanged (the curves denoted as No. 3 and No. 4), the temperature of the upper electrode was apparently higher by 0.01°C for roughly half the period. The apparent temperature difference as seen in is therefore likely to originate mainly from a slight difference in the response characteristics of the two thermistors. We did not observe any systematic temperature difference between the electrodes that exceeded the resolution limit of 0.01°C of the thermometer. Note that the temperatures shown in are for the back faces of the electrode plates having a thickness of 25 mm. It is considered that the temperature difference between the two opposing surfaces of the electrodes is somewhat smaller than that seen in , although we cannot know its exact value.
FIG. A1 Examples of temperature drift during the holding time on the back faces of the upper and lower electrodes. In the measurement of the No. 1 and No. 2 curves, thermistors A and B were used for the lower and upper electrodes, respectively, while in the measurements of the No. 3 and No. 4 curves, the two thermistors were exchanged.
It is conceivable that the temperature difference between the electrode surfaces is associated with a temporal drift in the overall temperature within the vessel that accommodates the electrodes. When the vessel temperature is either increasing or decreasing over time, the change in temperature of either one of the electrodes will precede that of the other, if there is a difference in the thermal conductivities along the heat paths. The sign of the temperature difference will accordingly depend on whether the vessel temperature is increasing or decreasing. In the experiment, the overall temperature of the electrodes was found to be increasing for some measurements of the survival rate, and decreasing for others. It is therefore expected that the major effect of thermophoresis will be to cause apparently random variations in the particle survival rates.
Acknowledgments
We would like to thank Ohbakiko Co., Ltd., Shizuoka, Japan, for providing silicon-gel materials as sealants for the electrodes. We would also like to thank JSR Corp., Tokyo, Japan, for providing PSL samples for density measurement. This work was supported in part by the Nanotechnology Material Metrology Project of the New Energy and Industrial Technology Development Organization.
Notes
1The density increment specified in JIS Z8901 is twice this value.
a Indicates whether a differential mobility analyzer was used upstream of the electrodes in the measurement.
b The particle mass calculated from m a = πD pa 3ρp/6.
c Angle-resolved light scattering.
d Since no direct information on the particle density was available, a tentative value of 1.055 g/cm3 was assumed.
e Diameters determined using the tentative value of particle density.
f The particle mass is not affected by the choice of ρp.
g The uncertainty associated with the tentative value of ρp was not included.
2Strictly speaking, the mass represented by the right-hand side of Equation (Equation7) is the mode of the particle mass distribution, and is not exactly equivalent to m a defined by Equation (Equation4). Also the effect of Brownian diffusion is not included in Equation (Equation7). These effects, if taken into consideration, would yield only higher order corrections to u s(m a), and can be neglected here.
3The effect of thermal expansion of particles can appear only in the buoyancy correction and the relaxation time. Because these two factors have only minor effects in EAB measurements, we can neglect the effect of thermal expansion on u(m a) as a higher order correction.
4The slight ρp dependence comes from the fact that m a is not exactly a parameter characterizing the particle mass distribution, but the mass corresponding to D pa.
5The JSR particles measured in the present study are old products. The nominal diameters of the particles currently available from JSR Corp. have been made traceable to the EAB results.
REFERENCES
- Allen , M. D. and Raabe , O. G. 1985 . Slip Correction Measurements of Spherical Solid Aerosol Particles in an Improved Millikan Apparatus . Aerosol. Sci. Technol. , 4 : 269 – 286 . [CSA]
- Brandrup , J. , Immergut , E. H. and Grulke , E. A. , eds. Polymer Handbook, , 4th Ed. , New York : John Wiley & Sons .
- Ehara , K. , Mulholland , G. W. and Hagwood , R. C. 2000 . Determination of Arbitrary Moments of Aerosol Size Distributions from Measurements with a Differential Mobility Analyzer . Aerosol. Sci. Technol. , 32 : 434 – 452 . [CROSSREF] [CSA]
- Ehara , K. , Takahata , K. and Koike , M. 2006 . Absolute Mass and Size Measurement of Monodisperse Particles Using a Modified Millikan's Method. I: Theoretical Framework of the Electro-gravitational Aerosol Balance . Aerosol. Sci. Technol. , 40 ( 7 ) : 514 – 520 . [CSA]
- Germer , T. A. , Mulholland , G. W. , Kim , J. H. and Ehrman , S. H. 2002 . Measurement of the 100 nm NIST SRM 1963 by Laser Surface Light Scattering . Proc. SPIE , 4779 : 60 – 71 . [CSA]
- Hinds , W. C. 1998 . Aerosol Technology, , 2nd Ed. , 173 New York : John Wiley & Sons .
- International Organization for Standardization . 1993 . Guide to the Expression of Uncertainty in Measurement Geneva, , Switzerland
- 1995 . Test powders and test particles , Tokyo : Japanese Standards Association . JIS Z8901
- Jung , K. Y. , Park , B. C. , Song , W. Y. O. , Song , B. -H. and Eom , T. B. 2002 . Measurement of 100-nm Polystyrene Sphere by Transmission Electron Microscope . Powder Technol. , 126 : 255 – 265 . [CROSSREF] [CSA]
- Kinney , P. D. , Pui , D. Y. H. , Mulholland , G. W. and Bryner , N. P. 1991 . Use of the Electrostatic Classification Method to Size 0.1 μ m SRM Particles . J. Res. Natl. Inst. Stand. Technol. , 96 : 147 – 176 . [CSA]
- Kitajima , M. , Fukai , Y. and Ehara , K. Size Determination of Nominal 100 nm PSL Particles by Electro-gravitational Aerosol Balance and Transmission Electron Microscopic Method . Proc. 16th Annual Tech. Meeting on Air Cleaning and Contamination Control . pp. 307 – 310 . in Japanese
- Kousaka , Y. , Okuyama , K. , Shimada , M. and Ohshima , K. 1988 . A Precise Method to Determine the Diameter of Airborne Latex Particles . J. Aerosol Sci. , 19 : 501 – 509 . [CROSSREF] [CSA]
- Kousaka , Y. , Okuyama , K. , Shimada , M. , Ohshima , K. and Hase , T. 1989 . Performance of a Nebulizer for Standard Aerosol Particle Generation . J. Aerosol Res. Japan. , 4 : 294 – 302 . [CSA]
- Knutson , E. O. and Whitby , K. T. 1975 . Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications . J. Aerosol Sci. , 6 : 443 – 451 . [CROSSREF] [CSA]
- Mizushima , S. , Kimura , I. and Hiraoka , Y. 2003 . Comparison of Absolute Gravimeters Between the NMIJ/AIST and the GSJ . AIST Bull. Metrology , 2 : 51 – 53 . [CSA]
- Mulholland , G. W. , Bryner , N. and Croarkin , C. 1999 . Measurement of the 100 nm NIST SRM 1963 by Differential Mobility Analyzer . Aerosol Sci. Technol. , 31 : 39 – 55 . [CROSSREF] [CSA]
- Pugh , T. H. and Heller , W. 1957 . Density of Polystyrene and Polyvinyltoluene Latex Particles . J. Colloid Sci. , 12 : 173 – 180 . [CROSSREF] [CSA]
- Stover , J. C. and Scheer , C. A. 2001 . Accurate Sizing of Deposited PSL Spheres From Light Scatter Measurements . Proc. SPIE. , 4449 : 147 – 150 . [CSA]
- Takahata , K. and Ehara , K. Performance Evaluation of Aerosol Generators for Particles Smaller than 100 nm . Proc. 21st Annual Meeting of Japan Assoc. Aerosol Sci. Technol. pp. 25 – 26 . in Japanese
- Yokochi , A. 1994 . Particle Size Measurements of Polystyrene Latex Standard Particles . Faculty-of-Engineering Bull. , 34 : 159 – 167 . (in Japanese), Tokai Univ.[CSA]