In livestock breeding, high bioaerosol concentrations in environments such as chicken houses are an occupational health concern. Two sampling methods (impinger and filter) and three non-culture methods [epifluoresence microscopy with fluorochrome (EFM/FL), flow cytometry with the fluorochrome (FCM/FL), and fluorescent in situ hybridization (FISH)] were used to monitor the total concentration, viability, and culturability of bioaerosols in chicken houses. These results were compared to the commonly used culture method. Total microbial cell concentrations measured by the non-culture methods averaged about 5 × 10 7 cells/m 3 . However, culture method underestimated bioaerosol concentrations by a factor of 10. For sampler comparison, viability determined following impinger collection was higher than that following filter method. In conclusion, impinger was considered an appropriate sampler, and EFM/FL, FCM/FL, and FISH approaches could adequately assess total microbial cell concentration and viability of bioaerosols in environmental samples.
INTRODUCTION
There are usually higher bioaerosol concentrations in chicken houses, resulting from animal dander, fecal matter, and feed materials (CitationChang et al. 2001). Bioaerosol exposure can cause upper respiratory irritation, chronic bronchitis, organic dust toxic syndrome, and other respiratory symptoms (CitationDonham et al. 2000). In Taiwan, broiler and native chickens are bred in the confinement and open-air style facilities. In confinement-style houses, the culturable concentrations of bioaersols ranged from 104 to 107 CFU/m3 for bacteria, from 102 to 104 CFU/m3 for gram-negative bacteria, and from 102 to 105 CFU/m3 for fungi (CitationBakutis et al. 2004; CitationRadon et al. 2002; CitationSeedorf et al. 1998; CitationVenter et al. 2004). The potentially pathogenic Salmonella spp. had also been detected in lower levels (101 to 102 CFU/m3; CitationVenter et al. 2004). However, culture method could not monitor the bioaerosol concentrations on a real-time basis, before the workers are exposed. Therefore, non-culture methods for real-time monitoring bioaerosols should be considered as an alternative.
In our previous investigation of bioaerosols as measured by real-time methods in Swine Buildings (CitationChi and Li 2005), non-culture methods [epifluorescence microscopy with fluorochrome (EFM/FL) and fluorescent in situ hybridization (FISH)] were compared to a traditional culture method. We found that the total microbial cell concentrations measured by the non-culture methods were 10–200 times higher than those by the culture method. Thus, culture method greatly underestimated the health threat for workers and swine. Therefore, it is necessary to apply non-culture methods to address bioaerosol profiles in chicken farms for the threat to ensure the health of the workers and the public.
MATERIALS AND METHODS
Sampling
We selected six broiler farms (three open-air and three confinement styles) and three native chicken farms (open-air styles) located in Taoyuan and Hsinchu. In native chicken farms, there were two flocks of different ages; 7 weeks (young) and 12 weeks (old). Airborne samples were collected from each of the six broiler and three native farms. Airborne samples were collected by the AGI-30 impinger (Ace Glass, Vineland, NJ) and filter to sample the air 1.5 m above the floor of the central location in each chicken house between 10:00 a.m. and 1:00 p.m. on each sampling day from April to October in 2005. By using direct-reading instruments, the temperature, relative humidity (Sekunden-Hygrometer 601; Testo, Tirana, Albania), wind velocity (VelociCheck, Model 8330; Brandt Instruments, Prairieville, LA), and CO2 concentration (Sibata Scientific Technology Ltd., Bejing, China) were recorded at the same floor height in the central location.
Bioaerosol Sampling and Analysis
Two sampling methods for collecting airborne microbe have been used in chicken farms: impingerment and filtration. Air samples were simultaneously collected with an AGI-30 impinger operated at 12.5 L/min (cut-off diameter: 0.31 μ m) and nuclepore filter (NF; polycarbonate membrane filter, 37-mm diameter, 0.4 μ m pore size; minipore), housed in a three-piece polystyrene cassette (37-mm diameter; SKC) operated at 4 L/min.
Before collection, 20 ml of sterile deionized water containing 1% peptone, 0.01% Tween 80, and 0.005% antifoam A (Sigma-Aldrich, St. Louis, MO) was placed into each autoclaved impinger as described previously (CitationThorne et al. 1992). After a 1-h sampling period, air samples were refrigerated and transported within 3 h to our lab for analysis, which was done on the same day. The final volume of each sample was measured and corrected for evaporation. After shaking, each sample was divided into two portions; one portion was not fixed for culture, FCM/FL, and EFM/FL analyses, while the other portion was fixed for FISH analysis with cold, fresh 4% paraformaldehyde in PBS to avoid rRNA degradation (CitationAmann et al. 1990).
Before sampling, filter and cellulose backing pads were autoclaved and three-piece polystyrene cassettes were sterilized by ethylene oxide. After the 1 h period of air sampling, filters were sealed inside the cassette and, within 3 h, transported to our laboratory at ambient temperature for the same-day analysis. The collected bioaerosols were removed from the filter by placing the filter in a test tube containing 5 mL of sterile deionized water and vortexing the tube for 60 s. Each vortexed suspension was also divided into two portions.
Culture-Based Method
Samples were vigorously shaken. Then, serial 10-fold dilutions (100 μ L) of each sample were prepared using phosphate-buffered saline. Aliquots of each dilution were plated on triplicate trypticase soy agar plates (TSA, Difco, Detroit, MI) for enumeration of bacteria. Each plate was incubated for 24 h at 37°C. As well, aliquots were added to triplicate malt extract agar (MEA, Difco, Detroit, MI) plates for enumeration of fungi. These plates were incubated for 48 h at 25°C (CitationJensen et al. 1992).
Non-Culture-Based Methods
EFM/FL
For EFM/FL, four fluorescent dyes were used. Acridine orange (AO) and 4′, 6-diamino-2-phenylindole dihydrochloride (DAPI) were used to determine total microbial cell concentration, and propidium iodide (PI) and YOPRO-1 were used to enumerate non-viable cells. PI and YOPRO-1 are impermeable, and so stain only non-viable cells. However, PI stains only completely damaged cells, whereas YOPRO-1 can stain partially damaged cells. Because bacteria and fungi cannot be distinguished using EFM/FL, only the total microbial cell concentration (bacteria and fungi), was, obtained using EFM/FL. Based on our previous studies (CitationChen and Li 2005a, Citation2005b), the optimal dye concentrations and incubation time were 50 μ g/mL for 5 min for AO, 15 μ g/mL for 30 min for DAPI, 3 μ M for 15 min for PI, and 25 μ M for 5 min for YOPRO-1. After being individually stained with AO, DAPI, PI, and YOPRO-1, each suspension was filtered through a 0.2 μ m black Isopore membrane filter (Millipore Corporation) and the filter was placed between a slide and a cover slip for observation. The number of biological particles on each filter surface was counted at 1000 × magnification using a Zeiss Axioskop 2 Microscope (Carl Zeiss Inc, Thornwood, NJ) fitted with a halogen 12 V × 100 W lamp and single fluorescence filters. The concentration of microbes in the liquid collection media and in the air was calculated by counting cells in multiple fields of the stained slides and using these values in a previously detailed series of enumerative equations (CitationChi and Li 2005).
FCM/FL
FCM was used to analyze the cell concentration in suspensions labeled with four dyes. AO and SYTO-13 were used to determine total microbial cell concentration, and PI and YOPRO-1 to determine non-viability. Based on our previous studies (CitationChen and Li 2005a, Citation2005b), the concentrations of bacteria and fungi could be calculated using the separated conditions of FCM/FL. Because the detection limits of FCM/FL were 105–108 cells/mL, measurement of low fungi concentrations (< 105 cells/mL) was unreliable. However, such low fungal numbers were typically irrelevant, with most, if not all, of the total microbial load being comprised of bacteria.
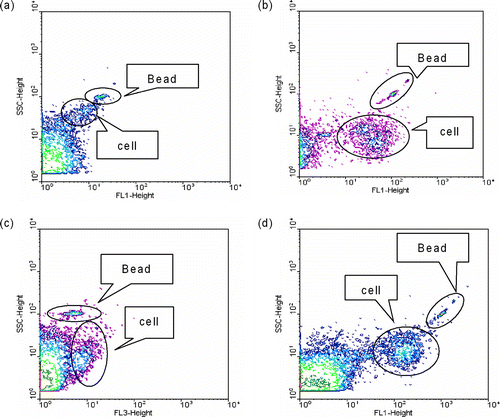
In FCM/FL, the population of bacteria could be clearly discriminated from the background of similar density and from the fluorescent polystyrene beads (). The optimal dye concentrations and incubation time were 5 μ g/ml for 5 min for AO, 2.5 μ M for 5 min for SYTO-13, 3 μ M for 15 min for PI, and 25 μ M for 5 min for YOPRO-1. FCM samples were prepared by mixing 0.5 mL of a stained cell suspension and 5 μ L of a fluorescent bead suspension (8.92 × 107 beads/mL) consisting of monodispersed fluorescein-tagged 1.0-μ m-diameter spherical polystyrene beads (Fluoresbrite; Polyscience, Inc., Warrington, PA). After staining, analysis was immediately done using a FacsCalibur flow cytometer (Becton Dickinson, San Jose, CA) equipped with an air-cooled argon laser (488 nm, 15 mW). Five readings were recorded for each cell: forward scatter (FSC), side scatter (SSC), green fluorescence (FL1, 515 to 545 nm), yellow-orange fluorescence (FL2, 564 to 606 nm), and red fluorescence (FL3, < 670 nm). The fluorescence of AO, SYTO-13, PI, and YOPRO-1 fell into green, green, red, and green fluorescence range, respectively. Samples were analyzed using FCM in triplicate (CitationChen and Li 2005a, Citation2005b).
FIG. 1 Multipapameter contour plots of air samples stained with (a) AO, (b) SYTO-13, (c) PI, (d) YOPRO-1, fluorescent beads, and background. AO and SYTO-13 were used for counting the total concentrations of microorganisms, PI and YOPRO-1 for counting the concentrations of non-viable microorganisms. SSC: side scatter, FL1: green fluorescence (515 to 545 nm), FL3: red fluorescence (< 670 nm).
FISH
Five probes were chosen to determine the total microbial cell concentration, bacteria, fungal, and Pseudomonas spp. Commercially synthesized probes (Bio-Synthesis, Inc., Lewisville, TX) were tagged at the 5′ end with either fluorescein (fl, green) or cy3 (cy, red). The five probe sequences were fl-Univ, fl-EUB, cy-EUK, fl-PSMg, and fl-NotEUB (CitationChi and Li 2005).
The collected bioaerosol sample was fixed with paraformaldehyde. Small portions (3 μ L) of the fixed samples in ethanol-PBS were spread on microscope slides, allowed to air dry, and then progressively dehydrated for 2 min in 50%, 80%, and 95% ethanol. Hybridization conditions were carefully examined as previously described (CitationChi and Li 2005). The total concentration of microbial cells was individually determined by staining with DAPI at 10 μ g/mL for 30 min and then viewing the slide by EFM (CitationAmann et al. 1990). All samples were analyzed in triplicate and the microbial cell concentrations were calculated as previously described (CitationChi and Li 2005).
Viability and Culturability
Viability was determined using suspensions of unfixed samples incubated with various dyes and by FISH examination of suspensions of fixed samples hybridized with various probes. The ability to culture recovered microbial cells was determined using suspensions of unfixed samples incubated on agar. Viability was calculated as described below.
Statistical Analyses
Data analysis was done using Excel (Windows XP, Version 7.0; Microsoft Corporation, Redmond, WA) and SigmaPlot (Windows, Version 3.06, SPSS, Chicago, IL). The differences between impinger (n = 12) and filter (n = 12) samples were assessed using Wilcoxon signed-rank test, as well as the differences between the two microbial species (culturable concentration of bacterial and fungal), two dyes (AO and DAPI, or PI and YOPRO-1) and two indicators (viability and culturability) (n = 24, impinger and filter). The differences between the concentrations in the open-air type (n = 6) and confinement type (n = 6) of broiler chicken houses, as well as those between old (n = 6) and young (n = 6) chicken houses in native chicken farms were assessed using the Mann-Whitney test. The relationships between viability as determined by the culture-based method, EFM/FL, FCM/FL, and FISH, as well as the relationships between the bioaerosol concentrations and environmental factors were analyzed by linear regression.
RESULTS AND DISCUSSION
In this study, the non-culture methods of EFM/FL, FCM/FL, and FISH were used to monitor the total concentration and viability of bioaerosols in chicken farms in Taiwan. In addition, the sampling effects of impinger were compared to a filter by these non-culture methods. The results were compared to a standard culture-based method.
Bioaerosol Characteristics
Total and Viable Concentrations of Bioaerosol
Bacterial concentrations measured by the culture method averaged 3.13 × 106 CFU/m3, a value that agrees with previous reports from Northern Europe (CitationSeedorf et al. 1998), Switzerland (CitationRadon et al. 2002), and Lithuania (CitationBakutis et al. 2004) (). Fungal average concentrations were 7.70 × 103 CFU/m3, which compares favorably with values from studies based in Northern Europe (CitationSeedorf et al. 1998) and Switzerland (CitationRadon et al. 2002). Significantly, the bacteria concentrations were much higher than the fungal concentrations (about 100 to 1000 times, p = 0.002). In addition, the concentrations of culturable bioaerosols were 30 times higher for bacteria and six times higher for fungi than those reported previously in swine buildings in Taiwan (CitationChi and Li 2005) (p < 0.05), which are consistent with reports from facilities in Northern Europe (CitationSeedorf et al. 1998) and Switzerland (CitationRadon et al. 2002).
TABLE 1 Total concentration and viability of bioaerosols in chicken houses by EFM/FL, FCM/FL, FISH, and culture method
By the non-culture methods (), the total microbial cell concentrations ranged from 5.54 × 106 to 1.58 × 108 cells/m3 with various dyes staining. Viable microbial concentrations as determined by EFM/FL and FCM/FL represented average levels of 1.56 × 107 cells/m3 with PI staining and 1.06 × 107 cells/m3 with YOPRO-1 staining. The total and viable microbial cell concentrations were 3.4 to 4.5 and 2.1 to 4.2 times higher than values measured in swine buildings in Taiwan, respectively (p < 0.05) (CitationChi and Li 2005). The significant differences in total concentrations between chicken and swine facilities in Taiwan echo with results from facilities in Switzerland (CitationRadon et al. 2002). The higher observed bioaerosols in chicken houses might be related to longer cleaning intervals and higher breeding density (CitationRadon et al. 2002). The differences of bioaerosol concentrations between chicken and swine facilities were larger by the culture method than those by the non-culture methods. This observation might be associated with higher temperature (27°C in chicken houses versus 20°C in swine buildings) and the generally darker environment of a chicken house (where plastic curtains cover both sides of the facility). A prolonged viability of microbes in the closed environment of the chicken could also reflect the protection from solar radiation (CitationHoerter et al. 2005; CitationHughes 2003). Therefore, our findings strongly confirm that the culture method is biased because growth conditions are never suitable for obtaining visible growth of all microorganism species present in the environmental sample.
Culturability and Viability of Bioaerosols
Viability of microbial cells was determined by EFM and FCM using suspensions of unfixed samples incubated with various dyes which stain non-viable cells, and by FISH using suspensions of paraformaldehyde-fixed samples hybridized with various probes. The ability to recover viable microbial cells upon culture was determined using suspensions of unfixed samples incubated on agar.
To our knowledge, the present evaluation of the viability of bioaerosols in chicken houses by EFM/FL, FCM/FL, and FISH is unique. The viability of bioaerosols in the chicken houses averaged 0.44 for PI staining, 0.29 for YOPRO-1 staining, 0.39 for FISH, and 0.09 for culturing (). As assessed by EFM/FL, the microbial viability in chicken houses was 0.8 times of those in swine buildings in Taiwan, a value that was insignificant (p > 0.05) (CitationChi and Li 2005). In contrast, FISH-based viability determinations revealed a significant difference in viability as compared to swine facilities (p < 0.05) (CitationChi and Li 2005). The stage of microorganism growth is an important variable for FISH, because incubation with the FISH probe does not permit microbial cells in post-log or stationary-phase growth to be distinguished from background fluorescence emissions (CitationLange et al. 1997). Therefore, longer cleaning intervals in chicken houses might result in bioaerosols containing microbes that have entered a quiescent state; their rRNA content might be insufficient for FISH hybridization, which detects microbial cells in the log-phase of growth (CitationAmann et al. 1995; CitationLange et al. 1997). Finally, the culture method estimated the bioaersol concentrations in chicken houses as 7.5 times higher than those in swine buildings in Taiwan (p < 0.05). In conclusion, these present and previous observations indicated that there were more culturable bioaerosols in chicken houses than those in swine buildings.
Non-Culture Method Intercomparisons
Total Concentrations of Bioaerosols
By using EFM/FL, the concentrations of AO-stained microorganisms were higher than those of DAPI-stained (p = 0.003, ). One possible explanation might be that the staining of AT-rich within the minor groove of B-DNA underestimates microorganisms containing richer CG base-pairs (CitationSaby et al. 1997). The higher microorganism concentrations observed by FCM/FL-AO staining as compared to SYTO-13 staining might be related to the failure AOFCM staining to distinguish microorganisms from the background, yielding an overestimate the total microbial bioaerosols (CitationChen and Li 2005). The total concentrations of AO-stained microorganisms determined by FCM/FL were 1.5 times higher than those by EFM/FL (p < 0.05), a result similar to that reported previously (CitationChen and Li 2005a). This result might be resulted from an overestimation of FCM/FL and underestimation of EFM/FL. The latter one might be explainable by loss of cells in the handling steps such as the filtration of stained samples, mounting of the filter onto the glass slide, and cover slip application (CitationHenningson et al. 1997). In summary, FCM/FL has rapid counting ability and lower propensity for handling-related problems. For EFM/FL and FISH, there was no significant difference in the total microbial cell concentrations of DAPI-stained samples (p > 0.05), as reported in a study of swine facilities (CitationChi and Li 2005). The total microbial cell concentrations by the non-culture methods were 5–50 times higher than those determined by the culture method (p < 0.000 by EFM, FCM, and FISH), clearly indicating that the use of growth in determining bioaerosol concentrations in chicken houses underestimates the actual number of viable cells.
Culturability and Viability of Bioaerosols
There were no differences in the viability of bioaerosols by PI staining and YOPRO-1 staining between ECM/FL and FCM/FL (p > 0.05) (). The viability determined by PI staining was 1.5 times higher than that evidence upon YOPRO-1 staining, using either EFM/FL or FCM/FL (p < 0.05). This result can be explained by the tendency of YOPRO-1 to stain partially damaged cells, whereas PI stains only completely damaged cells (CitationKaneshiro et al. 1993; CitationGlazer and Rye 1992). In agreement with previous studies (CitationChi and Li 2005; CitationRadon et al. 2002), the viability determined by EFM/FL, FCM/FL, and FISH was much higher than the viability based on culturing (p < 0.000).
Comparison of Bioaerosol Sampling Methods
This study is the first to evaluate impinger and filter sampling methods in chicken houses through the use of culture-based and non-culture-based assessments of viability. There was no difference in total concentrations measured by non-culture methods between the two samplers (p > 0.05, ). One explanation is that the non-culture methods detect both viable and non-viable microorganisms. The culturable concentrations of total microbial cells, bacteria, and fungi had significant differences between impinger and filter (p < 0.05, ). Filter recovery significantly underestimated the culturable concentrations compared with impinger sampling (64% for bacteria, 32% for fungi), similar to a previous study (CitationLi et al. 1999). Based on the results of viability, the viability and culturability of bioaerosols as measured by impinger were 1.3 to 3.8 times higher than those by filter sampling (p < 0.05; ), which agree with our previous investigation (CitationChen and Li 2005c). The poor recovery of filtration might be a consequence of higher method-associated sampling stress, dehydration by the dry filter medium, as well as extracting effects of the filter (CitationLi et al. 1999).
TABLE 2 Total concentration of bioaerosols in chicken houses by impinger and filter
TABLE 3 Viability of bioaerosols in chicken houses by impinger and filter
By using non-culture methods, our findings indicated that the two samplers can obtain the same total concentration but different viability due to sampling stress (CitationChen and Li 2005c). By using both impinger and filter, the total microbial concentration and viability in bioaerosols determined by non-culture methods were much higher than those by culture methods. Thus, the non-culture methods provide a more accurate bioaerosol profile for exposure and health assessment. The comparison of data derived from the culture and non-culture methods indicates that the impinger is preferable to filter for assessing total and viable bioaerosols.
Comparisons of Bioaerosols in Confinement and Open-Air Style Facilities, and the Influence of Chick Age
The highest culturable concentration (5.79 × 106 CFU/m3) was found in open-air broiler farms (). Use of both culture-based and non-culture methods revealed that total microbial concentration and viability were lower in the confinement facilities as compared to open-air style houses (p > 0.05). One explanation relates to the higher wind velocity in the enclosed space due to the use of continuous mechanical ventilation. In the native chicken farms (open-air style), no significant differences in the total concentrations and viability were evident between young and older populations (p > 0.05) (), perhaps reflecting the generally adequate ventilation in the open-air style houses. Environmental factors varied widely between facilities; temperature ranged from 20.2 to 31.0°C, wind velocity from 0.05 to 2.45 m/s, relative humidity from 54.7 to 83.7 %, and CO2 from 400 to 1050 ppm. Nonetheless, neither the bacterial nor fungal concentrations were correlated with environmental factors, consistent with previous reports (CitationChang et al. 2001; CitationChi and Li 2005).
TABLE 4 Total concentration and viability of bioaerosols in broiler houses by EFM/FL, FCM/FL, FISH, and culture method
TABLE 5 Total concentration and viability of bioaerosols in native chicken houses by EFM/FL, FCM/FL, FISH, and culture method
Bioaerosol Community Structure
By using different probes, FISH provides information on bioaerosol community structure by revealing the identity and viability of microorganisms in environmental samples (CitationChi and Li 2005; CitationKenzaka et al. 1998; CitationOkabe et al. 2003; CitationYang and Zeyer 2003; CitationNeef et al. 2003). In our current study, fl-Univ, fl-EUB, cy-EUK, and fl-PSMg probes hybridized to viable microbes, eubacteria, eukaryotes, and Pseudomonas spp., respectively. The percentage of specific viable microbial cells in the total (viable and non-viable) microbial cells averaged 39.29% for fl-Univ, 31.29% for fl-EUB, 1.28% for cy-EUK, and undetectable for fl-PSMg (). These values were significantly lower (p < 0.05) than those obtained in a previous study of swine facilities (CitationChi and Li 2005). As previously mentioned, the differences in the FISH determinations between the chicken and swine facilities may reflect longer cleaning intervals, which result in quiescent microbial cells and insufficient rRNA content for FISH hybridization. The percentage of viable eubacteria in the viable microorganisms, as determined by fl-Univ probe, ranged from 65.47 to 92.29%, with an overall average of 78.44% (data not shown). This indicates that eubacteria is the predominant component of bioaerosols in the chicken houses, in agreement with results from the culture-based method. The absence of Pseudomonas spp. in bioaerosols from chicken farms corroborates previous reports (CitationSeedorf et al. 1998; CitationRadon et al. 2002). However, high numbers of Pseudomonas spp. can be present in swine facilities (CitationChi and Li 2005). This result might be the practice of water washing of floors in the latter buildings, which presents a suitable environment for Pseudomonads spp. to grow. The occurrence of skin injuries in the swine population provide s more opportunity for Pseudomonas-related infections (CitationGreen et al. 1974).
TABLE 6 Bioaerosol community structure of chicken houses as percentages (%) of the total microorganisms (includes both viable and non-viable cells) by FISH
Presently, the use of FISH in conjunction with multiple probes permitted an accurate qualitative and quantitative representation of the viable microbial composition of environmental bioaerosol samples for chicken houses. Future research will involve using FISH to gain information on specific pathogens such as Legionella.
CONCLUSIONS
This was the first study to successfully apply three non-culture methods, EFM/FL, FCM/FL, and FISH, to assess the total concentration and viability of bioaerosols and sampling effects of impinger and filter in chicken houses. The total concentrations and viability of bioaerosol estimated by non-culture methods were much higher than those by the traditional culture method. For sampler comparisons, impinger is considered an appropriate sampler for assessing bioaerosol profiles. In conclusion, culture method seriously underestimates the microorganism concentration, and EFM/FL, FCM/FL, and FISH techniques can rapidly determine the viability based on membrane integrity and rRNA content, and precisely assess the bioaerosol exposure and consequent health effects of occupational worker.
Acknowledgments
This work was partially supported by grant NSC 93-2320-B-002-003 from the National Science Council, Republic of China. Miao-Ching Chi was supported by a graduate scholarship from the grant during part of this research effort.
Notes
a Mean (range).
b Significantly different from EFM (AO or DAPI), FCM (AO or SYTO-13), and FISH (p < 0.05).
c Significantly different from EFM (DAPI) and FCM (AO or SYTO-13) (p < 0.05).
d Significantly different from FCM (AO or SYTO-13) (p < 0.05).
e Significantly different from FCM (SYTO-13) (p < 0.05).
f Significantly different from EFM (YOPRO) and FCM (YOPRO) (p < 0.05).
g Significantly different from FCM (PI) (p < 0.05).
h Significantly different from FCM (YOPRO) (p < 0.05).
a Mean (range).
b Significantly different from NF sampling method (p < 0.05).
a Mean (range).
b Significantly different from NF sampling method (p < 0.05).
a Mean (range).
a Mean (range).
a Percentage of active cells in the total cells = {(active microbial cell concentration by FISH with different respective probes)/(total microbial cell concentration by DAPIFISH)} × 100.
b Mean (range).
REFERENCES
- Amann , R. I. , Binder , B. J. , Olson , R. J. , Chisholm , S. W. , Devereux , R. and Stahl , D. A. 1990 . Combination of 16S rRNA-targeted Oligonucleotide Probes with Flow Cytometry for Analyzing Mixed Microbial Populations . Appl. Environ. Microbiol. , 56 : 1919 – 1925 . [INFOTRIEVE] [CSA]
- Amann , R. I. , Ludwig , W. and Schleifer , K. H. 1995 . Phylogenetic Identification and in Situ Detection of Individual Mmicrobial Cells without Cultivation . Microbiol Rev. , 59 ( 1 ) : 143 – 169 . [INFOTRIEVE] [CSA]
- Bakutis , B. , Monstviliene , E. and Januskeviciene , G. 2004 . Analyses of Airborne Contamination with Bactyeria, Endotoxins and Dust in Livestock Barns and Poultry Houses . Acta Vet. Brno. , 73 : 283 – 289 . [CSA]
- Chang , C. W. , Chung , H. , Huang , C. F. and Su , H. 2001 . Exposure of Workers to Airborne Microorganisms in Open-air Swine Houses . J. Appl Environ Microbiol. , 67 ( 1 ) : 155 – 161 . [CSA] [CROSSREF]
- Chen , P. S. and Li , C. S. 2005a . Real-Time Quantitative PCR with Gene Probe, Fluorochrome, and Flow Cytometry for Bioaerosol Analysis . J. Envirn. Monit. , 7 ( 3 ) : 257 – 262 . [CSA] [CROSSREF]
- Chen , P. S. and Li , C. S. 2005b . Bioaerosol Characterization by Flow Cytometry with Fluorochrome . J. Envirn. Monit. , 7 ( 10 ) : 950 – 959 . [CSA] [CROSSREF]
- Chen , P. S. and Li , C. S. 2005c . Sampling Performance of Impingement and Filtration for Bioaerosols by Viability using Fluorochrome and Flow Cytometry . Aerosol Sci. Tech. , 39 ( 2 ) : 1 – 7 . [CSA] [CROSSREF]
- Chi , M. C. and Li , C. S. 2005 . Fluorochrome and Fluorescent In Situ Hybridization to Monitor Bioaerosols in Swine Buildings . Aerosol Sci. Tech. , 39 : 1101 – 1110 . [CSA] [CROSSREF]
- Donham , K. J. , Cumro , D. , Reynolds , S. J. and Merchant , J. A. 2000 . Dose-response Relationships between Occupational Aerosol Exposures and Cross-shift Declines of Lung Function in Poultry Workers: Recommendations for Exposure Limits . J. Occup. Environ. Med. , 42 ( 3 ) : 260 – 269 . [INFOTRIEVE] [CSA]
- Glazer , A. N. and Rye , H. S. 1992 . Stable dye-DNA Intercalation Complexes as Reagents for High Sensitivity Fluorescent Detection . Nature , 359 : 859 – 861 . [INFOTRIEVE] [CSA] [CROSSREF]
- Green , S. K. , Schroth , M. N. , Cho , J. J. , Kominos , S. K. and Vitanza-jack , V. B. 1974 . Agricultural Plants and Soil as a Reservoir for Pseudomonas aeruginosa . Appl Microbiol. , 28 ( 6 ) : 987 – 991 . [INFOTRIEVE] [CSA]
- Henningson , E. , Lundquist , M. , Larsson , E. , Sandström , G. and Forsman , M. 1997 . A Comparative Study of Different Methods to Determine the Total Number and the Survival Ratio of Bacteria in Aerobiological Samples . J Aerosol Sci. , 28 : 459 – 469 . [CSA] [CROSSREF]
- Hoerter , J. D. , Arnold , A. A. , Kuczynska , D. A. , Shibuya , A. , Ward , C. S. , Sauer , M. G. , Gizachew , A. , Hotchkiss , T. M. , Fleming , T. J. and Johnson , S. 2005 . Effects of Sublethal UVA Irradiation on Activity Levels of Oxidative Defense Enzymes and Protein Oxidation in Escherichia coli . J Photochem Photobiol B. , 81 ( 3 ) : 171 – 180 . [INFOTRIEVE] [CSA] [CROSSREF]
- Hughes , K. A. 2003 . Influence of Seasonal Environmental Variables on the Distribution of Presumptive Fecal Coliforms around an Antarctic Research Station . Appl. Environ. Microbiol. , 69 ( 8 ) : 4884 – 4891 . [INFOTRIEVE] [CSA] [CROSSREF]
- Jensen , P. J. , Todd , W. F. , David , G. N. and Scarpino , P. V. 1992 . Evaluation of Eight Bioaerosol Samplers Challenged with Aerosols of Free Bacteria . Am. Ind. Hygi. Assoc. J. , 53 : 660 – 667 . [CSA]
- Kaneshiro , E. S. , Wyder , M. A. , Wu , Y. P. and Cusion , M. T. 1993 . Reliability of Calcein Acetoxy Methyl Ester and Ethidium Homodimer or Propidium Iodide for Viability Assessment of Microbes . J. Microbiol. Methods , 17 : 1 – 16 . [CSA]
- Kenzaka , T. , Yamaguchi , N. , Tani , K. and Nasu , M. 1998 . rRNA-targeted Fluorescent in Situ Hybridization Analysis of Bacterial Community Structure in River Water . Microbiology. , 144 : 2085 – 2093 . [INFOTRIEVE] [CSA]
- Lange , J. L. , Thorne , P. S. and Lynch , N. 1997 . Applications of Flow Cytometry and Fluorescent in Situ Hybridization for Assessment of Exposure to Airborne Bacteria . Appl. Environ. Microbiol. , 63 : 1557 – 1563 . [INFOTRIEVE] [CSA]
- Li , C. S. , Hao , M. L. , Lin , W. H. , Chang , C. W. and Wang , C. S. 1999 . Evaluation of Microbial Samplers for Bacterial Microorganisms . Aerosol Sci. Tech. , 30 : 100 – 108 . [CSA] [CROSSREF]
- Neef , A. , Schafer , R. , Beimfohr , C. and Kampfer , P. 2003 . Fluorescence Based rRNA Sensor Systems for Detection of Whole cells of Saccharomonospora spp. and Thermoactinomyces spp . Biosens Bioelectron. , 18 ( 5–6 ) : 565 – 569 . [INFOTRIEVE] [CSA] [CROSSREF]
- Okabe , S. , Ito , T. and Satoh , H. 2003 . Sulfate-reducing Bacterial Community Structure and Their Contribution to Carbon Mineralization in a Wastewater Biofilm Growing under Microaerophilic Conditions . Appl. Microbiol. Biotechnol. , 63 ( 3 ) : 322 – 334 . [INFOTRIEVE] [CSA] [CROSSREF]
- Radon , K. , Danuser , B. , Iversen , M. , Monso , E. , Weber , C. , Hartung , J. , Donham , K. , Palmgren , U. and Nowak , D. 2002 . Air Contaminants in Different European Farming Environments . Ann. Agric. Environ. Med. , 9 ( 1 ) : 41 – 48 . [INFOTRIEVE] [CSA]
- Saby , S. , Sibille , I. , Mathieu , L. , Paquin , J. L. and Block , J. C. 1997 . Influence of Water Chlorination on the Counting of Bacteria with DAPI (4′,6-diamidino-2-phenylindole) . Appl. Environ. Microbiol. , 63 : 1564 – 1569 . [INFOTRIEVE] [CSA]
- Seedorf , J. , Hartung , J. , Schröder , M. , Linkert , K. H. , Pedersen , S. , Takai , H. , Johnsen , J. O. , Metz , J. H. M. , Groot Koerkamp , P. W. G. , Uenk , G. H. , Phillips , V. R. , Holden , M. R. , Sneath , R. W. , Short , J. L. , White , R. P. and Wathes , C. M. 1998 . Concentrations and Emissions of Airborne Endotoxins and Microorganisms in Livestock Buildings in Northern Europe . J. Agric. Engng. Res. , 70 ( 1 ) : 97 – 109 . [CSA] [CROSSREF]
- Thorne , P. S. , Kiekhaefer , M. S. , Whitten , P. and Donham , K. J. 1992 . Comparison of Bioaerosol Sampling Methods in Barns Housing Swine . Appl. Environ. Microbiol. , 58 : 2543 – 2551 . [INFOTRIEVE] [CSA]
- Venter , P. , Lues , J. F. and Theron , H. 2004 . Quantification of Bioaerosols in Automated Chicken Egg Production Plants . Poult Sci. , 83 ( 7 ) : 1226 – 1231 . [INFOTRIEVE] [CSA]
- Yang , Y. R. and Zeyer , J. 2003 . Specific Detection of Dehalococcoides Species by Fluorescence In Situ Hybridization with 16S rRNA-targeted Oligonucleotide Probes . Appl. Microbiol. Biotechnol. , 69 ( 5 ) : 2879 – 2883 . [CSA]