Abstract
A new instrument to study ice nucleation, the Zurich Ice Nucleation Chamber (ZINC), has been constructed. It is a continuous flow diffusion chamber following the design by CitationRogers (1988) but has a flat parallel plate geometry. The instrument can operate at temperatures as low as 236 K with the current setup with ice supersaturations of up to 50%. The typical sample flow is 1 lpm with a total flow of 10 lpm using twice 4.5 lpm for sheath flows. FLUENT simulations were performed and are presented to discuss the flow, temperature, and humidity profiles within the main chamber. Activation experiments with silver iodide particles were used to validate the instrument against literature data. We report the onset of freezing for an activated fraction of 2% of all particles. The data exhibit an almost linear trend between 257 K (111.5% RHi) and 237 K (119% RHi) with very good agreement with literature data.
1. INTRODUCTION
In the late 19th century, Coulier and Aitken recognized that aerosol particles are a prerequisite for fog and cloud formation (CitationSpurny 2000) and are thus named cloud condensation nuclei (CCN). Later it was found that the same applies to the formation of ice crystals in mixed phase and ice clouds where the aerosols in question are named ice nuclei (IN). While there is now a good understanding of particle properties that affect CCN activation, there is still a lack of understanding as to which intrinsic properties of aerosol particles determine their ice nucleation capabilities (CitationSzyrmer and Zawadzki 1997; CitationCantrell and Heymsfield 2005). While ice nucleation was studied intensely in the 1970s in the scope of weather modification programs (CitationBruintjes 1999; CitationFederer et al. 1986; CitationWilloughby et al. 1985), contemporary interest focuses more on the climate impact of natural and anthropogenic ice nuclei through the so-called aerosol indirect effect. The indirect effect traditionally refers to an increase in cloud droplets due to anthropogenic aerosols (CitationBaker 1997). Lately, also the effect of aerosol particles on mixed-phase and cirrus clouds have been addressed (CitationLohmann 2002; IPCC 2007).
One excellent tool to address this problem and to increase our understanding of ice forming processes in atmospheric clouds is a Continuous Flow Diffusion Chamber (CFDC), which was originally designed for ice nucleation studies by CitationRogers (1988). Among the different modes of ice nucleation that occur in the atmosphere (CitationRogers and Yau 1989), this type of instrument is mainly designed to study deposition nucleation but is also capable to measure condensation freezing and to some extend homogeneous freezing at temperatures below −35°C. This design has been enhanced continuously and was successfully deployed in many field campaigns over the last years (DeMott et al. 2003a; DeMott et al. 2003b; CitationChen et al. 1998). However, it bears a few drawbacks that motivated a completely new design which has been developed at the cloud physics group at ETH Zurich over the last two years. This instrument, the Zurich Ice Nucleation Chamber (ZINC) has been finished recently. It has been used successfully for laboratory experiments with silver iodide particles to demonstrate its capabilities as described below. This article discusses the new design concept of the ZINC instrument and highlights some improvements over the previously described instruments. It should be noted that the biggest change we made, the move from a concentric cylinder geometry to a flat parallel plate design, will be exploited to its full extend when the new in-situ detectors that are being developed currently for the ZINC instrument are ready and operable.
2. GENERAL FUNCTIONAL PRINCIPLE OF A CFDC TYPE IN COUNTER
An ice nucleus counter of the CFDC type consists of the following main parts: a main chamber where the aerosols are exposed to an environment with a defined temperature and supersaturation with respect to ice, an optional evaporation section, and a detector that counts the ice crystals that have formed in this chamber. Furthermore, electronics are needed to control the chamber settings and acquire data. These parts will now be discussed in further detail.
2.1. Main Chamber
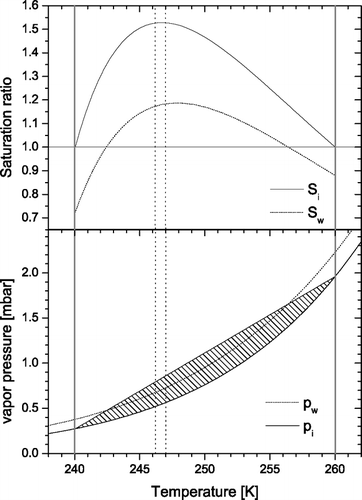
One of the challenges in constructing an IN counter is to maintain a defined supersaturation with respect to ice in which the aerosols under investigation can form ice crystals and then grow to sizes which are detectable. In a CFDC type instrument this is achieved in the following way: two opposing walls are cooled and held at different temperatures below the melting point of water. These walls are then covered with a thin layer of ice (please refer to the experimental section for details about the icing process). Above these ice layers a constant water vapor partial pressure is maintained according to the Clausius Clapeyron law. In steady state, diffusion of water vapor and heat leads to a linear gradient in the water vapor partial pressure and the temperature between the two walls. Because of the exponential relationship between temperature and saturation vapor pressure this leads to a supersaturation between the two walls with a maximum close to the center of the chamber. This is illustrated in . The aerosols under investigation are then exposed to this environment by pumping the particle loaded sample air through the chamber. The sample air is layered between two particle-free sheath flows and is typically set to 1/10th of the total flow to constrain the variance in temperature and humidity which the aerosol particles are exposed to. An example configuration is depicted in . The two walls are held at 240 K and 260 K, respectively. The hatched area indicates the supersaturation with respect to ice. In this example, a significant region is even supersaturated with respect to water (the hatched area above the water saturation line). The vertical dotted lines indicate the region of the chamber where the aerosols will be positioned at the flow conditions described above. Once an ice crystal has formed in the main chamber it grows to larger sizes until it reaches the exit of the main chamber where ice supersaturation which is maintained throughout the main chamber drops to zero.
FIG. 1 The relationship between temperature and saturation vapor pressure with respect to water (dotted line) and ice (solid line) as expressed by the Clausius Clapeyron law (lower panel). The straight line illustrates the steady state water vapor pressure which results from the diffusion of water vapor and heat between the two ice-coated walls of the main chamber (thick gray lines). In this example these are held at 240 K and 260 K, respectively. The resulting supersaturation is indicated by the hatched area and plotted as saturation ratios with respect to water (Sw) and ice (Si) in the upper panel. It can be seen that the whole chamber is supersaturated with respect to ice and a significant region (242–256 K) is also supersaturated with respect to water under these conditions. Dotted vertical lines denote the boundaries of the sample layer under standard flow conditions (sample: 1 lpm, sheath flows: 4.5 lpm each).
2.2. Ice Particle Detector
The ice crystals that form in the main chamber have to be detected after exiting the chamber. The important task of this detector is not only to count the ice crystals but also to distinguish them from dry aerosol particles and water droplets. The latter may form by CCN activation if the chamber is operated at humidities above water saturation (as in the example configuration illustrated in ). In this case, the detector needs to be able to distinguish between water droplets and ice crystals. The droplets can be evaporated prior to detection (see section 3.3) in a special chamber section. Other possibilities to differentiate water droplets and ice crystals are detectors that measure not only the intensity of the light which is scattered by a particle but also properties which are related to the shape of a particle, e.g., the 2D pattern of scattered light or the depolarization ratio (CitationHirst et al. 2001; CitationLiou and Lahore 1974; CitationNicolet et al. 2007).
3. DESIGN OF THE ZURICH ICE NUCLEATION CHAMBER
The ZINC instrument is an ice nucleus counter which has been designed in the cloud physics group at ETH Zurich. While the overall principle of the ZINC follows the concept of the Colorado State University (CSU) CFDC developed by CitationRogers (1988) we made some modifications and improvements to the existing design. Prominently, this is the move from the concentric cylinders geometry to a flat parallel plate design which has previously only been used for CCN instruments, e.g., CitationKumar et al. (2003) but not for IN counters. This geometry has some important implications and advantages for different parts of the chamber (e.g., cooling system, detector, inlet design). These will be discussed in detail in the following sections. In general, the new geometry allowed us to use aluminum as a constructing material for almost all parts of the chamber. Aluminum was chosen for its hardness while having a low specific weight and very good heat conductivity. All aluminum parts were anodized to increase chemical resistance against the cooling liquid and the deionized water, which is used for icing. In comparison to the copper tubes used in the CSU-CFDC the aluminum surfaces were sand blasted and anodized. The resulting surface showed a good wettability and thus a good cohesion of the ice layer. This avoids the complicated and toxic procedure of ebonizing, which is necessary to make the copper surfaces wettable. It has been reported that false ice nuclei can be generated by a loosening of the ebonizing layer on the copper walls in previous instruments (CitationCziczo et al. 2003). This is avoided with the sand-blasted and anodized aluminum which has a mechanically much harder surface.
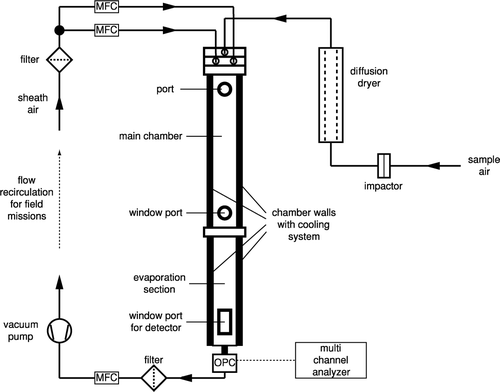
The whole setup of the ZINC instrument is depicted in . The sheath flows and the outgoing flow after the particle detector are regulated with mass flow controllers (MKS Type 1179B). The sample flow is calculated through the difference of the total outgoing flow minus the two sheath flows. The sampled air is drawn through a custom made diffusion dryer prior to entering the inlet. This reduces the humidity in order to avoid transient supersaturations upon cooling to the chamber temperature. Clogging of the inlet tubes due to condensation to the walls of the inlet section is also minimized in this way.
3.1. The Inlet Section
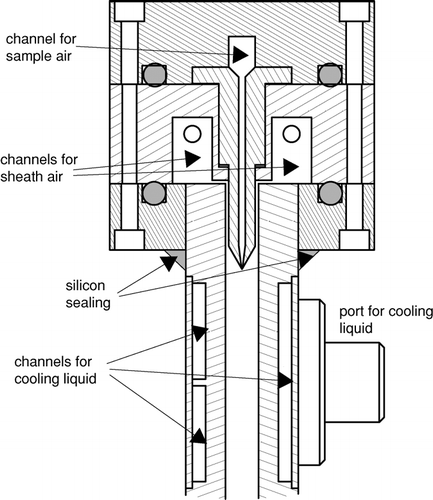
The inlet section of the ZINC instrument consists of three aluminum flanges which together form channels for the sample flow and the two sheath flows (cf. ). The uppermost flange contains a channel with two attached blades which distribute the sample flow almost over the whole width of the chamber and direct the flow downwards into the center of the main chamber. While the main chamber is 300 mm wide the width of the exit slit for the sample flow is only 270 mm. The 15 mm gap between sample flow and side walls on both sides was chosen to avoid particles to be exposed to regions where the flow and the humidity profiles deviate too much from the uniform conditions in the rest of the chamber by the influence of the side walls. The optimum width of the gap was found by analyzing humidity and flow profiles obtained by FLUENT simulations of the chamber. The second flange of the inlet section has two channels for the sheath flows which are fed with filtered pressurized air from both sides to ensure an even distribution over the whole chamber. The thin gaps between the channels and the main chamber fulfill the same purpose by providing a small pressure drop for the sheath flows. The sheath flow channels are in direct contact with the cooled plates forming the main chamber and are thus pre-cooled in an efficient manner. FLUENT simulations showed that even the sample flow is pre-cooled in the inlet section through heat conduction. Both sheath flows are regulated by mass flow controllers so that it is even possible to operate the ZINC with asymmetric flow settings for special purposes (e.g., quick changes in supersaturation). As is indicated in the incoming sample flow passes through a diffusion dryer to reduce the humidity. This will reduce clogging of the inlet section through condensation of water vapor to the walls.
FIG. 3 Cross section through the head and upper part of the main chamber of ZINC. The head consists of three flanges that form together the channels for the sample and sheath air flows. The sample flow is guided into the center of the main chamber by two blades. The walls of the main chamber contain channels and ports for the cooling liquid.
3.2. The Main Chamber
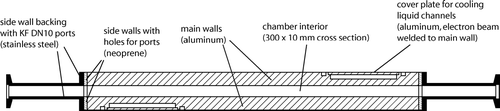
Two opposing aluminum plates 1000 × 300 mm form the main chamber. Channels for the cooling liquid are drilled into these plates from the outer surface. Thin aluminum plates cover the channels and are fixated to the plates by electron beam welding to minimize the deformation of the plates during welding. Each of the main walls is cooled individually by a bath cryostat (Lauda, RP 845C and RP 890C) using ethanol as a cooling liquid. For direct temperature measurements, three thermocouples (Type K) are attached to each wall in between the cooling channels. One is placed on the upper edge close to the inlet flange, one in the middle and one 100 mm above the lower connecting flange. The gap between the walls of the chamber is 10 mm and the side walls are made out of neoprene backed up with stainless steel plates for stability. Each side wall has two ports welded to 10 mm tubes terminated with KF DN10 flanges to connect sensors as well as windows for inspection of the interior of the chamber during experiments (cf. ). The tubes are slightly tilted upwards towards the outside to allow water to drain out of the ports entirely after the icing process.
FIG. 4 Horizontal cross section through the main chamber at the position of the ports on the side walls. The chamber interior has a cross section of 300 × 10 mm and is circumscribed by the cooled aluminum plates and neoprene side walls which are backed up by stainless steel plates for mechanical stability.
The uppermost port is used to connect a water level sensor needed for the icing routine, a pressure sensor, and a venting valve to the main chamber. The two opposing lower ports are equipped with windows to attach a light source while looking into the chamber from the other side to inspect the ice layers on the walls. The latter was especially useful to determine the quality of the ice layers on the walls for different icing conditions (see also section 4) and after different times of experimentation. All cooled parts of the ZINC chamber are isolated with 25 mm thick Armaflex foam sheets (Armacell).
3.3. Detection System
A new depolarization detector is currently being developed for ZINC which will be able to differentiate between ice crystals and water droplets by measuring the depolarization of scattered laser light at an angle of 175° (CitationNicolet et al. 2007). Until this new detector is available, ice crystals from the ZINC chamber are detected by means of an optical particle counter (Climet, CI 3100). The signal of the particle counter is amplified and directed to a multi channel analyzer (AmpTek, Pocket-MCA 8000A) to classify the detected particles by size. Typically, if a sample contains particles larger than a defined cutpoint (e.g., 2 μ m), they are removed before the inlet by using an impactor. Since the residence time of the ice crystals in the main chamber is chosen to be long enough to allow them to grow to sizes of several microns they can then be distinguished from aerosol particles simply by size. For lab studies with known particle sizes no inlet impactor may be needed to suppress particles with diameters larger than 2 μ m entering the chamber.
This method is not capable of distinguishing ice crystals from water droplets. Therefore, a short isothermal chamber is connected to the exit of the main chamber. The walls of this chamber are covered with a layer of ice as the main chamber and are thus subsaturated with respect to water. Hence, water droplets will evaporate and will not be counted as ice crystals by the detector. The bottom of this evaporation section is shaped as a funnel to direct particles towards the 10 mm diameter exit orifice where the particle counter is connected. Again, the 15 mm gap between the sample flow and the side walls ensures that no ice crystals are lost through sedimentation to the side walls of the funnel. This has been confirmed by calculating particle trajectories with 10 μ m ice crystals within the FLUENT model of the chamber. No particles originating from the sample flow inlet were lost in the funnel by deposition to the funnel side walls. The funnel itself contains horizontal channels terminated by KF DN50 flanges for windows where the depolarization detector will be placed in the future. The cooling system of the evaporation section is connected to the liquid cooling circuit of the warm wall of the main chamber and thus always has the same temperature as the warm wall.
Behind the detector a particle filter and a third MFC are placed to regulate the total flow through the chamber. In this way, the incoming sample aerosol flow is controlled indirectly because it must be the difference between total and the sum of the sheath flows which are all regulated by MFCs. A membrane pump (KNF Neuberger, N828KNE) behind the MFC is used to maintain the required pressure drop for the flow through the system. If the chamber is used in field campaigns where no pressurized air is available for the sheath flows, the outlet of the pump can be filtered and dried and then be recirculated for the sheath flows.
3.4. Computer Control and Data Handling
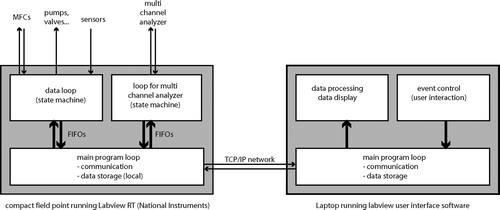
The heart of the ZINC instrument is a real-time controller (National Instruments, Compact Fieldpoint cFP-2020) running a LabView program which controls the hardware and samples data. It is equipped with several I/O modules to measure temperatures, flows, pressure, and some other housekeeping data and to control pumps, valves, and mass flow controllers. This allows for a high degree of automation, e.g., for the icing routine described later. The two coolers and the multi channel analyzer are connected to the cFP-2020 via RS232C serial ports. This real-time unit is connected to a laptop running the LabView graphical user interface for the instrument via TCP/IP networking (cf. for the computer setup). Both programs are written in such a way that temporary loss of the network connection does not result in a shutdown of the instrument or a loss of data. Instead, the real-time unit keeps running autonomously and caches the data to be sent to the laptop until the network connection is re-established. The real-time program consists of three major parts: a main loop and two time-critical loops (TCLs). The main loop communicates with the user interface via TCP/IP and with the two TCLs via FIFOs (first in first out buffers). It also stores data locally on the Fieldpoint. The TCLs are both state machines and receive commands from the main loop and send back data. One TCL is devoted to communicate with all sensors, the cooling baths, and the other hardware except for the MCA. The second TCL is solely responsible for the communication with the MCA since the serial port communication with this device is highly time critical and requires non-standard serial port procedures. The user interface provides time series plots of all measured data plus additional calculated data such as mean wall temperatures, sample flow, temperatures and humidities at the sample position as well as size spectra for the detected particles. The spectra can be integrated over a size range between two variable thresholds to define and calculate the total number of detected ice crystals. This is very helpful to detect the onset of freezing for activation experiments with different temperature settings. Sensor data are sampled with a rate of 1 Hz whereas size spectra are integrated over 4 seconds since readout of the MCA data is limited by the bandwidth of the serial port.
FIG. 5 Computer setup for controlling the ZINC hardware and sampling and storing data. A real-time unit (cFP-2020, National Instruments) running a custom made LabView program and equipped with several I/O-modules is the core of the system. The real-time unit communicates via a TCP/IP network with a Laptop running a LabView user-interface software.
4. FLUENT SIMULATIONS
During the design phase of the ZINC instrument, some variants of different parts of the chamber were tested by means of computational fluid dynamics simulations with the commercially available software FLUENT (FLUENT, 2005) to optimize the flow, the temperature, and the humidity profiles within the chamber. A selection of important results from these simulations will be presented in this section. These will be focused on the inlet section where the sheath flows and the sample flow are merged and on the uppermost part of the main chamber and the region where the laminar flow profile and the gradients in temperature and humidity develop along the flow. Some general remarks on the simulation parameters: the 3D version of FLUENT (rev. 6.2.16) was used with the segregated solver, k-ϵ turbulence scheme, energy equations but without radiation scheme. To simulate the ice layers on the main chamber walls the water vapor pressure parameterization from CitationMarti and Mauersberger (1993) was used as a user defined function in the boundary conditions settings for these walls to control the relative humidity. Wall temperatures are set to −20 and −40°C as in the example in . The side walls are assumed to be perfectly isolated to the surrounding resulting in a temperature gradient along the side wall that matches the gradient in the air gap. This assumption was quickly checked by measuring the inside center of one side wall with a thermocouple when there was a temperature gradient of 20 K between cold and warm wall. The thermocouple reading was, within the errors of the temperature measurement and positioning of the sensor, exactly at the mean temperature between both walls. The model grid was meshed with hexahedral cells in most sections except for the cylinder-shaped inlet tubes and the respective connected volumes where this was not possible. In addition to the fluid volumes the blades separating the sheath flows form the sample flow at the inlet nozzle were included in the model as solid parts to calculate the precooling of the sample flow through heat conduction.
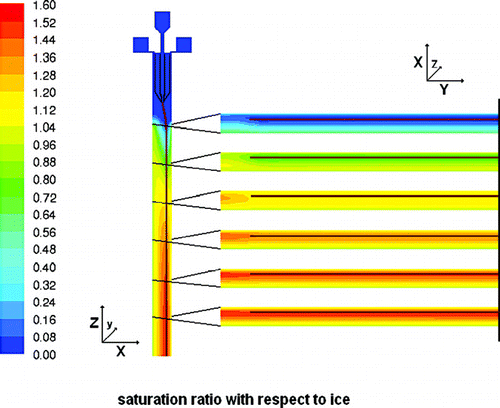
In , , and contours of flow velocity, temperature, and humidity for vertical cross sections of the inlet and upper part of the main chamber are shown with roughly the same field of view as in . In , contours of the flow velocity are shown with additional flow vectors for selected horizontal positions at 5, 10, 20, and 30 mm distance from the inlet nozzle where the sample flow is merged with the sheath flows. Pathlines for the sample flow are colored in red. One would expect a superposition of two flow profiles within the chamber: a true laminar parabolic profile, and a buoyant convective circulation caused by the temperature difference between the two cooled walls. Indeed, this expected profile develops very quickly within the first few centimeters behind the inlet nozzle for the sample flow (cf. ). As the sheath flows and the sample flow need to be distributed evenly over the whole width of 300 mm (or 270 mm for the sample flow), the sheath flow channels, with a cross section of 12 × 20 mm each, are narrowed to 1 mm and the aerosol nozzle from an 8 mm wide channel to about 400 μ m to provide a sufficient pressure drop for flow equalization. From horizontal cross sections through the inlet section which are not shown here one can see that this works as expected and the flow is distributed evenly over the whole width of the chamber. Only slight deviations can be observed very close to the side walls due to the friction with these walls. The temperature profiles for the inlet section are depicted in . On the right a contour plot for the center cross section is shown whereas the graph on the left presents individual horizontal profiles which are indicated as horizontal lines with matching colors in the contour plot. From the contour plot one can see that the precooling of the sheath flows is very efficient and the heat transport through the blades of the aerosol nozzle is sufficient to cool the sample flow rapidly. In reality this might be less ideal due to heat transport through the materials of the flanges and losses through the insulation. As the sheath flows merge with the sample flow a parabolic temperature profile emerges, that subsequently flattens down to the desired linear gradient between the two main walls. From the graph on the left in one can see that this happens within the first 100 mm of the chamber. shows a cross section depicting the humidities through the same region. However, the field of view is extended by roughly 60 mm into the main chamber as the water vapor profiles need a bit longer to develop. Humidities are expressed here in terms of the water vapor saturation ratio with respect to ice, which is one of the crucial parameters in ice nucleation studies. In addition, horizontal profiles are plotted on the right for several distinct levels to illustrate the homogeneity of this parameter over the width of the chamber and especially for the part where the aerosol layer is located (brown lines). The ice layer starts 10 mm below the inlet nozzle. This causes a sharp increase in the relative humidity at the walls at that level (cf. first horizontal cross section on the right in ). After 40 mm ice saturation is reached and after further 60 mm the maximum saturation ratio of about 1.6 for this temperature setting is reached. Ice saturation ratios are also critical in the region where the evaporation section is attached to the main chamber since one might expect a transient overshoot of supersaturation here. The simulations show that at the conditions discussed above, the ice saturation ratio overshoots from 1.55 to 1.58 for a short duration of 0.2 seconds. Directly afterwards the relative humidity relaxes to ice saturation so that any ice particle that may have formed solely due to this overshoot will not be able to grow to detectable sizes. Furthermore, the simulation assumes a perfect isolation between main chamber and evaporation section causing an abrupt temperature jump of about 20 K for one wall. In reality, heat conduction will lead to a smoother transition of temperatures and possibly to a less pronounced overshoot. Residence times have also been calculated for the whole instrument. Particles released from the sample inlet slit will stay in the main chamber for 12 seconds and another 8 seconds in the evaporation section.
FIG. 6 Contour plot of flow velocities in a vertical cross section through the center of the chamber. Individual vector-style profiles are depicted at the right for planes at 5, 10, 20, and 30 mm downstream of the aerosol nozzle where the sample and the two sheath flows are merged. Pathlines for aerosol particles within the sample flow are depicted as a red line originating from the aerosol nozzle.
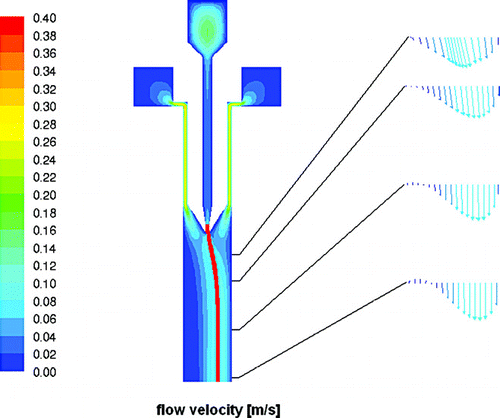
FIG. 7 Temperature profiles calculated with FLUENT along the vertical cross section of the inlet and the upper part of the main chamber. On the left, individual profiles for increasing distances from the inlet nozzle are plotted. The position of these profiles is depicted in the X/Z cross section on the right which is color coded by temperature. The precooling of the sheath flows can be clearly seen. The pathlines for aerosol particles are depicted as red lines originating from the aerosol nozzle.
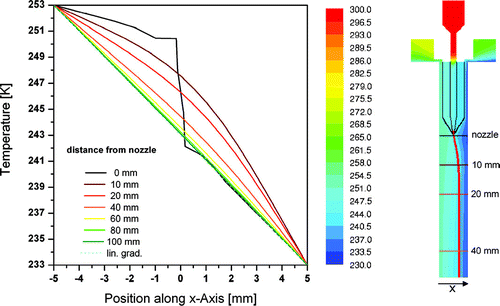
FIG. 8 Vertical (left) and horizontal (right) cross sections color coded by the saturation ratio with respect to ice. In the FLUENT simulation, the ice layer starts 10 mm below the aerosol nozzle (compare ). The horizontal profiles on the right at 10, 30, 50, 70, 90, and 110 mm below the inlet nozzle are shown from the center (the grey vertical line) to one of the side walls together with the position of the aerosol layer depicted as brown lines.
5. EXPERIMENTAL
Before an experiment can be performed with the ZINC instrument, the chamber has to be conditioned to obtain ice saturation at the walls. This procedure is essential for reliable operation and is described in the following section. To demonstrate the capabilities of the ZINC instrument and to validate it against literature data, activation experiments with the well known ice nucleus silver iodide were performed and will be discussed afterwards.
5.1. Icing the Chamber Walls
Prior to experimentation the walls of the ZINC chamber must be covered with a thin layer of ice. Generally, this is achieved by cooling the chamber to a temperature below 0°C. Then the chamber is flooded with purified water. After a certain time the remaining unfrozen water is pumped out. This procedure is automated and controlled by the Labview software. Water level sensors at the bottom and top of the chamber are used to automatically switch off the water pump when the chamber is filled or emptied.
It was found that the quality (smoothness) of the ice layer heavily depends on the conditions used during this procedure. Parameters that were varied to find the best conditions are: the initial temperature before flooding, the pump speed, the residence time of the water, and the cooling power of the cryostats (controlled via the pump level of the refrigerant pumps). Interesting findings of these tests are: at −5°C and low pump speed for the water pump, individual needlelike ice crystals form in the chamber resulting in an unusable topography of the ice layer. By replacing the initial membrane water pump with a powerful reversible gear pump (Cyclon INOX 24V), the pumping rate could be increased by a factor of about 20 (flooding the chamber now takes less than half a minute). This also allowed for a lower initial temperature of −10°C (being −5°C previously). At a residence time of only 30 seconds a visibly smooth layer of ice was obtained. After warming the chamber, the melted water was measured to be around 600 ml which corresponds to a homogeneous ice layer of about 600 μ m.
5.2. Activation Experiments with Silver Iodide
Silver iodide was used as a test aerosol to study ice nucleation since it is known to activate ice at high temperatures and low ice supersaturations and considerable literature data are available for comparison. Among the different methods which are available to produce silver iodide aerosols we chose the wet dispersion technique utilizing an atomizer. An aqueous suspension of silver iodide particles was obtained by mixing aqueous solutions of silver nitrate and potassium iodide. As aerosols produced from this suspension contain a significant amount of potassium nitrate, they were washed in the following way: first, the newly formed particles were allowed to precipitate. Then, the solution containing the remaining potassium nitrate was decanted carefully and the particles were then resuspended in pure water in an ultrasonic bath.
An atomizer was used to produce an aerosol out of this suspension. The wet aerosol was allowed to dry within an ETH-designed diffusion dryer filled with silica-gel as desiccant and directed into the ZINC chamber. Pressurized air from the house system was filtered through a HEPA filter and used for the sheath flows and the atomizer. Sheath flows were set to 4.5 lpm each and total flow was set to 10 lpm resulting in a sample flow of 1 lpm. Since the humidity at the sample position is controlled indirectly through the temperature gradient between the walls a series of activation experiments was carried out in the following way: the warm wall was gently cooled or heated at a rate of several degrees per hour. Simultaneously, the cold wall was cooled at about one degree per minute until activation could be observed visually in the MCA raw data (see below).
Temperatures for both walls were measured with 3 thermocouples per wall as described in section 3.2 and digitized with the appropriate Fieldpoint module (NI cFP-TC120/cFP-CB-3). Precision and accuracy of the thermocouples within this setup were determined with an ice/water mixture. All thermocouples had a precision of 0.03 K or better, the accuracy was 0.15 K or better for the six thermocouples attached to the main chamber walls and between 0.2 and 0.3 K for the two thermocouples attached to the evaporation section. The reduced accuracy in the latter case is probably caused by the position of the connectors of these sensors within the isothermal block, where the compensation temperature is measured. Due to heat conduction via the inlet section and isolation losses, the upper thermocouple could deviate from the other two sensors of one wall by a few degrees and thus was not considered for the calculation of temperatures and humidities of the sample. Hence, the wall temperatures were calculated by averaging the lower two thermocouples which are representative for the part of the main chamber where temperature and humidity profiles are fully developed. By knowing the flows and temperatures in the chamber, the sample position can be calculated by assuming a superposition of parabolic laminar and buoyant flow profiles (cf. ) as is indicated by vertical dotted lines in . Since the sample layer has a finite thickness, the temperature is not uniform within this layer. Hence, maximum and minimum temperature for the sample is calculated and plotted in (panel a) for a portion of the whole data set containing two individual activations. Panel (b) shows maximum and minimum calculated relative humidity with respect to ice as derived from profiles similar to the one in the upper panel of using the parameterizations from CitationMurphy and Koop (2005), respectively. As the sample layer roughly coincides with the maximum in supersaturation, the variance in relative humidity is much smaller than for the temperature. Panel (c) depicts the raw data from the MCA with individual size distributions every 5 seconds which are shown as a color coded intensity plot. The aerosol, in this case the silver iodide particles, are visibly in the lower half of the spectra reaching to roughly channel 110. The small number of background counts for larger particles is independent of the sample flow. These background concentrations have been measured occasionally by changing the sheath flow rates from 4.5 to 5.5 lpm causing a back flow of 1 lpm through the sample inlet. This ensures that only particle-free air is flowing through the chamber. The background was found to increase with decreasing chamber temperature and increasing experimental time which leads to the conclusion that it might be attributed to breakup of growing frost within the chamber.
FIG. 9 Course of a typical activation experiment with the ZINC instrument. Panels a) and b) show the devolution of temperature and relative humidity ranges for the sample layer. These data were calculated from measured wall temperatures and flows during the experiment. Panel c) shows the raw data from the particle counter in the form of color coded size spectra. Horizontal lines represent the thresholds used for integrating the total number and activated number of aerosols. The ratio of these, the activated fraction, is plotted in panel d). The threshold for the detection of the onset of freezing (2%) is indicated by a horizontal line. Vertical lines mark the positions at which activation data were evaluated.
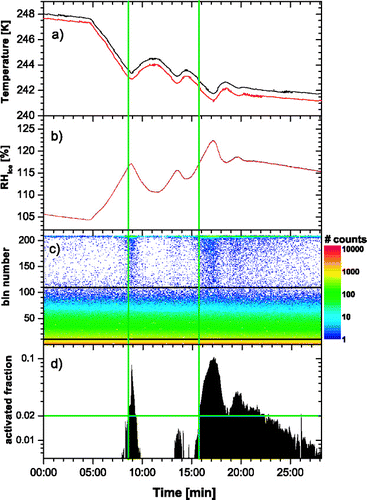
An activation was detected visually during the experiment by observing the counts for larger particles (above channel 110). When ice particles start to form, these counts increase significantly as can be seen in , panel (c) for t = 9 and 17 minutes experimental time. Once an activation was detected, the cold wall temperature was increased until ice crystals disappeared. This procedure was repeated subsequently for different warm wall temperatures to obtain the critical ice saturation (the relative humidity at which a significant portion of the aerosols activate ice) as a function of temperature. To determine the onset of ice formation in a more objective way, the size spectra from panel (c) were integrated from channel 10 (to dismiss electronic noise from the detector) on to calculate the total number of particles and from channel 110 (which roughly corresponds to 2 μ m particles) on to calculate the number of activated particles. We define the ratio of these numbers as the activated fraction of particles. This number is plotted in panel (d) of . As described earlier, at lower temperatures the background counts in channels above 110, which most likely stem from frost within the chamber, are significant and could be as a high as around 1% of all particles during an experiment. Consequently, a threshold to detect the onset of freezing must be set above this background level and was chosen as 2% activated fraction. The time at which this threshold level was passed is then used to retrieve temperatures and humidities for a particular activation. In , these times are indicated by vertical lines.
Errors were estimated as follows: since MCA data are available only every 5 seconds and particles can theoretically activate in different parts of the chamber (and thus need shorter or longer until they reach the detector), the inaccuracy at which the time for the onset of ice formation can be determined is estimated as 10 seconds at minimum. In some cases, the slope of increase in activated particles was very gentle and also noisy. The accuracy was then set to 20 seconds accordingly. Errors for temperature and saturation ratio were then set to the maxima and minima in the region of +/− 10 (or 20) seconds around the detected onset of freezing.
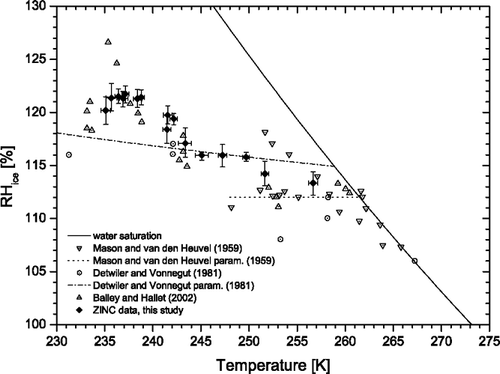
Mean temperatures and supersaturations for the onset of freezing are then collected for all activations and plotted in together with literature data. The solid line represents water saturation which was calculated using the new parameterizations for pw and pi from CitationMurphy and Koop (2005). The ZINC data show a constant trend towards higher critical supersaturations with decreasing temperatures. The overall agreement with literature data from CitationMason and van den Heuvel (1959), CitationDetwiler and Vonnegut (1981), and CitationBailey and Hallett (2002) is very good, especially if one considers the scatter between the different data sets. Interestingly, the feature in the Bailey dataset around 236 K seems to be missing in our data. Unfortunately, the cooling power of the warm wall cryostat limited us in going further down with temperatures to investigate this in more detail. A possible explanation might be the reduced pressure (≈ 300 mbar at T = 236 K) at which Bailey and Hallett performed their experiments. However, this “feature” can simply be an experimental artifact since only two points have critical supersaturations significantly above 20% and data points at temperatures only a couple degrees lower are again in agreement with our data. Another factor can be seen in the detection method for the ice crystals since they are based on different threshold sizes and numbers to determine the onset of freezing. CitationDetwiler and Vonnegut (1981) for example report their threshold supersaturations for 1% activated aerosols while the other sources do not relate their results to a defined fraction of activated particles. Furthermore, AgI particles are produced and dispersed differently in these studies possibly affecting their ice nucleating ability. The two lines (dashed and dash-dotted) represent parameterizations given by CitationMason and van den Heuvel (1959) based on their measurements and CitationDetwiler and Vonnegut (1981) based on theory constrained by their data. It appears that both parameterizations do not agree very well with the measurements, not only from this study.
FIG. 10 Critical saturation ratios for AgI particles from ZINC experiments using a 2% activated fraction as threshold for the onset of freezing (filled symbols). The temperatures were in the range 236–256 K. Data from different literature sources are also shown (open symbols). The water saturation (pw/pi) line is calculated using the parameterizations for pw and pi from CitationMurphy and Koop (2005).
6. CONCLUSIONS
A new instrument for ice nucleation studies, the ZINC chamber, has been constructed and designed with the aid of computational fluid dynamics software. It employs the functional principle of the CFDC by CitationRogers (1988) with major modifications to the geometry. The flat parallel plates, as opposed to the concentric cylinders of the CFDC, allow for a new and more direct way of actively cooling the chamber walls. It does not have the inherent problems of a curved surface or a poorly accessible inner wall. In contrast to copper tubes, aluminum was used to construct the chamber which made it possible to avoid a chemical treatment of the wall surfaces to make them sticky for the ice layer. Instead, the aluminum surface was only sandblasted and anodized. The ice layer sticks very well to the resulting rough and mechanically robust surface. A modern real-time unit running a custom-made Labview software controls the instrument and acquires the data. Test experiments with the well characterized aerosol silver iodide were carried out to validate the new instrument against existing data. The results show that the ZINC data agree very well with literature data and exhibit only little scatter. Temperatures as low as 236 K can be reached with the current setup. However, the range could be extended to lower temperatures with a more powerful cooling bath for the warm wall which is currently the limiting factor.
Acknowledgments
The authors would like to thank David Rogers, Paul DeMott, and Daniel Cziczo for very helpful discussions and suggestions. We thank Edwin Hausammann for his invaluable help in designing the mechanical parts. We thank the mechanical workshop at ETH Zurich for manufacturing the mechanical parts. Special thanks go to the workshop at Paul Scherrer Institute for the electron beam welding of the cooling channels.
REFERENCES
- Bailey , M. and Hallett , J. 2002 . Nucleation Effects on the Habit of Vapour Grown Ice Crystals from −18 to −42 degrees C . Quarterly Journal Of The Royal Meteorological Society , 128 : 1461 – 1483 .
- Baker , M. 1997 . Cloud Microphysics and Climate . Science , 276 : 1072 – 1078 .
- Bruintjes , R. T. 1999 . A Review of Cloud Seeding Experiments to Enhance Precipitation and Some New Prospects . Bull. Amer. Meteorol. Soc. , 80 : 805 – 820 .
- Cantrell , W. and Heymsfield , A. 2005 . Production of Ice in Tropospheric Clouds—A Review . Bulletin of the American Meteorological Society , 86 : 795
- Chen , Y. , Kreidenweis , S. M. , McInnes , L. M. , Rogers , D. C. and DeMott , P. J. 1998 . Single Particle Analyses of Ice Nucleating Aerosols in the Upper Troposphere and Lower Stratosphere . Geophysical Research Letters , 25 ( 9 ) : 1391 – 1394 .
- Cziczo , D. J. , DeMott , P. J. , Brook , C. , Hudson , P. K. , Jesse , B. , Kreidenweis , S. M. , Prenni , A. J. , Schreiner , J. , Thomson , D. S. and Murphy , D. M. 2003 . A Method for Single Particle Mass Spectrometry of Ice Nuclei . Aerosol Sci. Technol. , 37 : 460 – 470 .
- DeMott , P. J. , Cziczo , D. J. , Prenni , A. J. , Murphy , D. M. , Kreidenweis , S. M. , Thomson , D. S. and Borys , R. 2003 . Measurements of the Concentration and Composition of Nuclei for Cirrus Formation . Proc. Natl Acad. Sci. USA , 100 ( 25 ) : 14655 – 14660 .
- DeMott , P. , Sassen , K. , Poellot , M. , Baumgardner , D. , Rogers , D. , Brooks , S. , Prenni , A. and Kreidenweis , S. 2003 . African Dust Aerosols as Atmospheric Ice Nuclei . Geophys. Res. Lett. , 30 : 2041 – 2045 .
- Detwiler , A. and Vonnegut , B. 1981 . Humidity Required for Ice Nucleation from the Vapor Onto Silver-Iodide and Lead Iodide Aerosols Over the Temperature-Range −6 to −67-degrees-c . J. Appl. Meteorol. , 20 : 1006 – 1012 .
- Federer , B. , Waldvogel , A. , Schmid , W. , Schiesser , H. , Hampel , F. , Schweingruber , M. , Stahel , W. , Bader , J. , Mezeix , J. , Doras , N. , Daubigny , G. , Dermegreditchian , G. and Vento , D. 1986 . Main Results of Grossversuch-iv . J. Climate and Appl. Meteorol. , 25 : 917 – 957 .
- 2005 . FLUENT 6.2 User's Guide , Centerra Resource Park, 10 Cavendish Court, Lebanon, NH 03766, , USA : Fluent Inc. .
- Hirst , E. , Kaye , P. H. , Greenaway , R. S. , Field , P. and Johnson , D. W. 2001 . Discrimination of Micrometre-Sized Ice and Super-Cooled Droplets in Mixed-Phase Cloud . Atmos. Environ. , 35 : 33 – 47 .
- Pachauri , R. K. , Solomon , S. , Qin , D. and Manning , M. 2007 . The Physical Basis of Climate Change , International Panel on Climate Change . Technical report
- Kreidenweis , S. M. , Chen , Y. , Rogers , D. C. and DeMott , P. J. 1998 . Isolating and Identifying Atmospheric Ice-Nucleating Aerosols: A New Technique . Atmos. Res. , 46 : 263 – 278 .
- Kumar , P. , Broekhuizen , K. and Abbatt , J. 2003 . Organic Acids as Cloud Condensation Nuclei: Laboratory Studies of Highly Soluble and Insoluble Species . Atmos. Chem. Phys. , 3 : 509 – 520 .
- Liou , K. and Lahore , H. 1974 . Laser Sensing of Cloud Composition—Backscattered Depolarization Technique . J. Appl. Meteorol. , 13 : 257 – 263 .
- Lohmann , U. 2002 . A Glaciation Indirect Aerosol Effect Caused by Soot Aerosols . Geophysical Research Letters , : 29
- Marti , J. and Mauersberger , K. 1993 . A Survey and New Measurements of Ice Vapor Pressure at Temperatures between 170 and 250 k . Geophys. Res. Lett. , 20 ( 5 ) : 363 – 366 .
- Mason , B. and van den Heuvel , A. 1959 . The Properties and Behaviour of Some Artificial Ice Nuclei . Proc. Phys. Soc. London , 74 : 744 – 755 .
- Murphy , D. and Koop , T. 2005 . Review of the Vapour Pressures of Ice and Supercooled Water for Atmospheric Applications . Quarterly J. Royal Meteorol. Soc. , 131 : 1539 – 1565 .
- Nicolet , M. , Stetzer , O. and Lohmann , U. 2007 . Depolarization Ratios of Single Ice Particles Assuming Finite Circular Cylinders . Appl. Optics , 46 : 4465 – 4476 .
- Rogers , D. C. 1988 . Development of a Continuous Flow Thermal Gradient Diffusion Chamber for Ice Nucleation Studies . Atmos. Res. , 22 : 149 – 181 .
- Rogers , R. R. and Yau , M. K. 1989 . A Short Course in Cloud Physics, volume 113 of International Series in Natural Philosophy, , 3rd edition , Butterworth Heinemann by Elsevier .
- Spurny , K. 2000 . Atmospheric Condensation Nuclei P. J. Coulier 1875 and J. Aitken 1880 (historical review) . Aerosol Sci. Technol. , 32 : 243 – 248 .
- Szyrmer , W. and Zawadzki , I. 1997 . Biogenic and Anthropogenic Sources of Ice-Forming Nuclei: A Review . Bull. Amer. Meteorol. Soc. , 78 ( 2 ) : 209 – 228 .
- Willoughby , H. , Jorgensen , D. , Black , R. and Rosenthal , S. 1985 . Project Stormfury—A Scientific Chronicle 1962–1983 . Bull. Amer. Meteorol. Soc. , 66 : 505 – 514 .