Abstract
A Volatility-Tandem-Differential-Mobility-Analyzer (VTDMA) and a Differential Mobility Particle Sizer (DMPS) were used to determine the number and mass concentration of externally mixed aerosol particles in urban background air. In the VTDMA the less-volatile (LV) particle fraction was measured at 300°C for particles in the size range 20–250 nm. The LV particle fraction was converted to the number concentration of LV particles (NLV) and the mass concentration (MLV). MLV was compared with the mass concentration of black carbon (MBC) measured by a Multi-Angle Absorption Photometer (MAAP). The DMPS and VTDMA data were used for calculating scattering and absorption coefficients (σSP and σ AP) with a Mie model and compared with σ SP and σ AP measured with a TSI nephelometer and the MAAP. The model was run by assuming external and internal mixing of absorbing and scattering aerosol. The best fit of measured and modeled σ SP and σ AP was sought by varying the refractive index. During periods dominated by local emissions LV particle fraction ⟨ϕLV ⟩ was high ( >0.2). In these cases, the MLV and the modeled σ AP assuming external mixing agreed well with the measured MBC and σ AP, respectively. For the long-range transported aerosol ⟨ϕLV ⟩ was small ( <0.1) and MBC was higher than MLV. For the whole period the average (± std) refractive index was 1.55 (± 0.09) – 0.04 (±0.02)i when internal mixing was assumed. When ⟨ϕLV ⟩ was >0.2 the average refractive index of LV particles was 1.96 – 0.8 (±0.18)i when σ AP was modeled assuming external mixing.
1. INTRODUCTION
Soot particles mostly emitted from anthropogenic sources play an important role in the global radiative balance. Soot absorbs light efficiently leading to a warming effect of the atmosphere. Hence, soot particles may almost offset the cooling effect caused by scattering aerosols like sulfate (e.g., CitationCooke et al. 1999; CitationJacobson 2000, Citation2001). The majority of soot particles in urban regions is usually in particles smaller than 0.2 μ m. These small non-soluble soot particles may cause adverse health effects like respiratory and cardiovascular diseases (CitationIbald-Mulli et al. 2002; CitationOberdörster 2001).
Soot is externally mixed immediately after emission. However, during transport, soot particles may coagulate with other particles or get coated with chemical compounds like sulfates, nitrates or organics by condensation and thus get internally mixed. The mixing state of soot particles influences both light absorption (e.g., CitationJacobson 2000 Citation2001; CitationSchnaiter et al. 2003) and deposition in human airways. Information on the mixing state is thus necessary for an assessment of the climatic and health effects of soot particles.
Soot absorbs light efficiently and therefore often the term black carbon (BC) is used as a synonym of soot although the definition of BC is unclear (CitationAndreae and Gelencsér 2006). Soot contains both organic and elemental carbon (OC and EC). They are measured using thermal methods leading to an instrumental definition of these components. BC is in general measured with techniques based on light transmission through a filter (e.g., Aethalometer, the Multi-Angle Absorption Photometer (MAAP) and Particle Soot Absorption Photometer (PSAP)). With these instruments, the mass concentration of black or elemental carbon or the absorption coefficient can be determined. It is, however, not possible to retrieve the mixing state of soot particles from these techniques. Furthermore, mass size distributions can be determined by taking impactor samples and a subsequent EC analysis. Again, no information about the mixing state can be retrieved.
To determine the mixing state of non-volatile (NV) particles, it is convenient to use a method based on thermodesorption, Volatility-Tandem-Differential-Mobility-Analyzer (VTDMA) (e.g., CitationRader and McMurry 1986; CitationBurtscher et al. 2001). This technique has been used by other research groups, e.g., to measure volatility properties of the atmospheric, traffic-related, and combustion emission aerosols as well as differences between volatilization of diesel and spark ignition vehicle particulate emissions (CitationBurtscher et al. 2001; CitationKuhn et al. 2005a, Citation2005b). These investigations determined changes in number size distributions within temperature rise and fractions of more and less volatile particles in different particle diameters. In some reaction chamber experiments, the VTDMA technique has been used to quantify polymer fraction and volatility of secondary organic aerosol from the ozone initiated oxidation of α -pinene and limonene (CitationKalberer et al. 2004; CitationJonsson et al. 2007). A VTDMA can provide number and calculated volume fractions of the less volatile (LV) material for a narrow particle size fraction (Dp ± 10%) and can detect a possible volatile coating on the particles (CitationRader and McMurry 1986; CitationSmith and O'Dowd 1996; CitationOrsini et al. 1999). Based on this technique, a VTDMA was built and operated by CitationPhilippin et al. (2004). With this instrument the relative number size distribution of NV particles of a monodisperse aerosol can be measured at temperatures up to 300°C. More volatile and less volatile particle fractions can be determined comparing the measurement at 25°C with the corresponding one at 300°C. The VTDMA method itself provides thus relative values in terms of the mixing state for selected particle sizes.
Volatile compounds such as sulfates, nitrates and most of the organic species are evaporated at 300°C. Residual particles are either externally mixed soot particles or other NV material such as sea salt or crustal particles, which do not shrink significantly during heating (less volatile particle fraction), or particles that change their size to a greater amount due to evaporation of volatile compounds (more volatile particle fraction). In the present work a VTDMA was used to measure urban aerosol of Leipzig, Germany. At this site, far from the sea, it is most probable that the NV submicron particles are soot, not sea salt or crustal material. Another source of salt compounds could be road treatment during winter. However, the study of CitationHerrmann et al. (2006) found that the influence of the road treatment by NaCl is assumed to be negligible in the submicron size range.
CitationRose et al. (2005) presented the method to determine the number concentration of externally mixed NV particles in the fine particle size range using a combination of a VTDMA and a differential mobility size spectrometer (DMPS). The purpose of the present article is to evaluate the performance of this method for measuring soot concentrations and absorption coefficients by comparing it with optical measurements. Such a quantitative comparison has not been conducted for the present setup. First a simple method evaluation is made by comparing the mass concentration of the LV particles with the black carbon mass concentration obtained from the MAAP measurements. In another comparison the DMPS and VTDMA data are used for calculating scattering and absorption coefficients (σSP and σAP, respectively) with a Mie model. These are compared with measured σSP and σ AP.
2. METHODS
2.1. Instrumentation
2.1.1. Volatility and Size Distributions
The number concentrations of LV particles were determined by measuring the fraction of externally mixed particles using a VTDMA and combining these fractions with the total number concentrations of atmospheric aerosol particles measured by a mobility size spectrometer (Twin Differential Mobility Particle Sizer, Twin-DMPS) and Aerodynamic Particle Sizer (APS, TSI model 3321, St. Paul, MN, USA). Here, the Twin-DMPS (CitationBirmili, et al. 1999) measured the number size distribution of ambient aerosol particles in size range from 3 to 800 nm and the APS from 800 nm up to 10 μ m.
The VTDMA-system was described in detail by CitationPhilippin et al. (2004). It consists of two Differential Mobility Analyzers (DMA), two Condensation Particle Counters (CPC, TSI Model 3010), and a heating unit (). The heating unit includes two parallel heating tubes that are held at temperatures of 25° and 300°C. In the first DMA of the system, monodisperse particles are selected from the polydisperse aerosol (). The total number concentration of the initially selected particles is measured with a CPC. Next the monodisperse aerosol passes through the heating tubes. At 25°C the particles remain unchanged and the VTDMA measurement acts as a reference of behavior of untreated aerosol particles while at 300°C volatile compounds such as sulfates, nitrates, and most of the organic carbon will evaporate.
FIG. 1 Operation principle of the VTDMA instrument: (a) initial number size distributions measured by a first DMA/CPC combination, (b) the residual number size distribution divided into more and less volatile parts (NMV and NLV) after conditioning of the aerosol determined by second DMA/CPC combination and (c) a schematic diagram of the VTDMA system.
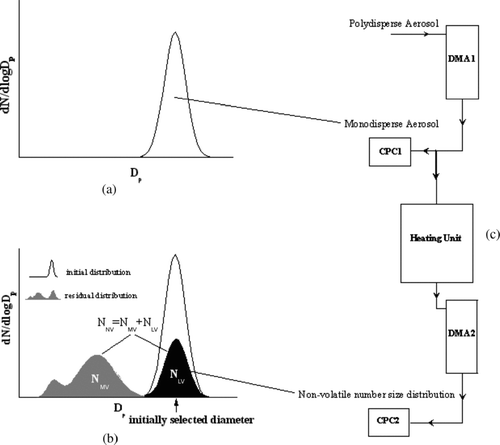
In the last step, the number size distribution of NV particles (NNV), divided into the number of more and less volatile particles (NMV and NLV), is measured by using a second DMA/CPC combination (). The residual particles that are non-volatile at 300°C are usually clearly separated in two modes (filled areas in , cf. CitationWehner et al. 2004), representing either externally mixed NV particles that do not shrink significantly during the heating (less volatile particles), or particles that evaporate partly in the heating unit and are then smaller than before heating. These so called more volatile residual particles may consist for instance of aged, coated soot or other NV particles such as polymers. In this study, the initial particle sizes 20, 30, 50, 80, 150, and 250 nm were chosen for the VTDMA treatment. Most traffic-related less volatile particles have been shown to be between 80 and 150 nm (e.g., CitationWehner et al. 2004).
The measured size distributions (at 25 and 300°C) were corrected for the CPC counting efficiency and for particle losses due to thermophoresis and diffusion in the VTDMA using an empirically determined loss function that depends on the particle size and heater temperature. The transport efficiency depends on the heating temperature and it was determined by the ratio of the number concentrations before and after the heating unit. The majority of the particle losses actually occur in the path between the heater exit and the second DMA. Right after the heater, aerosol is in the heater temperature but it rapidly cools to room temperature when it exits the heating unit (CitationOrsini et al. 1999). Thus, the path between the heater and the second DMA was included in the loss function determination. Ag particles were used as the test aerosol because Ag is non-volatile at the temperatures used here. An exponential function was fitted to the empirically measured transport efficiencies () according to:
FIG. 2 Transport efficiencies of the VTDMA heating unit as a function of particle size at different temperatures.
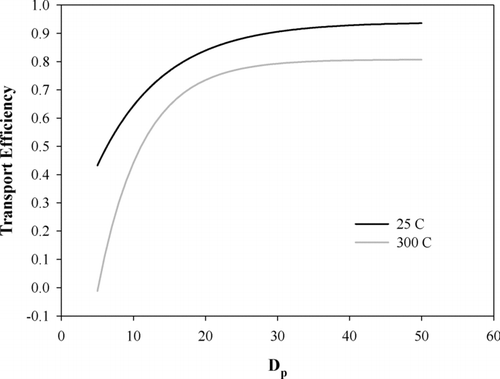
TABLE 1 The coefficients of the fitted exponential functions (Equation [1]) for the VTDMA transport efficiencies
2.1.2. Optical Instrumentation
The absorption coefficient σAP was measured with a Multi-Angle Absorption Photometer (MAAP) (Thermo Electron Corporation, Model 5012). The MAAP determines σAP of the particles deposited on a filter by a simultaneous measurement of transmitted and backscattered light (CitationPetzold and Schönlinner 2004). The wavelength λ of the MAAP was approximately 637 nm instead of 670 nm given by the manufacturer. The real wavelength was determined during a GAW absorption photometer workshop held at IfT in November 2005, using a high-resolution spectrometer (Ocean Optics, Model HR2000). The wavelength range of the spectrometer was from 300 to 1200 nm with an optical resolution about 0.3 nm. The spectrometer was connected to the MAAP photometer using an optical fiber with a diameter of 200 μ m.
The MAAP actually measures σAP, but the output data are black carbon (BC) mass concentrations (MBC) calculated by the instrument firmware using the mass absorption cross section of 6.6 m2g–1. Thus, the output values of the instrument were multiplied with this constant to obtain the values in σAP. Using a constant factor in calculation causes uncertainty to the BC mass concentrations since the BC mass absorption cross section is a function of several parameters, for instance particle size, morphology, and wavelength (e.g., CitationSchnaiter et al. 2003). However, for the absorption coefficient this is not a source of uncertainty.
Scattering coefficients σSP at λ = 450, 550, and 700 nm were measured using a TSI nephelometer (Model 3563). The scattering coefficients were corrected for truncation error according to CitationAnderson and Ogren (1998). The scattering coefficient was interpolated logarithmically to the MAAP wavelength 637 nm by σ SP(637) = σSP(700) × (700/637)α using the Ångström exponent calculated from α = −log(σSP(550)/σSP(700))/log(550/700).
2.2. Data Processing
2.2.1. Combination of the VTDMA, Twin-DMPS, and APS
The method combining the VTDMA and the ambient size distribution measurements yielded the concentration of externally mixed NV particles. The externally mixed number and mass concentration derived from the VTDMA/Twin-DMPS-APS combination will be referred to as NLV and MLV, respectively.
The number fraction of the less volatile particles (φLV) is the ratio of NLV and the integral over the 25°C scan (monodisperse size distribution) (N1):
The volume-weighted average fraction of less volatile particles < ϕLV > was calculated from:
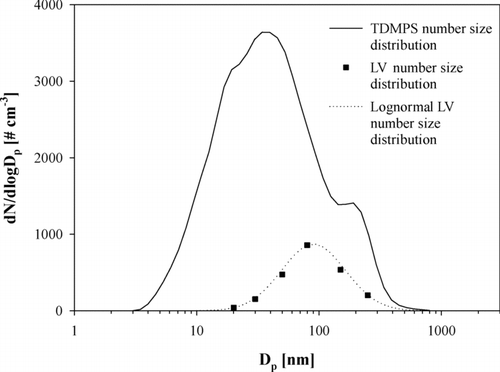
As mentioned above, the determination of the number concentration of externally mixed NV particles was based on the simultaneous measurement of particle number size distribution using a mobility size spectrometer. The concentration of LV particles was calculated by multiplying the LV number fraction for each selected size by the corresponding number concentration measured with the Twin-DMPS and the APS. To combine the two particle size distributions from these instruments, it has to be considered that in the DMPS data particle diameters are electrical mobility diameters and in the APS, they are aerodynamic diameters. For calculation of the optical properties of the aerosol particles, the geometric diameter has to be used. Conversion to the geometric diameter was done assuming that particles were spherical and that the dry particle density was 1.7 g cm–3 (CitationWex et al. 2002). Finally, the size distribution of LV particles was achieved by applying a lognormal function through all data points available from the VTDMA measurements. A lognormal function was fitted to each of the hourly averaged data points separately. All data points were not always distributed log normally which resulted in some uncertainty to the fittings. The uncertainty of the VTDMA measurements also increased the uncertainty of lognormal fitting. Thus, the uncertainty of the lognormal fitting of the mass size distribution including the VTDMA uncertainty was determined to be approximately 10%. An example of the number size distribution of ambient aerosol measured with a DMPS and number size distribution of externally mixed NV particles is shown in .
FIG. 3 An example of a total number size distribution measured by the Twin-DMPS-APS combination (after 10 μ m inlet) and a less-volatile particle number size distribution according to the data based on the VTDMA/Twin-DMPS-APS combination. The dotted line describes the lognormal fit based on the VTDMA data points.
The number size distributions of LV particles were converted to volume size distributions assuming spherical shape of the particles. The mass size distributions () and the total mass concentrations of LV particles (MLV) were calculated using the density of 1.0 g cm–3 (CitationHitzenberger et al. 1999). This density was chosen according to the density of loosely packed soot clusters (CitationOuimette & Flagan 1982). Soot particles are however generally agglomerates with sometimes complex shapes. Therefore, the mass concentration was additionally computed taking the dynamic shape factor χ into account. Following CitationDeCarlo et al. (2004)the volume equivalent diameter was calculated from
FIG. 4 Examples for the lognormal number and mass size distributions based on the VTDMA measurements for shape factors of 1.0 and 1.15.
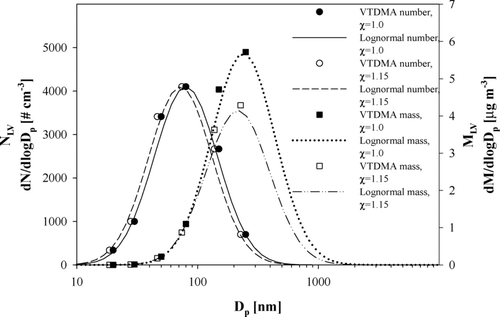
Diesel soot particles have fractal dimensions that influence the volume of the particles. However, the fractal dimensions of the LV particles were not taken into account. This increases the uncertainty of the calculation while the fractal dimension especially for the soot particles can have a significant effect to the particle size and it can vary with particle size (e.g., CitationPark et al. 2004; CitationSlowik et al. 2004).
2.2.2. Scattering and Absorption Modeling
The measured optical properties were compared with modeled ones calculated by using a Mie code over the particle size range from 3 nm to 10 μ m with 76 size channels of the Twin-DMPS-APS system. The comparison was made for the wavelength of the MAAP (637 nm). The modeling was done by assuming that the aerosol was (1) internally mixed and (2) externally mixed. In case (1) the scattering and absorption coefficients (σSP and σ AP) were calculated from
Scattering and absorbing particles were first assumed to be internally mixed and the modeling was done using number size distributions from the Twin-DMPS-APS and by varying the complex refractive index. The real refractive index n r was varied in the range 1.42–1.80 and the imaginary refractive index ki in the range 0.01 and 0.1. Larger n r values do exist in the constituents of some atmospheric particles, for instance CitationOuimette and Flagan (1982) report n r = 1.96 for soot particles. However, the majority of the light scattering particles in urban air consist of a combination of secondary aerosols such as sulfates, nitrates, and organics, possibly NaCl used for de-icing road surfaces in winter, and dust that often contains silica. For all of these combinations, n r fits in the above range (e.g., CitationSeinfeld and Pandis 1998). For ki the upper limit 0.1 is arbitrary in the sense that if a large enough fraction of the aerosol mass were soot, certainly ki would be larger than that. However, this upper limit proved to be high enough for obtaining σAP for the whole measurement period when internal mixing was assumed.
When the aerosol was assumed to be externally mixed, it was assumed that the LV particles were soot and for them the complex refractive index mLV was varied around the values 1.75–0.44i and 1.96–0.66i presented by the studies of Citationd'Almeida et al. (1991) and CitationOuimette and Flagan (1982). The imaginary refractive index of the LV particles ki,LV was varied in the range between 0.4 and 0.9. Values of ki,LV larger than 0.9 can be considered to be unrealistic at λ = 637 nm comparing with literature values for soot (e.g., CitationOuimette and Flagan 1982; CitationSmyth and Shaddix 1996; CitationMüller et al. 2006). The volatile particles were assumed to be purely scattering and for them the real refractive index n r,V was again varied between 1.42–1.80.
2.3. Measurement Site
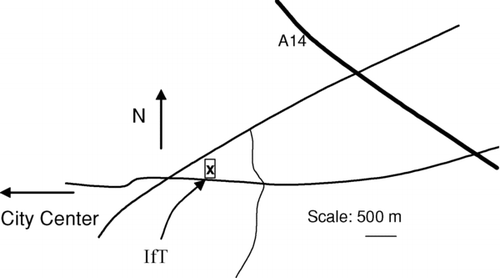
The measurements were done from December 22, 2005 to January 15, 2006 on the roof of the Leibniz Institute for Tropospheric Research (IfT) in Leipzig, Germany (). The site is located on the outskirts of Leipzig, between heavily trafficked roads but far enough of these so that for most of the time the air can be considered representing urban background. A PM10-inlet was used before all the instruments to allow sampling of particles with diameters below 10 μ m.
3. RESULTS
3.1. Number and Mass Size Distribution of Less-Volatile Particles
The average (± standard deviation, std) geometric mean particle diameter and the average (± std) geometric standard deviation of the LV particle number size distribution were 90 ± 20 nm and 1.9 ± 0.1, respectively. Mass size distributions peaked at the particle sizes approximately from 190 to 450 nm. When the shape factor χ was taken into account, the geometric mean particle diameter shifted to a smaller volume equivalent diameter. For χ = 1.15 the shift was on the average 6 nm. In some former studies it has been found that the maximum NV particle fraction from vehicle emissions concentrates in particle sizes around 80 nm (CitationRose et al. 2005; CitationWehner et al. 2004). Using the shape factor correction with χ = 1.15 the mass concentrations of the less-volatile particles were on average 80 (± 3)% of those of the spherical particles. In the frame of this investigation, it was not possible to determine the real shape of the particles.
3.2. Comparison between MLV and MBC
An example of a typical period of MLV and MBC during the measurement campaign is presented in . shows a seven-day time series of hourly averaged MBC and MLV calculated using the density of 1.0 g cm–3 and the shape factor 1.0. MBC was usually higher than MLV suggesting a significant fraction of soot particles to be internally mixed. However, there were also periods when MBC and MLV closely followed each other which suggests that a large fraction of non-volatile particles was externally mixed soot. An example of such a period is on December 25–26, 2005 (). Supporting information is provided by the wind speed and direction measured next to the aerosol inlet (). The dominating wind direction was south-southwest (220°–270°), i.e., from the urban area of Leipzig. The good agreement of MBC and MLV suggests that the NV fraction was dominated by freshly emitted soot particles from traffic emissions, such as diesel vehicles. When wind blew from the direction of the city center the volume-weighted average fraction of less volatile particles ⟨ϕLV⟩ varied mainly in values > 0.1, up to > 0.4 (). On December 26, the wind direction turned to north and later northeast and MBC was clearly higher than MLV, and ⟨ϕLV⟩ decreased to less than 0.1, suggesting that a larger fraction of aged, internally mixed soot particles was observed. On December 29, wind direction turned to southwest and south again and fresh soot was measured.
FIG. 6 Hourly averaged (a) MLV and MBC concentrations, (b) wind speed and direction, and (c) volume-weighted less-volatile particle fraction < φLV > measured between December 24 and 31, 2005.
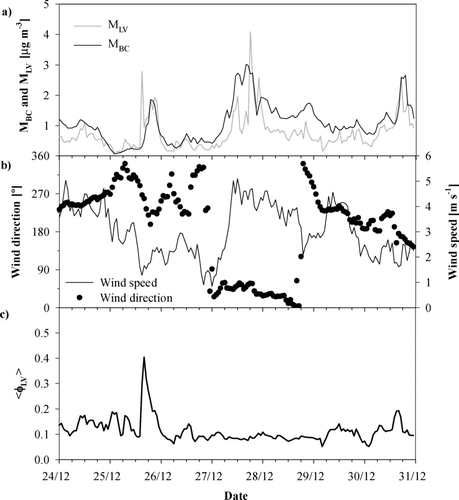
On December 25 and December 27 there were some hours when MLV was significantly higher than MBC. They are individual data points, however, outliers in the surrounding time series. This fact suggests that the reasons are probably more of technical origin than in the aerosol itself.
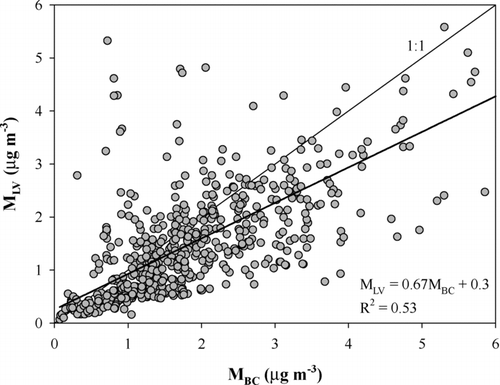
In the whole data obtained between December 22, 2005 and January 15, 2006 there was a weak linear correlation between MLV and MBC (). Using the shape factor correction with χ = 1.15 the correlation was even weaker having the regression line of MLV = 0.55MBC + 0.2 (R2 = 0.52). One of the reasons for the low correlation of MLV and MBC is that the MAAP determines both the internally mixed and the externally mixed BC concentration whereas the VTDMA method gives only the externally mixed low-volatile particle fraction. The different size range of the MAAP and VTDMA measurements is another reason why MLV was lower than MBC. Earlier studies have shown that BC often has a mass mode also in the accumulation size range (e.g., CitationBerner et al. 1996) even though externally mixed soot particles are mainly smaller than 300 nm. Since the VTDMA measured only up to 250 nm part of the soot particles that were measured with the MAAP were not measured with the VTDMA. However, according to the number size distributions measured with the TDMPS and the APS the number concentration of particles larger than 1 μ m was not significant (less than 1% of the particles below 10 μ m) as is shown in the typical size distribution during the campaign in . Therefore the possible non-measured soot particles were mostly smaller than 1 μ m. Particles in the size range 0.25–1.0 μ m comprised on the average approximately 4% of the total submicron aerosol number but much higher fraction of aerosol mass. In particles larger than 250 nm a significant fraction of the soot particles can be assumed to be internally mixed and thus, the MBC is larger than MLV.
FIG. 7 Hourly averaged MLV as a function of MBC with a linear regression based on the data of the whole campaign. No shape factor correction was applied.
A reasonable hypothesis is that the larger the volume-weighted average fraction of less-volatile particles ⟨φLV⟩ (Equation [3]) is the better MLV and MBC agree. To study this, the MLV–to–MBC ratio was plotted against ⟨φLV⟩. shows the ratios of hourly-averaged concentrations and the medians and the 95% ranges in nine ⟨φ LV⟩ bins from < 0.1 to > 0.4. Here, the variation within the bins was large but results were in a fair agreement with the hypothesis: on the average, MBC was clearly higher than MLV at low ⟨φLV⟩ but at higher ⟨φLV⟩ close to MBC. The average (±std) and median MLV–to–MBC ratio was 0.6 ± 0.3 and 0.5 for ⟨φLV⟩ < 0.1 and 1.0 ± 0.5 and 0.9 for ⟨φLV⟩ > 0.2, respectively. Of all the ratios 21% were larger than one but mainly within some tens of percents larger. However, there were several such cases as in on December 26 and December 27 where MLV was significantly larger than MBC. The reason for these could not be explained from the available data. A possible explanation is that the LV particles measured in these cases were not BC. On the other hand, these cases were such as those in : individual data points where the ratio did not stay above one during several successive hours one. This suggests that the explanation is not in the aerosol but in the measurement methods.
FIG. 8 The ratios of hourly-averaged MLV and MBC concentrations as a function of volume-weighted less-volatile particle fraction ⟨φLV⟩. The filled squares are the median ratios and the vertical error bars the 95% ranges in nine ⟨φLV⟩ bins shown by the horizontal error bars.
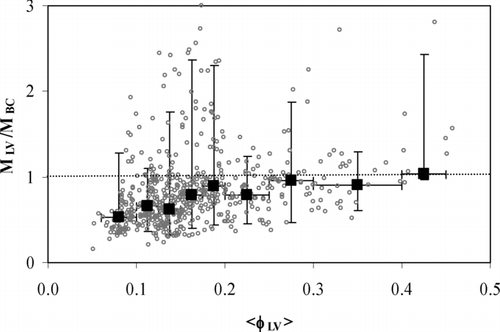
The average (± std) ⟨φLV⟩ was 17 ± 10 % during the measurement period, and 95% of ⟨φLV⟩ values were between 8 and 36%. The highest values were associated with air from the city center and diesel vehicles from the highly frequented roads. This result is in agreement with CitationPhilippin et al. (2004) who showed that the proximity of the emission sources increases the fraction of non-volatile particles.
3.3. Measured and Modeled Optical Properties
In addition to the comparison of MBC and MLV, the conditions under which σAP derived from the VTDMA data agree with measured σAP were examined. An example of modeled and measured σAP, ⟨φLV⟩, and derived effective imaginary refractive indices is shown in . shows the corresponding measured and modeled σSP and the effective real refractive indices.
FIG. 9 Hourly averaged (a) measured and modeled σAP assuming internally mixed particles, (b) measured and modeled σAP assuming externally mixed particles -modeled σAP is given for the data based on the fitted refractive index (Modeled1) and a literature value for the refractive index of soot, m = 1.96 – 0.66i (Modeled2), (c) imaginary part ki of externally and internally mixed particles and (d) less volatile particle fraction ⟨φLV⟩ between January 10 and 15, 2006.
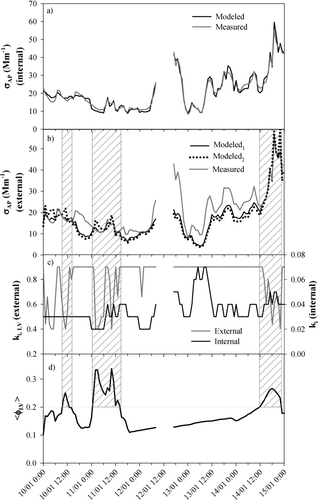
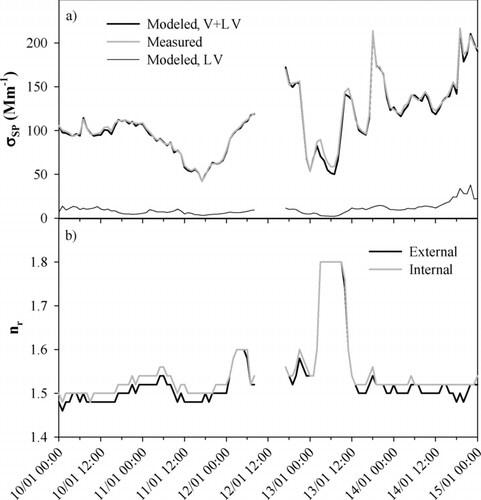
FIG. 10 Hourly averaged (a) measured and modeled σSP of externally mixed particles—for the modeled σSP both the sum of σSP of volatile and less volatile particles and σSP of only less volatile particles are shown and (b) effective real refractive index nr when assuming internal mixing of absorbing and scattering particles and nr,V of volatile particles when assuming external mixing.
When it was assumed that the particles were internally mixed, it was always possible to find a combination of n r and k i so that the measured and modeled σAP agreed (). The average and standard deviation of the refractive index was 1.55 ± 0.09 – (0.04 ± 0.02)i. The corresponding median value was 1.52–0.03i.
When it was assumed that the absorption is due to the externally mixed LV particles only the modeled σAP was most of the time even tens of percents lower than the measured one with ki < 0.9. When the fraction of less-volatile particles ⟨φLV⟩ was > 0.2 (hatched areas in the based on the ), it was possible to find reasonable values of ki that would make the modeled and measured σAP agree. In these cases the fitting procedure resulted in an average (± standard deviation) mLV = 1.96 – (0.80 ± 0.18)i and median k iLV = –0.90i. This refractive index is very close to the literature value given for soot, m = 1.96 – 0.66i (CitationOuimette and Flagan 1982). Modeled σAP for externally mixed particles using refractive index of soot agreed well with the modeled σAP based on the fitting of the refractive index ().
The message of is that it was most of the time possible to find such values for n r within the range 1.42–1.80 that the modeled σSP agreed with the measured ones. There were some periods, however, when the modeled σSP remained lower than the measured one even with n r = 1.8, for instance on January 13. The number concentrations, σSP or σAP were not in any way anomalous during this period so the reason for this disagreement cannot be explained from the available data. The best fit between the modeled and measured σSP was usually found when nr varied around 1.5. When it was assumed that the particles were externally mixed the average (± standard deviation) refractive index of the scattering particles was nr,V = 1.53 ± 0.10 with median value of 1.50. The externally mixed LV particles were responsible of approximately 10% of the light scattering.
4. SUMMARY AND CONCLUSIONS
A system to determine less volatile particle number and mass concentrations was tested. The technique consisted of a Volatility TDMA and a Twin-DMPS combination. The VTDMA measured the non-volatile number size distribution for certain particle sizes selected from the initial polydisperse aerosol. The principle is based on the volatilization of particles in a heating unit at 300°C. Particles that did not change their size within the heating were assumed to consist mostly of externally mixed soot. The fractions of externally mixed soot particles in six particle diameters in the size range 20–250 nm were converted to number concentrations using ambient air number size distribution data measured in parallel with the VTDMA. The number size distribution of less volatile particles was determined by a lognormal fitting to the less volatile number concentration points. Mass concentrations of less volatile particles (MLV) were calculated assuming density for loosely packed soot clusters. These results were compared with the BC mass concentrations (MBC) obtained with a monitor for soot mass concentration (MAAP). In addition, the absorption coefficients (σAP) and the scattering coefficients (σSP) were calculated from the size distribution data using a Mie model assuming that the soot particles were either internally or externally mixed with the scattering particles. The modeled σAP and σSP were fitted to the measured ones by varying the refractive index.
When fresh emissions from local traffic sources dominated the measurement site the mass concentrations retrieved from the number size distributions of less volatile particles were in good agreement with the BC concentrations, within the uncertainties. This relation was analyzed by plotting the MLV-to-MBC ratios as a function of the fraction of less-volatile particles ⟨φLV⟩ in the size range of the VTDMA. The MLV-to-MBC ratio increased from about 0.5 to ∼ 1 when ⟨φLV⟩ increased from less than 0.1 to about 0.2. The aerosol that had the highest fractions of externally mixed residual material came most probably from the city center or from other near-by pollution sources like diesel vehicles near the sampling site. During periods with wind coming from regions out of the city ⟨φLV⟩ was < 0.1. A large fraction of the aerosol was long-range transported and thus dominated by internally mixed particles and aged soot particles played a significant role in the absorption measurements. These internally mixed particles could for example be influenced by other sources of carbonaceous emissions, e.g., domestic heating (CitationHerrmann et al. 2006). In these cases the MBC was clearly higher than MLV.
There are several reasons for the higher MBC than MLV. The most obvious one is that the MAAP measured total soot concentration, both internally and externally mixed, whereas the VTDMA method gave only the externally mixed fraction. Another reason is that the VTDMA measured concentrations of LV particles of particle sizes only up to 250 nm. This is large enough a size when only externally mixed soot particles are measured. However, soot inclusions are present also in larger internally mixed particles, which could be measured by the MAAP. In principle the VTDMA/Twin-DMPS method could be extended to larger particles as well. If the VTDMA/Twin-DMPS measurements were done exactly for the same size range as a BC monitor is measuring, for instance for submicron particles after a preimpactor, the procedure could be modified for calculating the total non-volatile particle number concentration, i.e., the sum of internally and externally mixed non-volatile particles. Then the VTDMA method and the BC monitor would most probably agree much better also for aged aerosol. This could also be a method to study how the state of mixing affects the absorption efficiency of particles.
Another source of uncertainty in comparing MBC and MLV is that a constant mass absorption cross section is used in converting the filter-based raw measurements, i.e., the absorption coefficients to BC mass concentrations. BC mass absorption cross section is a function of several parameters, for instance particle size, morphology, and state of mixing.
The volume-averaged less volatile particle fraction ⟨φLV⟩ was calculated in order to see, whether it is possible to find an explanation for the differences of the MBC and MLV. ⟨φLV⟩ proved not to be a perfect quantity for predicting when MBC and MLV agree. However, the relationship of ⟨φLV⟩ and the MLV–to–MBC ratio behaved statistically in the right direction: at low ⟨φLV⟩ MLV was clearly lower than MBC and at higher ⟨φLV⟩ the average MLV–to–MBC ratio approached one.
The σAP and σSP measured by the MAAP and the nephelometer were compared with the modeled ones. Mie modeling is usually conducted so that the size-dependent aerosol chemical composition is first obtained from some method and this chemical information is then used for calculating the size-dependent refractive index and subsequently for modeling σAP and σSP, aiming for a radiative closure. In the present campaign no information on aerosol chemistry was available so the above method was no possible and another approach was used. The real and imaginary part of the complex refractive index m = nr + ki i were varied so that the measured and modeled σAP and σSP agreed. An important source of uncertainty of this method is that the combination of nr and ki is not unambiguous, the same σAP and σ SP can be obtained with different nr − ki combinations. Therefore the aim was not to find the best possible nr − ki combination but rather to study the conditions under which σAP derived from the VTDMA data agree with measured σAP and to study which assumption, internal or external mixing of absorbing and scattering aerosol yields the modeled σSP and σAP closer to the measured ones with more realistic refractive indices.
When it was assumed that the absorbing and scattering particles were internally mixed a result of the procedure was an effective refractive index m = nr + ki i that yields the measured σSP and σ AP when applied to the whole particle number size distribution. When it was assumed that the absorbing and scattering particles were externally mixed a result of the procedure was an effective real refractive index nr,V for the volatile particles that were assumed to be purely scattering and a complex refractive index m LV = n r,LV + ki,LV i for the less-volatile particles that were assumed to be the only light-absorbing particles.
When it was assumed that the absorption is due to the externally mixed LV particles only the modeled σAP was most of the time even tens of percents lower than the measured one. When the fraction of less volatile particles ⟨φLV⟩ was higher than 0.2 the modeled and measured σAP agreed well and the fitting procedure resulted in an average soot refractive index of 1.96 – 0.8 that is close to the literature values. When it was assumed that the particles were internally mixed, it was almost always possible to find a combination of nr and ki so that the measured and modeled σAP and σSP agreed. The procedure showed that the aerosol came from varying sources during the measurement period since the resulting refractive index was not constant but had to be varied to fit the measured σAP and σSP.
The fractal-like nature of soot particles creates uncertainty in the modeling of σSPand σAP, especially when modeling is done for the externally mixed soot particles. The optical properties of irregular clusters differ significantly from those of spheres and therefore for a rigorous treatment a more sophisticated model should be used, such as the COSIMA by CitationNaumann (2003). Such a model was not available. However, the goal of the present study was the evaluation of the applicability of the VTDMA method for estimating absorption coefficients by comparing it with a regular filter-based soot monitor. For this purpose it proved out that a standard Mie model yields reasonable results. One of the possible explanations is that even though fresh soot particles are fractal-like aggregates, they may get restructured and collapse at high relative humidities (e.g., CitationWeingartner et al. 1995, Citation1996; CitationMikhailov et al. 2006).
The combination of a volatility TDMA and a mobility size spectrometer is a convenient system to measure the fraction of externally mixed soot and its number concentration. In addition, despite all the uncertainties, the combination of a soot monitor such as the MAAP and a VTDMA is a good method of estimating the fraction of internally and externally mixed soot since the externally mixed soot particles are mainly in the particle size range of the present VTDMA.
Acknowledgments
This work has been supported by the European Commission within the frame of the Marie Curie Programme and the project AEROTOOLS (No. EVK2-CT-2002-57005) and a grant from the Maj and Tor Nessling Foundation, Helsinki, Finland (grant no. 2004172).
REFERENCES
- D'Almeida , G. A. , Koepke , P. and Shettle , E. P. 1991 . Atmospheric Aerosols. Global Climatology and Radiative Characteristics , 561 Hampton, Va : A. Deepak .
- Anderson , T. L. and Ogren , J. A. 1998 . Determining Aerosol Radiative Properties Using the TSI 3563 Integrating Nephelometer . Aerosol Sci. Technol. , 29 : 57 – 69 .
- Andreae , M. O. and Gelencsér . 2006 . Black Carbon or Brown Carbon? The Nature of Light-Absorbing Carbonaceous Aerosols . Atmos. Chem. Phys. , 6 : 3131 – 3148 .
- Berner , A. , Sidla , S. , Galambos , Z. , Kruisz , C. and Hitzenberger , R. 1996 . Modal Character of Atmospheric Black Carbon Size Distributions . J. Geophys. Res. , 101 ( D14 ) : 19559 – 19565 .
- Birmili , W. , Stratmann , F. and Wiedensohler , A. 1999 . Design of a DMA-Based Size Spectrometer for a Large Particle Size Range and Stable Operation . J. Aerosol Sci. , 30 : 549 – 553 .
- Burtscher , H. , Baltensperger , U. , Bukowiecki , N. , Cohn , P. , Hüglin , C. , Mohr , M. , Matter , U. , Nyeki , S. , Schmatloch , V. , Streit , N. and Weingartner , E. 2001 . Separation of Volatile and Non-Volatile Aerosol Fractions by Thermodesorption: Instrumental Development and Applications . J. Aerosol Sci. , 32 : 427 – 442 .
- Cooke , W. F. , Liousse , C. , Cachier , H. and Feichter , J. 1999 . Construction of a 1°× 1° Fossil Fuel Emission Data Set for Carbonaceous Aerosol and Implementation and Radiative Impact in the ECHAM4 model . J. Geophys. Res. , 104 : 22137 – 22162 .
- DeCarlo , P. F. , Slowik , J. G. , Worsnop , D. R. , Davidovits , P. and Jimenez , J. L. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory . Aerosol Sci. Technol. , 38 : 1185 – 1205 .
- Herrmann , H. , Brüggemann , E. , Franck , U. , Gnauk , T. , Löschau , G. , Müller , K. , Plewka , A. and Spindler , G. 2006 . A Source Study of PM in Saxony by Size-Segregated Characterisation . J. Atmos. Chem. , 55 : 103 – 130 .
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurements of Airborne Particles , 10 New York : John Wiley & Sons, Inc. . 51–52, 320–322, 408–410
- Hitzenberger , R. , Jennings , S. G. , Larson , S. M. , Dillner , A. , Cachier , H. , Galambos , Z. , Rouc , A. and Spain , T. G. 1999 . Intercomparison of Measurement Methods for Black Carbon Aerosols . Atmos. Environ , 33 : 2823 – 2833 .
- Ibald-Mulli , A. , Wichmann , H.-E , Kreyling , W. and Peters , A. 2002 . Epidemiological Evidence on Health Effects of Ultra Fine Particles . J. Aerosol Med. , 15 : 189 – 201 .
- Jacobson , M. Z. 2000 . A Physically-Based Treatment of Elemental Carbon Optics: Implications for Global Direct Forcing of Aerosols . Geophys. Res. Lett. , 27 : 217 – 220 .
- Jacobson , M. Z. 2001 . Strong Radiative Heating Due to the Mixing State of Black Carbon in Atmospheric Aerosols . Nature , 409 : 695 – 697 .
- Jonsson , Å. M. , Hallquist , M. and Saathoff , H. 2007 . Volatility of Secondary Organic Aerosols from the Ozone Initiated Oxidation of α -Pinene and Limonene . J. Aerosol Sci. , 38 : 843 – 852 .
- Kalberer , M. , Paulsen , D. , Sax , M. , Steinbacher , M. , Dommen , J. , Prevot , A. S. H. , Fisseha , R. , Weingartner , E. , Frankevich , V. , Zenobi , R. and Baltensperger , U. 2004 . Identification of Polymers as Major Components of Atmospheric Organic Aerosols . Science , 303 : 1659 – 1662 .
- Kuhn , T. , Biswas , S. , Fine , P. M. , Geller , M. and Sioutas , C. 2005a . Physical and Chemical Characteristics and Volatility of PM in the Proximity of a Light-Duty Vehicle Freeway . Aerosol Sci. Technol. , 39 : 347 – 357 .
- Kuhn , T. , Krudysz , M. , Zhu , Y. , Fine , P. M. , Hinds , W. C. , Froines , J. and Sioutas , C. 2005b . Volatility of Indoor and Outdoor Ultrafine Particulate Matter Near a Freeway . J. Aerosol Sci. , 36 : 291 – 302 .
- Mikhailov , E. F. , Vlasenko , S. S. , Podgorny , I. A. , Ramanathan , V. and Corrigan , C. E. 2006 . Optical Properties of Soot–Water Drop Agglomerates: An Experimental Study . J. Geophys. Res. , 111 : D07209 doi:10.1029/2005JD006389
- Müller , T. , Müller , D. and Dubois , R. 2006 . Particle Extinction Measured at Ambient Conditions with Differential Optical Absorption Spectroscopy. 2. Closure Study . Appl. Opt. , 40 ( 10 ) : 2295 – 2305 .
- Naumann , K.-H. 2003 . COSIMA-A Computer Program Simulating the Dynamics of Fractal Aerosols . J. Aerosol Sci. , 34 : 1371 – 1397 .
- Oberdörster , G. 2001 . Pulmonary Effects of Inhaled Ultrafine Particles . Int. Arch. Occup. Environ. Health , 74 : 1 – 8 .
- Ouimette , J. R. and Flagan , R. C. 1982 . The Extinction Coefficient of Multicomponent Aerosols . Atmos. Environ , 16 : 2405 – 2419 .
- Orsini , D. A. , Wiedensohler , A. , Stratmann , F. and Covert , D. S. 1999 . A New Volatility Tandem Differential Mobility Analyzer to Measure the Volatile Sulfuric Acid Aerosol Fraction . Journal of Atmospheric and Oceanic Technology , 16 ( 6 ) : 760 – 772 .
- Park , K. , Kittelson , D. and McMurry , P. 2004 . Structural Properties of Diesel Exhaust Particles Measured by Transmission Electron Microscopy (TEM): Relationships to Particle Mass and Mobility . Aerosol Sci. Technol. , 38 ( 9 ) : 881 – 889 .
- Petzold , A. and Schönlinner , M. 2004 . Multi-Angle Absorption Photometry—A New Method for the Measurement of Aerosol Light Absorption and Atmospheric Black Carbon . J. Aerosol Sci. , 35 : 421 – 441 .
- Philippin , S. , Wiedensohler , A. and Stratmann , F. 2004 . Measurements of Non-Volatile Fractions of Pollution Aerosols with an Eight-Tube Volatility Tandem Differential Mobility Analyzer (VTDMA-8) . J. Aerosol Sci. , 35 : 185 – 203 .
- Rader , D. J. and McMurry , P. H. 1986 . Application of the Tandem Differential Mobility Analyzer to Studies of Droplet Growth or Evaporation . J. Aerosol Sci. , 17 : 771 – 787 .
- Rose , D. , Wehner , B. , Ketzel , M. , Engler , C. , Voigtländer , J. , Tuch , T. and Wiedensohler , A. 2005 . Atmospheric Number Size Distributions of Soot Particles and Estimation of Emission Factors . Atmos. Chem. Phys. , 6 : 1021 – 1031 .
- Schnaiter , M. , Horvath , H. , Möhler , O. , Naumann , K.-H , Saathoff , H. and Schöck , O. W. 2003 . UV-VIS-NIR spectral optical properties of soot and soot-containing aerosols . J. Aerosol Sci. , 34 : 1421 – 1444 .
- Seinfeld , J. H. and Pandis , S. N. 1998 . Atmospheric Chemistry and Physics: From Air Pollution to Climate Change , Hoboken, NJ : John Wiley .
- Slowik , J. , Stainken , K. , Davidovits , P. , Williams , L. , Jayne , J. , Kolb , C. , Worsnop , D. , Rudich , Y. , DeCarlo , P. and Jimenez , J. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 2: Application to Combustion-Generated Soot Aerosols as a Function of Fuel Equivalence Ratio . Aerosol Sci. Technol. , 38 ( 12 ) : 1206 – 1222 .
- Smith , M. H. and O'Dowd , C. D. 1996 . Observations of Accumulation Mode Aerosol Composition and Soot Carbon Concentrations by Means of a High-Temperature Volatility Technique . J. Geophys. Res. , 101 ( D14 ) : 19583 – 19591 .
- Smyth , K. C. and Shaddix , C. R. 1996 . The Elusive History of m = 1.57 – 0.56i for the Refractive Index of Soot. . Combustion and Flame , 107 : 314 – 320 .
- Wehner , B. , Philippin , S. , Wiedensohler , A. , Scheer , V. and Vogt , R. 2004 . Variability of Non-Volatile Fractions of Atmospheric Aerosol Particles with Traffic Influence . Atmos. Environ. , 38 : 6081 – 6090 .
- Weingartner , E. , Baltensperger , U. and Burtscher , H. 1995 . Growth and Structural Change of Combustion Aerosols at High Relative Humidity . Environ. Sci. Technol. , 29 ( 12 ) : 2982 – 2986 .
- Weingartner , E. , Baltensperger , U. and Burtscher , H. 1996 . Hygroscopic Properties of Carbon and Diesel Soot Particles . Atmos. Environ. , 31 : 2311 – 2327 .
- Wex , H. , Neusüss , C. , Wendisch , M. , Stratmann , F. , Koziar , C. , Keil , A. and Wiedensohler , A. 2002 . Particle Scattering, Backscattering, and Absorption Coefficients: An in Situ and Sensitivity Study . J. Geophys. Res. , 107 ( D21 ) : 8122 doi:10.1029/2000JD000234 2002