Abstract
The Aerodyne Aerosol Mass Spectrometer (AMS) is a useful tool to study ambient particles. To be quantitative, the mass or (number) of particles detected by the AMS relative to the mass (or number) of particles sampled by the AMS, or the AMS collection efficiency (CE), must be known. Here we investigated the effect of particulate phase on AMS CE for ammonium nitrate, ammonium sulfate, mixed ammonium nitrate/ammonium sulfate, and ammonium sulfate particles coated with an organic liquid. Dry, solid ammonium sulfate particles were sampled with a CE of 24 ± 3%. Liquid droplets and solid particles that were thickly coated with a liquid organic were collected with a CE of 100%. Mixed phase particles, solid particles thinly coated with liquid organic, and metastable aqueous ammonium sulfate droplets had intermediate CEs. The higher CEs for liquid particles compared with solid particles were attributed to wet or coated particles tending to stick upon impact with the AMS vaporizer, while a significant fraction of solid particles bounced prior to vaporization/detection. The consistency of single particle signals indicated that the phase (and hence CE) of mixed component particles did not affect the AMS sensitivity to a particular chemical species once volatilization occurred. Particle phase might explain a significant fraction of the variable AMS CEs reported in the literature. For example, ambient particles that were liquid (e.g., composition dominated by ammonium nitrate or acidic sulfate) have been reported to be sampled with 100% CE. In contrast, most ambient particle measurements report CEs of < 100% (typically~ 50%).
INTRODUCTION
Atmospheric aerosols play important roles in human health, visibility, acid deposition, and global climate. The effects of aerosols depend upon the chemical composition, phase, size, shape, and number and mass concentrations of the particles which comprise the aerosol. Although there are several types of instruments designed to investigate aerosols, very few are capable of providing real-time, size-resolved, quantitative measurements of both chemical composition and mass on a fast time scale. The Aerodyne aerosol mass spectrometer (AMS, developed by Aerodyne Research Inc., Billerica, MA, USA) is capable of providing these measurements, and therefore represents a powerful tool for the study of ambient atmospheric aerosols. Although great advances have been made in using the AMS as a quantitative tool (CitationAllan et al. 2003a,Citationb; CitationJimenez et al. 2003; CitationCanagaratna et al. 2007), there remain some questions concerning the collection efficiency (CE) of particles sampled into the AMS system. In simplest terms, the CE is defined as the ratio of the mass (or number) of particles detected by the AMS to the mass (or number) of particles introduced to the AMS inlet.
Comparisons between the AMS and other co-located aerosol instruments report high degrees of correlation between measured particle chemistry and mass, though the slopes of the correlations are typically less than unity. The observed discrepancies, after accounting for varying transmission efficiencies among the instruments and other potential issues, appear to be due to collection efficiencies of less than 100% in the AMS. Apparent CE values for field data have been determined by comparing the AMS mass loadings for a given chemical species with mass loadings obtained from other particulate chemical measurement techniques, such as particle into liquid samplers (PILS) with ion chromatography analysis (CitationWeber et al. 2001; CitationTakegawa et al. 2005), and the AMS total mass loadings (sum of all chemical species) with other number and mass based instrument techniques, such as SMPS (CitationQuinn et al. 2006) and TEOM (CitationDrewnick et al. 2004; CitationWeimer et al. 2006). In early field campaigns, the mass of sulfate detected by the AMS was often low by a factor of 1.5–2.3, implying an apparent CE value for sulfate between 40 and 70% (CitationAllan et al. 2004; CitationDrewnick et al. 2004; CitationHogrefe et al. 2004a; CitationWeimer et al. 2006). Allan and co-workers (2004) demonstrated that there was a relative humidity (RH) influence on the CE, Quinn and co-workers (Citation2006) observed a dependence on particle acidity, and Crosier and co-workers (Citation2007) observed a dependence on nitrate content. In addition, chamber studies have shown that secondary organic aerosol can coat ammonium sulfate particles and lead to a change in the observed sulfate mass loadings detected by the AMS (A. Kiendler-Scharr, personal communication 2004; CitationBahreini et al. 2005). Other laboratory studies of pure organic species indicated that the collection efficiency depended on the phase of the particles, with 100% CE for liquids and 20–50% CE for solids (CitationAlfarra 2004).
In the aerodynamic lens, spherical particles are focused with higher efficiency than nonspherical particles (CitationLiu et al. 1995; CitationJayne et al. 2000; CitationKane and Johnston 2000; CitationTobias and Ziemann 2000). Hence, it was initially suspected that CEs less than 100% for sulfate particles observed in ambient measurements was caused by dry, nonspherical sulfate-containing particles not being focused into a narrow enough beam to allow all the particles to impact the AMS vaporizer (CitationAllan et al. 2004; CitationHogrefe et al. 2004a). The observed increases in apparent CE values at high relative humidity (RH) by Allan and co-workers (Citation2004) were attributed to the solid sulfate particles becoming spherical at high RH due to deliquescence, allowing the particles to be more efficiently focused by the aerodynamic lens inlet system. However, it has recently been reported in laboratory and field studies that the aerodynamic lens in the AMS is capable of focusing many types of particles, including dry sulfate particles, into a beam narrow enough to allow all the particles to strike the AMS vaporizer (CitationHuffman et al. 2005; CitationWeimer et al. 2006; CitationQuinn et al. 2006; CitationSalcedo et al. 2007).
New laboratory and field results indicate that AMS CE values less than 100% are likely due to solid particles bouncing off the vaporizer, resulting in these particles not being detected by the AMS system (CitationQuinn et al. 2006). Particle bounce or “reflection” has long been known to influence measurements from traditional impactors and particle mass spectrometers utilizing flat surfaces for volatilization; various approaches have been used to minimize its effects including vaporizer design and RH control (CitationMyers and Fite 1975; CitationStoffels and Lagergren 1981; CitationVasiliou et al. 1999). The kinetic energy required for bounce to occur for the same incident normal velocity is proportional to the particle diameter (CitationDahneke 1971). Furthermore, the higher the incident velocity or the harder the material, the less likely the particle's kinetic energy can be transferred to the impaction surface and more likely the particle will bounce (CitationHinds 1982). According to CitationCheng and Yeh (1979), the maximum velocity at which 100 nm latex particles collide and stick to (i.e., not bounce off) an uncoated metal surface at atmospheric pressure is 20 m/s. The impaction conditions in the AMS differ significantly from traditional impactors: the pressure at the vaporizer is very low (< 10−5 mbar), particle velocities are very high (typically 90–250 m/s) (CitationJayne et al. 2000), and the vaporizer is a porous tungsten cylinder machined into an inverted cone and heated to 600°C. While the details differ, the collection efficiency of the AMS is determined, in part, by particle bounce. Here, we focused on particulate phase and its effect on particle bounce and the AMS collection efficiency.
The AMS CE is dependent upon particle transmission into vacuum, particle focusing onto the vaporizer, and particle vaporization (or particle bounce at the vaporizer). The AMS CE as defined by Huffman and co-workers (2005) for a given vacuum aerodynamic diameter (CE(d va )) is:
For particles falling within the vacuum aerodynamic size range for which the standard lens was designed (60–600 nm), E L (d va ) is 100% (CitationJayne et al. 2000; CitationLiu et al. 2007). Because ambient particle distributions could fall outside the optimum transmission range of the lens, E L (d va ) may need to be considered when interpreting field data. The losses associated with E s (d va ) appear to be less than 20% for ambient particles (CitationHuffman et al. 2005; CitationQuinn et al. 2006; CitationSalcedo et al. 2007). Thus, E b (d va ) may be the dominant term in determining CE (d va ) for particle sizes that fall within the transmission range of the lens.
This article investigated the effect of particle phase on CE (d va ), focusing on solid or liquid laboratory particles to provide insight into understanding the CE (d va ) of the AMS during ambient sampling. To simplify the experiments, we chose particle sizes (200 to 400 nm in diameter) and types (ammonium sulfate, ammonium nitrate, and organic liquids) that have been shown to be transmitted and detected efficiently by the AMS inlet system (CitationHuffman et al. 2005). Thus, with E L (d va ) and E s (d va ) equal to 100% and a narrow size range studied, CE should be equal to E b . In all experiments, we determined the overall value of CE as a measure of E b , and did not attempt to measure E L , E s , or E b individually. The underlying assumption of this study was that particles not detected by the AMS have bounced off the vaporizer prior to vaporization and subsequent detection.
EXPERIMENTAL SECTION
A schematic diagram of the experimental apparatus is shown in . Four basic types of experiments were conducted: (1) hydration of ammonium sulfate or ammonium nitrate particles, (2) dehydration of ammonium sulfate or ammonium nitrate particles, (3) dry, mixed ammonium nitrate/sulfate particles with varying compositions, and (4) coating dry, solid ammonium sulfate particles with liquid dioctyl sebacate.
FIG. 1 Schematic diagram of the experimental apparatus. The thick, solid lines represent the aerosol flow path. After generation, particles were sized with a differential mobility analyzer (DMA). For the hydration experiments, the aerosol stream was next passed through a drying relative humidity controller (RHC #1), while for the dehydration experiments, the aerosol was passed above water. The relative humidity was conditioned with the second controller (RHC #2). For the mixed ammonium sulfate/ammonium nitrate experiments, the sized particles were passed through RHC #1 to dry them and RHC #2 was removed. When coating particles with dioctyl sebacate (DOS), RHC #1 was used as a dryer and RHC #2 was replaced with the glass DOS condensing chamber (see text). In all experiments, particles entering the AMS were counted with a condensation particle counter (CPC).
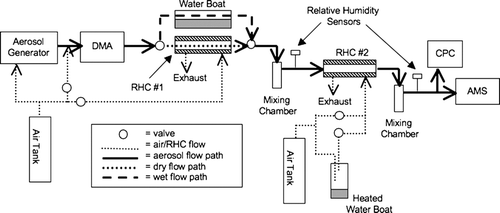
For the hydration and dehydration experiments, particles were generated with a custom built atomizer, dried, and then sized with a custom-built differential mobility analyzer (DMA). Filtered, recirculated air was used for the DMA inlet and outlet sheath flows, and the aerosol flow exiting the DMA typically had a relative humidity of 10–20% RH. By monitoring the particle distribution with the AMS, the aerosol generator operating pressure and solution concentrations were optimized to minimize the occurrence of multiply charged particles. For hydration experiments, the particles were dried further by passing them through an RH controller (RHC, see below) operating as a dryer. For dehydration experiments, the aerosol stream was instead passed through the headspace of a vessel containing water (water boat) which served to hydrate the aerosol. Next, the dried (hydration experiments) or fully hydrated (dehydration experiments) particles were passed through a mixing chamber and the initial RH was measured. Then, the particles were passed through a second RHC (which adjusted the RH of the aerosol stream) and a second glass mixing chamber after which the final RH was measured. The aerosol stream was then split with an isokinetic tee and sampled by a condensation particle counter (CPC, TSI model 3022A) as well as the AMS. All connecting tubing was stainless steel with some short sections of black conductive tubing (TSI). For hydration experiments, the dried particles were exposed to increasing RH to mimic particles undergoing deliquescence (solid-to-liquid phase transition). For dehydration experiments, the fully hydrated particles were exposed to decreasing RH conditions to study potential efflorescence (liquid-to-solid phase transition).
The RH controllers (RHC) were made from microporous Teflon tubing enclosed in stainless steel tubing. The aerosol stream passed through the center of the microporous tubing while dry (or humidified) sheath air passed through the space between the microporous tubing and stainless steel tubing in the opposite direction. In the first RHC, used only for drying, water vapor was removed by diffusion from the aerosol stream through the microporous tubing to the dry sheath air. The second RHC worked in the same manner, except water vapor diffused in either direction depending on the difference in RH between the aerosol and sheath flows. The humidity of the sheath air in the second RHC was controlled by manually mixing different amounts of dry and humidified air while keeping the total sheath flow constant. Using this system, relative humidity values from less than 10% RH to more than 90% RH measured downstream of the second RHC were achieved for the hydration experiments while values between 18% RH to more than 90% RH were achieved for the dehydration experiments. The RH measurements (Vaisala Humitter model 50Y) were precise within a few percent and accurate within ± 5% RH. The relative humidity reported in the figures was from the second sensor, representing the sample line prior to entering the CPC and the AMS.
The experiments for the mixed ammonium nitrate/ammonium sulfate particles were conducted analogous to the hydration experiments discussed above, except the relative humidity was held constant at 10% RH. The mixed composition particles were produced by atomizing solutions containing a fixed amount of ammonium nitrate and varying amounts ammonium sulfate. Particles for the coating experiments were generated by first aerosolizing aqueous solutions of ammonium sulfate, drying and sizing them with the DMA, further drying them with the first RHC, and then condensing dioctyl sebacate (DOS) onto them. The DOS condensation system was identical to that used previously for coating organic liquid particles with sulfuric acid (CitationMiddlebrook et al. 1997). Here, the dry particles passed through a bent glass tube containing a small drop of pure liquid DOS encased in a temperature controlled aluminum heater block. By controlling the temperature, and therefore the amount of DOS in the gas phase, the amount of DOS condensing on the particles was controlled. The amount of DOS on the coated particles was measured with the AMS, using a relative ionization efficiency (RIE) of 1.4 (the standard value for organic mass, CitationCanagaratna et al. 2007). The DOS layer thickness was calculated from the organic mass fraction and material densities (ρDOS = 0.914 g cm−3 and ρ(NH4)2SO4 = 1.77 g cm−3) and assuming uniform coatings on spherical (NH4)2SO4 particles.
For all of these experiments, a quadrupole aerosol mass spectrometer (Q-AMS) was used. The operation of the Q-AMS has been discussed in detail elsewhere (CitationJayne et al. 2000; CitationAllan et al. 2003a, CitationJimenez et al. 2003; CitationDrewnick et al. 2004), but a brief discussion of its operation is provided here. The AMS measured particles by sampling them through a critical orifice followed by a low pressure (2.0 mbar) aerodynamic lens which focused the particles into a narrow beam. The particles passed through the lens into a high vacuum region, traversed the particle time-of-flight chamber and then impacted onto a vaporizer, producing gas phase species, some of which were converted to ions via electron impact ionization. The vaporizer was a 3.8 mm diameter, porous (80% dense) tungsten cylinder machined with an inverted cone (60° angle and 3 mm deep) into the end facing the particle beam. The vaporizer temperature for these experiments was between 575 and 600°C, which is the recommended operating temperature range for ambient aerosol measurements (CitationJimenez et al. 2003). All AMS instruments currently use the same vaporizer design.
For these experiments, the resulting ions were analyzed with a quadrupole mass spectrometer. In mass spectrum (MS) mode, complete mass spectra from all of the particles and gas molecules interacting with the vaporizer were measured along with the instrument background spectra. In particle time-of-flight (PToF) mode, the particle beam first passed through a 2% chopper wheel located at the entrance of the time-of-flight region which allowed particles of different sizes to be separated based on their velocity in the time-of-flight chamber. For a given set of experimental conditions and particle compositions in this work, PToF data as well as bulk mass spectra were averaged for 5 min and recorded.
In the laboratory, the most accurate method for determining CE is through direct particle counting statistics and dividing the Q-AMS particle counts by those from the CPC. CE can also be determined through average particle mass concentrations compared to other instruments as has been done for field studies (CitationAlfarra et al. 2004; CitationAllan et al. 2004; CitationDrewnick et al. 2004; CitationHogrefe et al. 2004a). The mass-based CE for these laboratory experiments was determined by dividing the measured AMS particulate mass by the particulate mass calculated from CPC counts of monodisperse particles (size selected using a DMA), particle densities, and the assumption of particle sphericity (CitationLiu et al. 2007). The mass method works well when there are few multiply charged particles from the DMA or when the greater mass of the multiply charged particles is taken into account theoretically. For the mass measurements here, the RIE for sulfate, nitrate, and ammonium were 1.15, 1.1, and 4, respectively (CitationCanagaratna et al. 2007).
Individual particles were detected in PToF mode and counted with the Q-AMS by monitoring the signal at specific mass to charge ratios (m/z) for selected particle components (e.g., m/z = 30 or 46 for nitrate and m/z = 48, 64, or 80 for sulfate). A particle was counted whenever a pulse of the specific m/z ratio was detected above an established background level, and the particle count data were stored as PToF particle number histograms for each m/z as a function of particle flight time. PToF data also included the summed integrated ion signals for each m/z analyzed from all counted and uncounted particles as a function of particle flight time. The average particles per cubic centimeter counted by the AMS was determined by integrating the PToF particle number histogram for a given m/z value, and then taking the average value for all the m/z used for a given species.
The threshold for counting individual particles varied for each m/z depending on the signal background (which could change between experiments), and so the minimum particle size for counting individual particles varied with the amount of material in the particles as well as the m/z used. For example, at m/z 30 all of the dry, ammonium nitrate particles larger than d va = 220 nm were counted whereas at m/z 46 all were counted from particles larger than d va = 160 nm due to the lower background. At m/z 30 none of the particles smaller than d va = 100 nm were counted, so the particle counting statistics increased as a function of size for the intermediate sizes. While the noise of one PToF scan placed limits on the size of individual particles counted and detected by the Q-AMS, in normal operation of both MS and PToF modes the total integrated signals are used and in the example discussed above the integrated signal from both m/z 30 and 46 was measured from particles as small as 40 nm d va .
For the count-based collection efficiency measurements, it was important that all of the particles entering the instrument were included in the Q-AMS particle counts. These counting statistics were determined at each m/z by dividing the PToF signal where particles were counted by the total PToF signal integrated over several chopper cycles during the sampling interval which increased the signal to noise. So if the ratio of the counted to integrated signal for a given m/z was less than 80% due to high background signals or insufficient signal for counting, the derived Q-AMS number concentrations were increased by the inverse of this ratio to account for the drop in counting statistics. Although most of the particles were large enough for this not to be important, the counts from some of the mixed composition particles needed to be adjusted for uncounted particles with low signals arising from the minor chemical component in the particles.
The uncertainty associated with the count-based CE measurements was calculated as ± 20% based upon one standard deviation of the average CE from the experiments with 199 nm ammonium nitrate particles where the CE remained essentially unchanged as a function of relative humidity. The uncertainty in the CE measurements increased above the DRH during the hydration/dehydration experiments due to the combined effects of the sharp decrease in E L (d va ) (particle transmission through the lens) and particle size variations due to uncertainties in RH. Averages of the CE measurements are reported here with the associated standard deviations.
Ammonium nitrate (99.99+%), ammonium sulfate (99.99+%), and dioctyl sebacate (DOS) (95%) were acquired from Aldrich Chemicals and used without further purification. Nanopure water was used to prepare solutions of the ammonium salts and for adjusting the aerosol RH. Filtered Ultra Zero air (General Air Service and Supply, Denver, CO) was used for the generation of particles in all experiments.
RESULTS AND DISCUSSION
Ammonium Nitrate Particles
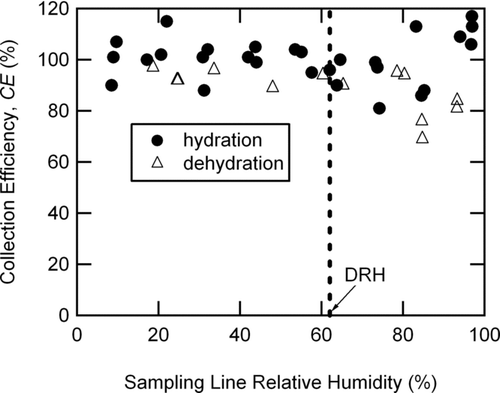
The collection efficiencies for ammonium nitrate (NH4NO3) particles were measured as a function of relative humidity as particles were exposed to increasing (hydration) or decreasing (dehydration) relative humidity. depicts the CE results for both hydration and dehydration experiments with initial dry mobility diameters (d m ) = 199 nm. CE was 96 ± 20% and did not demonstrate a dependence upon RH within experimental uncertainty. These particle count-based results indicate that NH4NO3 particles transmitted into the AMS (E L and E S = 100%) were collected within the AMS with CE = 100%. The wider range of CE values observed above 80% RH (from 69 to 117%) was due to the combination of variations in the deliquesced particle size and a sharp decrease in particle transmission through this lens at sizes close to the deliquesced particle sizes. Literature reports that submicron NH4NO3 particles remain as liquid or metastable liquid droplets throughout the full relative humidity range accessible in our laboratory (CitationTang 1980; CitationLightstone et al. 2000).
FIG. 2 Count-based collection efficiencies for NH4NO3 particles (initial d m = 199 nm) for the hydration (solid circles) and dehydration (triangles) experiments as a function of sampling line relative humidity. The vertical dashed line indicates the deliquescence relative humidity (DRH at 62% RH) for NH4NO3 (CitationTang 1980).
Ammonium Sulfate Particles
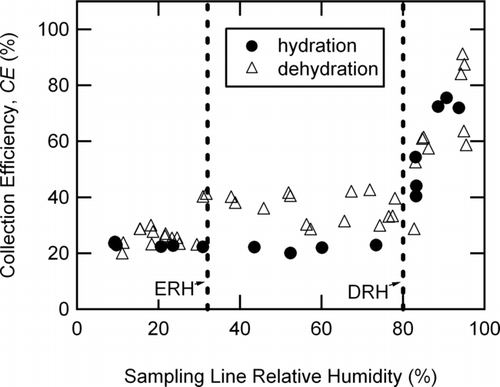
Unlike the ammonium nitrate case, ammonium sulfate ((NH4)2SO4) particles exhibited obvious phase transitions as a function of relative humidity. In fact, shows that the CE for (NH4)2SO4 particles with initial dry d m = 199 nm demonstrated a strong RH dependence. In the hydration experiment, the CE values were 22 ± 2% for RH less than the deliquescence relative humidity (DRH = 80% RH) (CitationTang 1980). However, at RH greater than the DRH, the CE values rose quickly towards 100%, with the last experimental points showing a CE of 73 ± 2% at 90% RH. This change in CE around the DRH was consistent with the predicted (NH4)2SO4 solid-to-liquid phase transition occurring in the sampling line upstream of the AMS inlet. During the dehydration experiment (also in ), CE = 76 ± 15% above 90% RH and decreased with decreasing RH to the DRH. Below the DRH, the average CE = 36 ± 5% until the relative humidity reached 30% RH. Below 30% RH, the CE = 25 ± 3%, in close agreement with the results from the hydration experiment up to the DRH. (NH4)2SO4 particles have been shown to have well defined deliquescence (DRH) and efflorescence relative humidity (ERH) points (80% RH and 36% RH, respectively) (CitationTang 1980). Further, the measured ERH for submicron (NH4)2SO4 particles is slightly lower than for supermicron particles (32% RH rather than 36% RH) (CitationOnasch et al. 1999). The results in show that the AMS was sensitive to both of these phase transitions for submicron ammonium sulfate particles occurring upstream of the AMS. At low RH where the (NH4)2SO4 particles were dry solids, both experiments indicated a constant CE of 24 ± 3%. These low CE values were likely due to particles bouncing off the vaporizer prior to full vaporization causing particle mass to go undetected by the AMS. Note that the CEs of dry, pure ammonium sulfate particles did not show a clear size dependence over the range of sizes measured here (CE = 24 ± 3%, 29 ± 3%, and 29 ± 6% for d m = 199, 276, and 343 nm, respectively).
FIG. 3 Count-based collection efficiencies for (NH4)2SO4 particles (initial d m = 199 nm) for the hydration (solid circles) and dehydration (triangles) experiments as a function of sampling line relative humidity. The vertical dashed lines indicate the efflorescence and deliquescence relative humidities (ERH at 32% RH and DRH at 80% RH) for (NH4)2SO4 (CitationOnasch et al. 1999).
Above the deliquescence point, (NH4)2SO4 particles were liquid solution droplets. Hence, there should have been a step function in the observed CE at 80% RH with deliquescence changing the solid particles into liquid droplets, thus increasing the CE to 100%. However, during the hydration experiment, the observed CE above 80% RH appeared to increase with increasing RH, rather than jumping directly to 100% at the point where the particles were liquid droplets in the sampling line. Phase changes due to water evaporation in the inlet might be the reason that CE did not increase sharply to 100% at the deliquescence point during this experiment. The dehydration experiment exhibited the same behavior as the relative humidity decreased. For both experiments above 87% RH the average CE was 75 ± 11%. As for the NH4NO3 particles, the same two factors contributed to the wide range of CE observed (from 58 to 90%) for the points above the DRH: variations in the deliquesced particle size due to uncertainties in RH and a sharp decrease in particle transmission through this particular lens near the deliquesced particle sizes.
Under typical conditions, (NH4)2SO4 particles undergoing dehydration will remain liquid (metastable with respect to the thermodynamically stable solid) until the system reaches the ERH where particles effloresce. As mentioned above, the (NH4)2SO4 particles in these experiments effloresced at 30% RH. However, in the metastable regime (between ERH and DRH), where the droplets in the sampling line remained liquid, the observed CE was 36 ± 5%. This value was experimentally different than the 22 ± 2% observed over the same RH range in the hydration experiment; however, it was significantly lower than the CE for liquid droplets. These results were further confirmed with the mass-based CE values in this relative humidity range (not shown for clarity) which were similar to the count-based CEs.
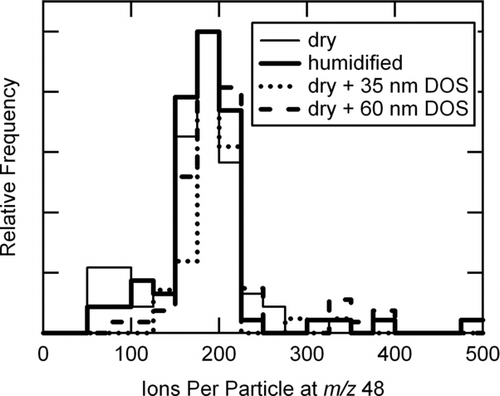
The observed increase in the measured sulfate mass with increasing RH (RH > 80% DRH) was due to an increase in the number of particles detected and not due to an increase in the number of sulfate ions per particle. To verify this, we examined the ions per particle (IPP) at one m/z, for dry and hydrated (NH4)2SO4 particles with the same initial dry dm. For particles with an initial dry d m = 276 nm, the average at m/z = 48 was 175 ± 56 IPP and for subsequently hydrated particles was 191 ± 73 IPP (thin and thick lines in , respectively). For the particles depicted in (d m = 199 nm), the average signal per particle at m/z = 48 was 63 ± 26 IPP and 59 ± 12 IPP for dry and hydrated particles, respectively. Since the values were nearly identical for the same initial dry particle size (i.e., mass of ammonium sulfate), the presence of water did not appear to significantly alter the number of sulfate ions detected per particle at high RH and could not account for the large changes in CE values observed in for RH > 80%.
FIG. 4 Histograms of the integrated peak area at m/z 48 for individual (NH4)2SO4 particles (initial d m = 276 nm) that were dry, humidified to > 90% RH, and dry and coated with varying layer thicknesses of dioctyl sebacate (DOS).
A potential explanation for the difference in the CE of the dehydrating particles in the metastable regime (between ERH and DRH) compared to that of the solid particles (dehydrating particles below the ERH or hydrating particles up to the DRH) might be found in an analysis of the particulate water content and the observed particle morphologies. The AMS particle water content was estimated using a relative ionization efficiency of 1 for water and was corrected for the gas phase water concentration. The gas phase correction was determined by measuring the water signal relative to air as a function of RH in particle free air, which resulted in the following linear fit: (m/z = 18)/(m/z = 28) = (1.0 × 10−3 × P H 2 O ), where P H 2 O was the water vapor pressure in mbar.
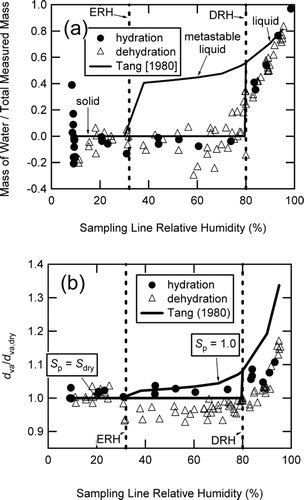
As can be seen in , although somewhat noisy, the water mass fraction of the particles for less than 30% RH averaged to 0 ± 0.1 for both the hydration and dehydration experiments and for less than 80% RH it averaged to 0 ± 0.2, implying that the dehydrated particles had little remaining water content after sampling into the vacuum system of the AMS. The noise in these data points reflected the relatively high background signal at m/z 16, 17, and 18 in the AMS from water in the vacuum chamber and the accuracy in determining the particulate water mass fraction with the AMS, mainly due to the accuracy of accounting for the water in the air as well as uncertainties in the fragmentation pattern of sulfate which generated signal at m/z 18 where water was measured. Note that the water content measurements shown in were independent of CE since the water content was normalized by the total aerosol mass (water plus inorganic mass). For comparison, the calculated water content from (NH4)2SO4 data in the literature (CitationTang 1980, also shown in ) had a distinct departure from the equilibrium solid data for the metastable liquid particles, due to the metastable liquid particles containing more water than the solid particles. The AMS estimated particulate water content was lower than the literature values and only approached the literature values at the very highest sampling line RH (above 95% RH) or below the ERH. This fact remained true in the metastable region, even if the estimated relative ionization efficiency of water was underpredicted by a factor of 2. This indicated that a significant fraction of particulate water was being lost from the aqueous (and metastable) droplets during sampling into the low-pressure regime inside the aerodynamic lens inlet.
FIG. 5 (NH4)2SO4 (a) particulate water content and (b) growth curves as a function of sampling line relative humidity for experiments initiated with dry d m = 199 nm particles. The vertical dashed lines indicate the efflorescence and deliquescence relative humidities (ERH at 32% RH and DRH at 80% RH, respectively) for (NH4)2SO4 (CitationOnasch et al. 1999). (a) Particulate water content is reported as the ratio of measured water to the total measured mass. The solid lines are the ratios of measured water to the total measured mass calculated from the literature data (CitationTang, 1980), with the upper and lower branches between the ERH and DRH indicative of metastable liquid and crystalline solids, respectively. (b) Growth curves for (NH4)2SO4 in terms of d va /d va, dry as measured by the AMS. The solid curve is the literature (CitationTang 1980) physical growth data converted to vacuum aerodynamic diameter (d va /d va, dry ) using Equation (Equation3) and S p = S dry = 0.96 for the lower horizontal branch of the curve between 30 and 80% RH where the dry particles have not deliquesced and S p = 1.0 for the upper branch of the curve between 30 and 95% RH where the particles are liquid or metastable liquid.
Since the metastable particles exhibited different CE values than the dry solid ammonium sulfate particles, it was possible that the metastable particles solidified in the aerodynamic inlet with different morphologies than those that solidified outside the system. One measurement of particle morphology in the AMS was provided by a comparison of the vacuum aerodynamic diameter (d va ) (CitationDeCarlo et al. 2004; CitationSlowik et al. 2004; CitationZelenyuk et al. 2006a) to the mobility diameter (d m ) for a monodisperse particle sample. The relationship between the d va and dm for particles without voids was given by equations 32 and 44 from DeCarlo and co-workers (2004) as:
The growth factor ratio of the measured d va to the dry d va (or d va /d va, dry ) is plotted as a function of sampling line RH for the (NH4)2SO4 hydration and dehydration experiments in . Also shown in the figure are the CitationTang (1980) literature values for physical growth factors () converted to using the following equation:
Upon closer examination of the d va /d va, dry growth factors in , the dehydrating particles in the metastable regime of 30–80% RH consistently had a lower d va /d va, dry than the particles undergoing hydration. A lower implied that the dehydrating particles in this regime had a lower effective density ρ eff = ρ o × d va /d m = S p × ρ p , CitationDeCarlo et al. 2004 than particles that solidified outside the AMS system (CitationSlowik et al. 2004; CitationZelenyuk et al. 2006a; CitationSlowik et al. 2007). For the data shown in , the derived ρ eff is 1.66 g cm−3, which was 4% lower than the measured ρ eff of 1.70 g cm−3 for the dry, solid ammonium sulfate particles. Both of these effective density measurements were slightly higher than Zelenyuk and co-workers (Citation2006a) reported (ρ eff of 1.64 g cm−3) for 200 nm dry solid ammonium sulfate particles.
The observed lower effective density, lower particulate water content estimates, and lower AMS CE than predicted or observed for other liquid droplets suggested that the aqueous and metastable ammonium sulfate particles were affected by the sampling of these particle types into the low pressure regime of the aerodynamic lens inlet. The water content results showed that the inlet relative humidity (and possibly temperature) were different from those in the sampling line and varying these conditions might affect the evaporation rate and resulting morphology of dried particles (CitationLeong 1987a,Citationb). These observations differed from the results for ammonium nitrate particles, where both the hydration and dehydration particles were likely initially aqueous/metastable and apparently did not change phase or shape significantly upon sampling. The current study could not conclusively identify the phase or morphology changes of the sampled metastable ammonium sulfate particles that were responsible for the observed CE and ρ eff . However, since the aqueous/metastable particles exhibited an AMS CE significantly less than 100%, they likely underwent a phase transition (i.e., solidified).
There are several potential explanations for these observed CE and ρ eff changes. The first potential scenario is that the sampled ammonium sulfate metastable droplets solidified due to water loss and/or latent cooling inside the aerodynamic lens into an amorphous solid with a near spherical shape, but lower density than dry ammonium sulfate. A 4% decrease in the particle density due to the presence of some water remaining in the metastable/glassy particles was less than, but comparable to, the 7 –10% decrease previously observed for the difference in particle densities for anhydrous amorphous particles relative to crystalline solid particles (CitationZelenyuk et al. 2005). Furthermore, the measurable particulate water content on the aqueous particles changed smoothly with decreasing RH suggesting that the particles still contained some water content (at least to the DRH) and thus likely had a lower particle density than dry ammonium sulfate particles.
A second explanation is that the metastable droplets solidified into dry, solid ammonium sulfate particles with different morphologies (e.g., nonspherical or hollow) than dry, solid ammonium sulfate particles that effloresced outside of the AMS, but with similar material densities. The more nonspherical a particle, the greater the surface to volume ratio and thus the more likely the particle is to “stick” to the surface of the AMS vaporizer and be fully vaporized. While the effective density was observed to decrease slightly (4%), the CE was observed to increase 50% from 24 ± 3% to 36 ± 5%, however, remained significantly below 100%. A third explanation is simply that the metastable droplets solidified with different morphologies and densities than dry ammonium sulfate particles.
A fourth potential explanation for the observations could be that some of the metastable particles effloresced and some did not, causing an ensemble CE which was between the values for solid and aqueous liquid ammonium sulfate. While this explanation could not be directly discounted based on the current work, the observed changes in effective densities and the results obtained from coating dry ammonium sulfate particles with liquid organic (discussed in detail in the next section) suggested that this was not a likely scenario.
A thorough and definitive investigation of the potential reasons underlying these interesting observations is beyond the scope of this article. A measure of particle sphericity or shape (independent from density) is required to conclusively determine whether the decrease in observed effective density was due to changes in particle density, shape, or a combination of the two. In previous laboratory work, high-resolution optical d va distributions for ammonium sulfate particles exhibited a greater width (10%) than spherical polystyrene latex spheres showing that the ammonium sulfate particles were slightly aspherical (CitationZelenyuk et al. 2005). For the experiments shown here with the AMS, similar determinations of particle asphericity could be obtained using a wire beam width probe (CitationHuffman et al. 2005; CitationSalcedo et al. 2007). Unfortunately, a beam width probe was not available during this study.
Mixed Ammonium Nitrate/Sulfate Particles
Experiments were conducted with mixed NH4NO3/ (NH4)2SO4 particles to measure CE as a function of the fraction of NH4NO3 (ammonium nitrate mass fraction or ANMF) to determine how the particles behave in the AMS system as the composition varies from pure (NH4)2SO4 to NH4NO3. This experiment might be particularly relevant considering recent ambient measurements of individual particles showing nitrate to be internally mixed with sulfate when sulfate was fully neutralized by ammonium (CitationDrewnick et al. 2005; CitationMurphy et al. 2006). The water activity and phase of the mixed H+/NH4 +/HSO4 −/SO4 2−/NO3 − system has been modeled by CitationPotukuchi and Wexler (1995) and included in the online Aerosol Inorganics Model (see http://www.hpc1.uea.ac.uk/~e770/aim.html) by CitationWexler and Clegg (2002). In this system for dry conditions, solid (NH4)2SO4 is predicted to be thermodynamically stable for an ANMF from 0 up to 0.5. Solid NH4NO3 is predicted to be thermodynamically stable above an ANMF of > 0.6. Other predicted thermodynamically stable solid phases that may exist in the particle phase are the double salts (NH4)2SO4·2NH4NO3 and (NH4)2SO4·3NH4NO3. These double salt species dominate the composition (mole fraction basis) from an ANMF of 0.33 to 0.65. However, as observed in many aerosol systems, free floating particles on occasion might not form the thermodynamically predicted solid.
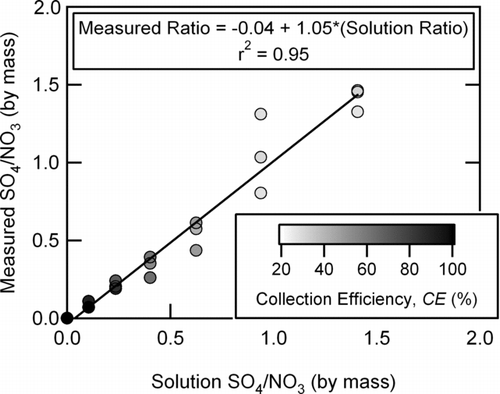
Solutions containing varying ANMF were atomized, dried to a relative humidity of 10% RH, size-selected with a DMA, and sampled with the AMS. A DMA mobility diameter of 343 nm was selected for this experiment as there was sufficient mass of each species for the studied concentrations to allow direct measurement of the count-based CE from either sulfate or nitrate individual particle signals. Since the mixed particles were generated by atomizing solutions containing both species, the resulting particles were composed of internal mixtures with the same proportions as the solutions. A linear regression of the sulfate to nitrate mass ratio measured by the AMS versus the solution concentration had a slope of 1.05, an offset of –0.04, and an r2 of 0.95 (see ). As for the pure ammonium sulfate particles where the single particle signal was constant for the hydrated or dehydrated particles, these mixed ammonium nitrate and sulfate particles have the correct relative mass ratios despite having varying collection efficiency from 29 to 100% (as described below). A lack of matrix effects on the sensitivity to sulfate and nitrate in mixtures was previously noted by Hogrefe and co-workers (2004b).
FIG. 6 The mass ratio of sulfate-to-nitrate measured by the AMS (using RIE = 1.1 and 1.15 for nitrate and sulfate, respectively) versus the mass ratio of the solution used to generate the mixed particles, shaded by the measured collection efficiency.
shows a strong dependence of CE on ANMF present for dry particles, where CE was determined from particle counts in the sulfate or nitrate signals (triangles) or from the total AMS mass divided by the calculated mass loading using the mobility diameter, number concentration from the CPC, and an estimated density for mixed particles (ρ = 1.75 g cm−3) (circles). Up to an ANMF of 0.6, the CE was 29 ± 6%, which was within the observed variation of 24 ± 3% for the CE of solid (NH4)2SO4 (). These particles were essentially behaving (in terms of AMS CE) as pure (NH4)2SO4, even though the predicted thermodynamically stable phase consisted of a changing combination of internally-mixed dry solid salts. This result suggested that even if the double salt, (NH4)2SO4·2NH4NO3, formed as predicted, it apparently exhibited a similar AMS CE as pure, solid (NH4)2SO4. At higher ANMF, CE increased from a value of 29 ± 6% at a mass fraction of 0.55 to 99 ± 6% at a mass fraction > 0.9.
FIG. 7 Count- and mass-based collection efficiencies for dry, mixed ammonium nitrate and ammonium sulfate particles (d m = 343 nm) as a function of the mass fraction of ammonium nitrate present in the particles. Solid triangles are the CE calculated from nitrate peaks at m/z = 30 and 46 and open triangles are the CE calculated from sulfate peaks at m/z = 48, 64, and 81. Circles are the mass-based CE, using the total measured AMS mass divided by the calculated mass from d m = 343 nm, CPC data, and an estimated density of 1.75 g cm−3 for the mixed particles. The dotted line is a guide for the eye and the solid line is the observed ambient AMS CE reported by Croiser et al. (2007).
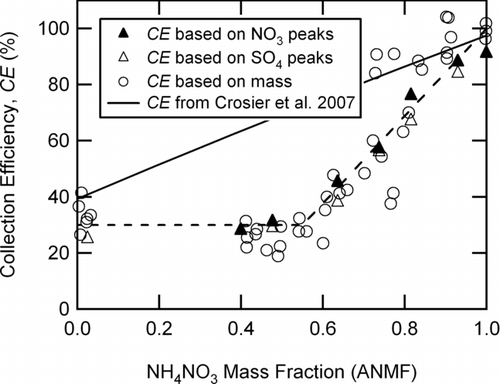
Above an ANMF of 0.5, solid (NH4)2SO4 was not predicted to exist in the mixed particle. At an ANMF of 1, the CE was 100% as reported above for pure, dry NH4NO3 particles. The regime between ANMF of 0.5 to 1 was predicted to consist of varying compositions of two different solid salts (a double salt and pure ammonium nitrate). If the NH4NO3 solid salt did not crystallize as predicted by observations in the literature (CitationLightstone et al. 2000) but the double salt did, the particles might consist of mixed phases for ANMF > 0.6. The behavior of mixed phase particles might explain the observed increase in AMS CE values with increasing ANMF above 0.5 (i.e., increasing nitrate composition). Alternatively, there could be a mixed population of particles with an increasing fraction that were not solid causing the CE to gradually increase as the ANMF was increased.
The deliquescence and efflorescence properties of mixed ammonium nitrate and ammonium sulfate particles has been studied by CitationDougle et al. (1996), who reported that when the ammonium nitrate to ammonium sulfate mass fraction was more than 1.5 (ANMF = 0.6) the particles did not effloresce down to 10% RH whereas particles effloresced at 30% RH when the mass fraction was less than 1.3 (ANMF = 0.56). The CEs measured here showed that when the ANMF was less than 0.56 the dried mixed particles were solid and when the ANMF was more than 0.6 they were not all solid, consistent with the laboratory work by CitationDougle et al. (1996).
A general observation can be drawn from this experiment in terms of AMS CE. In dry, mixed composition NH4NO3/(NH4)2SO4 particles, internally-mixed particles with significant concentrations of (NH4)2SO4 exhibit a lower CE, whereas particles dominated by NH4NO3 exhibit a higher CE. Furthermore, small amounts of NH4NO3 have a minimal effect whereas small amounts of (NH4)2SO4 have a significant effect on the overall AMS CE value. Although these observations strictly only apply to mixed inorganic NH4NO3/(NH4)2SO4 particles, they provide a potential explanation for observed changes in AMS CE measurements from ambient studies with relatively high particulate ammonium nitrate compositions (e.g., CitationCrosier et al. 2007).
Ammonium Sulfate Particles Coated with Dioctyl Sebacate
The above results have been interpreted with the a priori understanding that solid phase particles would likely bounce, lowering the measured CE, whereas liquid particles would not. To directly test this hypothesis, we studied the CE for dry, solid (NH4)2SO4 particles as a function of a liquid organic coating that did not dissolve the solid. The organic used for these coating experiments was dioctyl sebacate (DOS), a liquid diester at room temperature which was a poor solvent for (NH4)2SO4 and has been shown to exhibit an AMS CE = 100% for pure particles (CitationAlfarra 2004).
In these experiments, DOS was condensed onto dry, pure (NH4)2SO4 particles (dm = 276 nm). For the (NH4)2SO4 particles to be coated with a layer of DOS more than 80 nm thick, the temperature of the reservoir had to be increased to the point where pure DOS particles started to homogeneously nucleate in the sample stream. While these nucleated particles did not complicate particle counting or mass statistics obtained from the AMS, these small particles did render useless the counting statistics of the CPC for particles with the thickest coatings. The average number of ions per particle (IPP) at m/z = 48 for sulfate from dry particles was 175 ± 56 IPP, for particles coated with 35 nm of DOS was 221 ± 179 IPP, and for those coated with 60 nm of DOS was 208 ± 71 IPP, which indicated that the sulfate ion signals from individual particles were constant and independent of the amount of DOS within experimental uncertainty (see ). Hence, the total sulfate mass was used to determine a mass-based CE for these experiments in lieu of the count-based CE. We compared the total measured sulfate in the coated particles to the mass of sulfate measured for humidified particles with the same initial dry size and nominally the same initial number concentration.
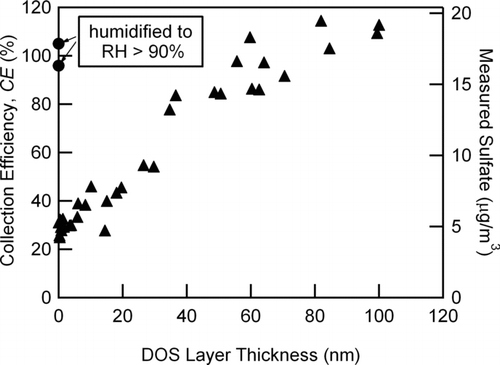
The mass-based CE and the mass of sulfate measured for the DOS coated particles as a function of DOS layer thickness along with the measured sulfate from uncoated dry and humidified (92% RH) (NH4)2SO4 particles (initial dry mobility diameters of 276 nm) are shown in . The CE noticeably increased with increasing DOS layer thickness. This increase could be due to coated solid (NH4)2SO4 particles bouncing less as the solid core of the particles became cushioned upon impact on the vaporizer by the surrounding organic liquid. Another possible explanation for this observation was that an increasing fraction of particles became coated with CE = 100%, leaving a fraction uncoated with a CE = 29 ± 3%. If true, sulfate would in theory appear at two different sizes in the PToF data due to the two different externally mixed particle types (uncoated and coated). However, the width of AMS mass distributions for monodisperse particles from the DMA was 19 ± 3% and d va increased more slowly than dp with increased coating thickness due to the lower density of DOS (0.914 g cm−3) compared to ammonium sulfate (1.77 g cm−3). Because of these two factors, there was not enough resolution to detect a potential difference in size if these two particle types did co-exist.
FIG. 8 Mass-based collection efficiencies and measured mass of sulfate (μ g/m3) for dry ammonium sulfate particles (initial d m = 276 nm) coated with dioctyl sebacate (DOS) as a function DOS layer thickness. Solid triangles are particles coated with DOS, while solid circles are from the same dry-size, uncoated ammonium sulfate particles humidified to RH > 90% RH.
An investigation of the variability in the mass measurements of DOS from the individual particle signals was similar to the variability in measured mass for sulfate (as depicted in ), suggesting that all ammonium sulfate particles were coated with DOS. This result supported the hypothesis that liquid coatings decreased the potential of solid particles to bounce due to more elastic collisions at the vaporizer surface.
The observed increase in CE (measured by sulfate mass) for solid (NH4)2SO4 particles with increasing DOS coating was consistent with the surface of the particle being converted from a solid to liquid phase. Furthermore, it required a layer thickness > 60 nm on the existing 276 nm particles (which corresponded to DOS mass fractions > 0.5) to increase the measured sulfate mass to that observed for the humidified (NH4)2SO4 aqueous droplets. The addition of thicker DOS coatings beyond 60 nm thickness did not further affect the measured sulfate mass (i.e., the measured particulate sulfate mass asymptotically reached the value observed for high RH pure ammonium sulfate particles of the same initial dry diameters).
USING LABORATORY RESULTS TO INTERPRET AMS CEs
These results strongly support the argument that phase plays an important role in how efficient particles are detected in the AMS. Adhesion of particles to surfaces has long been known to be a problem for solid particles that cannot be easily deformed (CitationHinds 1982). A major difference in ambient particles, compared with the laboratory particles studied here, is that organic matter is typically internally mixed with any present inorganic compounds and in significant amounts (CitationMurphy et al. 2006; CitationZhang et al. 2007). Also, the relative composition on an individual particle basis could vary (CitationDrewnick et al. 2005). Therefore, the addition of internally mixed organic (and other inorganic) compounds such as observed on almost all ambient particles (CitationMurphy et al. 2006) may complicate the simple laboratory picture.
CitationAlfarra (2004) previously examined six different pure organic compounds and found that the three liquid organic compounds (dioctyl sebacate, oleic acid, and nonylaldehyde) were sampled with 100% CE in the AMS, whereas the three solid compounds (myristic acid, succinic acid, and pyrene) were sampled with AMS CEs of 20–50%. The measured sulfate mass changes in other laboratory experiments involving organic coatings (A. Kiendler-Scharr, personal communication 2004; CitationBahreini et al. 2005) were due to changes in the particle phase. Hence, the organic phase also affected the AMS CE and the combined laboratory results strongly indicated that ambient particles sampled with 100% CE in the AMS were liquid droplets. Applying this knowledge can help interpret AMS data and provide insight into the phase of ambient particles.
The AMS CE determined in many previous field studies was around 40–50% (CitationAllan et al. 2004; CitationDrewnick et al. 2004; CitationHogrefe et al. 2004a; CitationWeimer et al. 2006) and AMS measurements using a CE of 50% compared favorably with other collocated data in several different environments (CitationAllan et al. 2003b; CitationAlfarra et al. 2004; CitationTopping et al. 2004; CitationTakegawa et al. 2005; CitationZhang et al. 2005; CitationSalcedo et al. 2006). In most of these studies (CitationDrewnick et al. 2004; CitationTopping et al. 2004; CitationTakegawa et al. 2005; CitationZhang et al. 2005; CitationWeimer et al. 2006; CitationSalcedo et al. 2006), the sulfate was fully neutralized, the ANMF was less than 0.6, and the average organic mass fraction was less than 0.6. Combined, these results imply that those found in geographically different environments (urban to rural, continental to marine) were generally not liquid (i.e., solid or mixed solid-liquid phase) a significant fraction of the time.
Mass closure studies between the AMS and other aerosol instruments suggest that while sampling internally mixed ambient aerosol, particle bounce could be corrected using a single chemistry-dependent collection efficiency applied to all chemical species (CitationQuinn et al. 2006; CitationDrewnick et al. 2004; CitationWeimer et al. 2006). For example, in several field studies where the same CE, often derived from sulfate comparisons, was applied to all of the species (Citationde Gouw et al. 2005; Takagawa et al. 2005; CitationQuinn et al. 2006; CitationVenkatachari et al. 2006; CitationKondo et al. 2007; Citationde Gouw et al. 2008), the AMS organic mass was linearly correlated with other organic carbon measurements and had slopes ranging between 1.24–2.22, which were comparable to the values of 1.6–2.1 reported from other methods (CitationTurpin and Lim 2001). Had the organic species been actually present in an externally-mixed mode with CE = 20% or 100%, the organic mass reported would have been too low or high, respectively. Obviously, externally mixed particles might not exhibit similar particle bounce collection efficiencies.
These laboratory results might explain field observations that specifically note when the AMS sampled ambient particles with CE = 100%. CitationAllan et al. (2004) observed a factor of 2 increase in the mass loadings of ambient aerosol dominated by sulfate when the sampling line temperature approached the dew point (i.e., RH approached 100%) compared to when it was 10°C higher. This dramatic change in sampled aerosol mass as a function of sampling line relative humidity could be explained by the deliquescence of the ambient particles due to water uptake by the sulfate. As CitationAllan et al. (2004) did not specifically measure the AMS CE for these particles, it was not known whether the humidification of the sulfate dominated ambient particles generated stable or metastable solution droplets. Ambient measurements of freshly formed ammonium nitrate particles and particles containing a large mass fraction of ammonium nitrate had a CE as high as 100% (Middlebrook and Matthew, unpublished data; Crosier et al. 2007). As shown in , the laboratory results of internally mixed ammonium sulfate and ammonium nitrate provide a framework for understanding the changing AMS CE (solid line in ) observed by Crosier and co-workers (2007) in the outflow from the Po Valley (Italy) where relatively high particulate ammonium nitrate mass fractions were present. CitationQuinn et al. (2006) observed that the measured AMS CE increased from an average 45% to 100% when the chemical composition of the ambient particles was dominated by acidic sulfate. For the last two of these field studies, a composition-dependent CE was derived and applied.
Laboratory and theoretical work indicated that the organic fraction in tropospheric aerosols might remain liquid and prevent the inorganic fraction from solidifying (CitationMarcolli et al. 2004). It is possible that the derived CEs from a 2002 study in the Gulf of Maine were higher than 80% due to the presence of organic material (Citationde Gouw et al. 2005; CitationBates et al. 2005) since the relatively low sampling line relative humidity and inorganic composition would be more consistent with a lower CE. However, in Mexico City the aerosol was dominated by slightly non-spherical particles containing mostly organic species (CitationSalcedo et al. 2007) and exhibited a CE = 50% (CitationSalcedo et al. 2006), strongly suggesting that these ambient organic particles were solid or mixed phase.
An important factor for phase affecting the AMS CE might well be the liquid water content of atmospheric aerosols. These laboratory results for aqueous and metastable ammonium sulfate solution droplets, which were predicted and observed to be liquid prior to sampling into the AMS through the observations of the deliquescence and efflorescence phase transitions, show that some aqueous particles might change phase during sampling into the low pressure AMS system and exhibit a CE < 100%, in contrast to other liquid droplets, including metastable ammonium nitrate, sulfuric acid solution droplets (which could contain significant water at high relative humidity) and neat organic liquids (no particulate water present). The evaporation of water and subsequent latent cooling of solution droplets as they enter and pass through the aerodynamic lens inlet appeared to play a role in controlling the particle phase and measured AMS CE for some aqueous droplets. Ammonium sulfate solution droplets were the only inorganic submicron droplets examined in this work that exhibited an apparent phase transition during sampling into the low pressure regime inside the aerodynamic lens inlet under typical relative humidity conditions. More work is required to fully understand the effects of particulate water on the measured AMS CE of ambient particles and the ability of the AMS to provide meaningful information on the phase of ambient particles.
Changes in the particle size and measurements of water content as a function of relative humidity for aqueous particles showed that water evaporation occurs in the AMS instrument. Hence, although the AMS could detect water in atmospheric particles, the water content was reduced compared to ambient sampling conditions and in some cases the aqueous particles might change phase upon sampling. It is important to note that this behavior would hold true for any aerosol instrument that samples aqueous particles into a low pressure regime (in this explicit case any instrument that uses a low pressure aerodynamic lens inlet). Thus, while the observed phase transition of the aqueous ammonium sulfate particles might reduce the AMS CE, it also likely broadened the particle beam divergence (e.g., CitationHuffman et al. 2005), potentially affecting aerosol instruments that rely on well-defined particle trajectories (e.g., single particle laser ablation systems). As ambient relative humidity (i.e., particulate water) appeared to play a significant and varying role in the ability to sample ambient particles using aerosol instruments such as the AMS, we suggest that ambient particles should be dried prior to sampling. Drying of ambient particles would remove the potential for ambient particles to exhibit significantly varying CEs (e.g., CitationAllan et al. 2004) with changing RH, even while it might decrease the AMS CE for some types of particles (e.g., pure aqueous ammonium sulfate particles).
Various practices are actively being studied to reduce or measure the amount of particle bounce during ambient sampling such as coating particles with an “inert” and chemically distinct liquid such as Fomblin® and changing the vaporizer geometry. In the case of particle coating, E L must be considered since larger particles may not be transmitted efficiency through the aerodynamic lens. The addition of an inert liquid such as Fomblin® could increase the background for organic measurements. With a change in vaporizer geometry, particles may be trapped allowing for multiple collisions with the surface in order to vaporize them at the expense of size resolution. A light scattering module for the AMS has been recently developed that optically counts and sizes particles prior to entering the vaporizer chamber (CitationCross et al. 2007). This technique provides a direct count-based method for determining AMS CE in situ by correlating the optical and chemical detection of the same particles within the AMS.
In the absence of suitable techniques to prevent particle bounce during sampling or of carrying out direct particle counting, an estimate of the particle phase based on the composition and sampling line relative humidity could be applied to ambient data sets to account for particle bounce. Work is in progress to applying a composition-dependent CE based on this laboratory work and the field work by Quinn and co-workers (Citation2006) to various data sets and comparing the results to other aerosol measurements. However, caution must be used when attempting to apply these laboratory results to field data since the ubiquitous presence of organics (and other inorganic components) on ambient particles may complicate this simple understanding. Indeed, the collection efficiencies from ambient measurements, such as those by Crosier and co-workers (2007) depicted in , have never been as low as that observed for pure, dry, ammonium sulfate particles (24 ± 3%).
CONCLUSIONS
The Aerodyne aerosol mass spectrometer (AMS) is based on a detection scheme that separates particles from the ambient gas phase species through an aerodynamic lens into high vacuum and impacts the particles on a heated vaporizer. Particles that interact with the vaporizer surface long enough to thermally equilibrate will result in the vaporization of the nonrefractory fraction of the particles, leading to quantitative detection of the mass and chemical composition. Particles that strike the vaporizer and bounce off prior to temperature equilibration, however, may not get detected and can lead to an underestimation of the ambient mass loadings.
The phase of particles being sampled into the AMS affects the collection efficiency, CE. Liquid and liquid-coated droplets were collected with nearly 100% efficiency, while solid particles were collected with efficiencies as low as 20%. Mixed phase particles, solid, nonspherical particles, and metastable aqueous ammonium sulfate droplets exhibited a CE that fell between these values. Measured collection efficiencies of internally mixed aerosols were independent of which species was analyzed, demonstrating that the same collection efficiency should be used for internally mixed species. Furthermore, the consistency of single particle signals indicated that the phase (and hence collection efficiency) of mixed component particles did not affect the AMS sensitivity to a particular chemical species and that relative mass measurements of internally-mixed particles were independent of the particles' collection efficiency. Although the results presented here are from the Aerodyne AMS, the issues of particle bounce, water evaporation, and phase changes could be relevant to other particle mass spectrometers depending on their design.
Acknowledgments
We thank James Allan for developing the Q-AMS data analysis software, Roya Bahreini, Peter Liu, and Astrid Kiendler-Scharr for sharing their preliminary data, Jose-Luis Jimenez, John Jayne, Doug Worsnop, Dan Cziczo, Frank Drewnick, and Dan Murphy for fruitful discussions, and Thomas Koop and Becky Garland for assistance with literature searches.
REFERENCES
- Alfarra , M. R. 2004 . In Insights Into Atmospheric Organic Aerosols Using an Aerosol Mass Spectrometer , PhD thesis under Prof. H. Coe 94 – 95 . Univ. of Manchester .
- Alfarra , M. R. , Coe , H. , Allan , J. D. , Bower , K. N. , Boudries , H. , Canagaratna , M. R. , Jimenez , J. L. , Jayne , J. T. , Garforth , A. A. , Li , S.-M. and Worsnop , D. R. 2004 . Characterization of Urban and Rural Organic Particulate in the Lower Fraser Valley Using Two Aerodyne Aerosol Mass Spectrometers . Atmos. Environ. , 34 : 5745 – 5758 .
- Allan , J. D. , Bower , K. N. , Coe , H. , Boudries , H. , Jayne , J. T. , Canagaratna , M. R. , Millet , D. B. , Goldstein , A. H. , Quinn , P. K. , Weber , R. J. and Worsnop , D. R. 2004 . Submicron Aerosol Composition at Trinidad Head, California, during ITCT 2K2: Its Relationship with Gas Phase Volatile Organic Carbon and Assessment of Instrument Performance . J. Geophys. Res. Atmos , 109 (D23) doi:10.1029/2003JD004208
- Allan , J. D. , Jimenez , J. L. , Williams , P. I. , Alfarra , M. R. , Bower , K. N. , Jayne , J. T. , Coe , H. and Worsnop , D. R. 2003a . Quantitative Sampling Using an Aerodyne Aerosol Mass Spectrometer. Part 1: Techniques of Data Interpretation and Error Analysis . J. Geophys. Res.—Atmos , 108 (D3): doi:10.1029/2002JD002358
- Allan , J. D. , Alfarra , M. R. , Bower , K. N. , Williams , P. I. , Gallagher , M. W. , Jimenez , J. L. , McDonald , A. G. , Nemitz , E. , Canagaratna , M. R. , Jayne , J. T. , Coe , H. and Worsnop , D. R. 2003b . Quantitative Sampling Using an Aerodyne Aerosol Mass Spectrometer. Part 2: Measurements of Fine Particulate Chemical Composition in Two UK Cities . J. Geophys. Res.—Atmos. , 108 (D3): doi:10.1029/2002JD002359
- Bahreini , R. , Keywood , M. D. , Ng , N. L. , Varutbangkul , V. , Gao , S. , Flagan , R. C. , Seinfeld , J. H. , Worsnop , D. R. and Jimenez , J. L. 2005 . Measurements of Secondary Organic Aerosol from Oxidation of Cycloalkenes, Terpenes, and m-Xylene Using an Aerodyne Aerosol Mass Spectrometer . Environ. Sci. Technol. , 39 ( 15 ) : 5674 – 5688 .
- Bates , T. S. , Quinn , P. K. , Coffman , D. J. , Johnson , J. E. and Middlebrook , A. M. 2005 . Dominance of Organic Aerosols in the Marine Boundary Layer over the Gulf of Maine During NEAQS 2002 and Their Role in Aerosols Light Scattering . J. Geophys. Res. Atmos , 110 (D18202): doi:10.1029/2005JD005797
- Canagaratna , M. R. , Jayne , J. T. , Jimenez , J. L. , Allan , J. D. , Alfarra , M. R. , Zhang , Q. , Onasch , T. B. , Drewnick , F. , Coe , H. , Middlebrook , A. , Delia , A. , Williams , L. R. , Trimborn , A. M. , Northway , M. J. , Kolb , C. E. , Davidovits , P. and Worsnop , D. R. 2007 . Chemical and Microphysical Characterization of Ambient Aerosols with the Aerodyne Aerosol Mass Spectrometer . Mass Spec. Rev. , 26 : 185 – 222 .
- Cheng , Y.-S. and Yeh , H.-C. 1979 . Particle Bounce in Cascade Impactors . Environ. Sci. Technol , 12 ( 11 ) : 1392 – 1396 .
- Crosier , J. , Allan , J. D. , Coe , H. , Bower , K. N. , Formenti , P. and Williams , P. I. 2007 . Chemical Composition of Summertime Aerosol in the Po Valley (Italy), Northern Adriatic and Black Sea . Q. J. R. Meterol. Soc , 133 ( S1 ) : 61 – 75 .
- Cross , E. S. , Slowik , J. G. , Davidovits , P. , Allan , J. D. , Worsnop , D. R. , Jayne , J. T. , Lewis , D. K. , Canagaratna , M. and Onasch , T. B. 2007 . Laboratory and Ambient Particle Density Determinations Using Light Scattering in Conjunction with Aerosol Mass Spectrometry . Aerosol Sci. Technol. , 41 : 343 – 359 .
- Dahneke , B. 1971 . The Capture of Aerosol Particles by Surfaces . J. Colloid Interface Sci. , 37 ( 2 ) : 342 – 354 .
- de Gouw , J. A. , Middlebrook , A. M. , Warneke , C. , Goldan , P. D. , Kuster , W. C. , Roberts , J. M. , Fehsenfeld , F. C. , Worsnop , D. R. , Canagaratna , M. R. , Pszenny , A. A. P. , Keene , W. C. , Marchewka , M. , Bertman , S. B. and Bates , T. S. 2005 . Budget of Organic Carbon in a Polluted Atmosphere: Results from the New England Air Quality Study in 2002 . J. Geophys. Res.—Atmos , 110 (D16305): doi:10.1029/2004JD005623
- de Gouw , J. A. , Brock , C. A. , Atlas , E. L. , Bates , T. S. , Fensenfeld , F. C. , Goldan , P. D. , Holloway , J. S. , Kuster , W. C. , Lerner , B. M. , Matthew , B. M. , Middlebrook , A. M. , Onasch , T. B. , Peltier , R. E. , Quinn , P. K. , Senff , C. J. , Stohl , A. , Sullivan , A. P. , Trainer , M. , Warneka , C. , Weber , R. J. and Williams , E. J. 2008 . Sources of Particulate Matter in the Northeastern United States in Summer: 1. Direct Emissions and Secondary Formation of Organic Matter in Urban Plumes . J. Geophys. Res , 113 D08301, doi:10.1029/2007JD009243
- DeCarlo , P. F. , Slowik , J. G. , Worsnop , D. R. , Davidovits , P. and Jimenez , J. L. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory . Aerosol Sci. Technol. , 38 : 1185 – 1205 .
- Dougle , P. G. , Vlasenko , A. L. , Veefkind , J. P. and Ten Brink , H. M. 1996 . Humidity Dependence of the Light Scattering by Mixtures of Ammonium Nitrate, Ammonium Sulfate, and Soot . J. Aerosol Sci , 27 : 513 – 514 . (S1): doi: 10.1016/0021-8502(96)00329-1
- Drewnick , F. , Schwab , J. J. , Jayne , J. T. , Canagaratna , M. , Worsnop , D. R. and Demerjian , K. L. 2004 . Measurement of Ambient Aerosol Composition during the PMTACS-NY 2001 Using an Aerosol Mass Spectrometer. Part I: Mass Concentrations . Aerosol Sci. Technol. , 38 ( S1 ) : 92 – 103 .
- Drewnick , F. , Hings , S. S. , DeCarlo , P. F. , Jayne , J. T. , Gonin , M. , Fuhrer , K. , Weimer , S. , Jimenez , J. L. , Demerjian , K. L. , Borrmann , S. and Worsnop , D. R. 2005 . A new Time-of-Flight Aerosol Mass Spectrometer (ToF-AMS)—Instrument Description and First Field Deployment . Aerosol Sci. Technol. , 39 : 637 – 658 .
- Hinds , W. C. 1982 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , 131 New York : John Wiley and Sons .
- Hogrefe , O. , Schwab , J. J. , Drewnick , F. , Lala , G. G. , Peters , S. , Demerjian , K. L. , Rhoads , K. P. , Felton , H. D. , Rattigan , O. V. , Husain , L. and Dutkiewicz , V. A. 2004a . Semicontinous PM2.5 Sulfate and Nitrate Measurements at an Urban and a Rural Location in New York: PMTACS-NY Summer 2001 and 2002 Campaigns . J. Air Waste Manag. Assoc. , 54 : 1040 – 1060 .
- Hogrefe , O. , Drewnick , F. , Lala , G. G. , Schwab , J. J. and Demerjian , K. L. 2004b . Development, Operation and Applications of an Aerosol Generation, Calibration and Research Facility . Aerosol Sci. Technol. , 38 ( S1 ) : 196 – 214 .
- Huffman , J. A. , Jayne , J. T. , Drewnick , F. , Aiken , A. C. , Onasch , T. , Worsnop , D. R. and Jimenez , J. L. 2005 . Design, Modeling, Optimization, and Experimental Tests of a Particle Beam Width Probe for the Aerodyne Aerosol Mass Spectrometer . Aerosol Sci. Technol. , 39 ( 12 ) : 1143 – 1163 .
- Jayne , J. T. , Leard , D. C. , Zhang , X. , Davidovits , P. , Smith , K. A. , Kolb , C. E. and Worsnop , D. R. 2000 . Development of an Aerosol Mass Spectrometer for Size and Composition Analysis of Submicron Particles . Aerosol Sci. Technol. , 33 : 49 – 70 .
- Jimenez , J. L. , Jayne , J. T. , Shi , Q. , Kolb , C. E. , Worsnop , D. R. , Yourshaw , I. , Seinfeld , J. H. , Flagan , R. C. , Zhang , X. , Smith , K. A. , Morris , J. W. and Davidovits , P. 2003 . Ambient Aerosol Sampling Using the Aerodyne Aerosol Mass Spectrometer . J. Geophys. Res.—Atmos , 108 (D7): doi:10.1029/2001JD001213
- Kane , D. B. and Johnston , M. V. 2000 . Size and Composition Biases on the Detection of Individual Ultrafine Particles by Aerosol Mass Spectrometry . Environ. Sci. Technol. , 34 ( 23 ) : 4887 – 4893 .
- Kondo , Y. , Miyazaki , Y. , Takegawa , N. , Miyakawa , T. , Weber , R. J. , Jimenez , J. L. , Zhang , Q. and Worsnop , D. R. 2007 . Oxygenated and Water-Soluble Organic Aerosols in Tokyo . J. Geophys. Res. , 112 D01203, doi:10.1029/2006JD007056
- Leong , K. H. 1987a . Morphological Control of Particles Generated from the Evaporation of Solution Droplets: Theoretical Considerations . J. Aerosol Sci. , 18 ( 5 ) : 511 – 524 .
- Leong , K. H. 1987b . Morphological Control of Particles Generated from the Evaporation of Solution Droplets: Experiment . J. Aerosol Sci , 18 ( 5 ) : 525 – 552 .
- Lightstone , J. M. , Onasch , T. B. , Imre , D. and Oatis , S. 2000 . Deliquescence, Efflorescence, and Water Activity in Ammonium Nitrate and Mixed Ammonium Nitrate/Succinic Acid Microparticles . J. Phys. Chem. A , 104 ( 41 ) : 9337 – 9346 .
- Liu , P. S. K. , Deng , R. , Smith , K. A. , Williams , L. R. , Jayne , J. T. , Canagaratna , M. R. , Moore , K. , Onasch , T. B. , Worsnop , D. R. and Deshler , T. 2007 . Transmission Efficiency of an Aerodynamic Focusing Lens System: Comparison of Model Calculations and Laboratory Measurements for the Aerodyne Aerosol Mass Spectrometer . Aerosol Sci. Technol. , 41 ( 9 ) : 721 – 733 .
- Liu , P. , Ziemann , P. J. , Kittelson , D. B. and McMurry , P. H. 1995 . Generating Particle Beams of Controlled Dimensions and Divergence: II. Experimental Evaluation of Particle Motion in Aerodynamic Lenses and Nozzle Expansions . Aerosol Sci. Technol. , 22 ( 3 ) : 314 – 324 .
- Marcolli , C. , Luo , B. P. and Peter , T. 2004 . Mixing of the Organic Aerosol Fractions: Liquids as the Thermodynamically Stable Phases . J. Phys. Chem. A , 108 : 2216 – 2224 .
- Middlebrook , A. M. , Thomson , D. S. and Murphy , D. M. 1997 . On the Purity of Laboratory-Generated Sulfuric Acid Droplets and Ambient Particles Studied by Laser Mass Spectrometry . Aerosol Sci. Technol. , 27 ( 3 ) : 293 – 307 .
- Murphy , D. M. , Cziczo , D. J. , Froyd , K. D. , Hudson , P. K. , Matthew , B. M. , Middlebrook , A. M. , Peltier , R. E. , Sullivan , A. , Thomson , D. S. and Weber , R. J. 2006 . Single-Particle Mass Spectrometry of Tropospheric Aerosol Particles . J. Geophys. Res.—Atmos , 111 (D32S32): doi:10.1029/2006JD007340
- Myers , R. L. and Fite , W. L. 1975 . Electrical Detection of Airborne Particulates Using Surface Ionization Techniques . Environ. Sci. Technol , 9 ( 4 ) : 334 – 336 .
- Onasch , T. B. , Siefert , R. L. , Brooks , S. D. , Prenni , A. J. , Murray , B. , Wilson , M. A. and Tolbert , M. A. 1999 . Infrared Spectroscopic Study of the Deliquescence and Efflorescence of Ammonium Sulfate Aerosol as a Function of Temperature . J. Geophys. Res.—Atmos. , 104 ( D17 ) : 21,317 – 21,326 .
- Perry , R. H. and Green , D. W. 1997 . Perry's Chemical Engineers' Handbook , New York : McGraw-Hill . Table 2-37
- Potukuchi , S. and Wexler , A. S. 1995 . Identifying Solid-Aqueous Phase Transitions in Atmospheric Aerosols. II. Acidic Solutions . Atmos. Environ. , 29 ( 22 ) : 3357 – 3364 .
- Quinn , P. K. , Bates , T. S. , Coffman , D. , Onasch , T. B. , Worsnop , D. , Baynard , T. , de Gouw , J. A. , Goldan , P. D. , Kuster , W. C. , Williams , E. , Roberts , J. M. , Lerner , B. , Stohl , A. , Pettersson , A. and Lovejoy , E. R. 2006 . Impacts of Sources and Aging on Submicrometer Aerosol Properties in the Marine Boundary Layer Across the Gulf of Maine . J. Geophys. Res.—Atmos. , 111 (D23S36): doi:10.1029 /2006JD007582
- Salcedo , D. , Onasch , T. B. , Dzepina , K. , Canagaratna , M. R. , Zhang , Q. , Huffman , J. A. , DeCarlo , P. F. , Jayne , J. T. , Mortimer , P. , Worsnop , D. R. , Kolb , C. E. , Johnson , K. S. , Zuberi , B. , Marr , L. C. , Volkamer , R. , Molina , L. T. , Molina , M. J. , Cardenas , B. , Bernabé , R. M. , Márquez , C. , Gaffney , J. S. , Marley , N. A. , Laskin , A. , Shutthanandan , V. , Xie , Y. , Brune , W. , Lesher , R. , Shirley , T. and Jimenez , J. L. 2006 . Characterization of Ambient Aerosols in Mexico City during the MCMA-2003 Campaign with Aerosol Mass Spectrometry: Results from the CENICA Supersite . Atmos. Chem. Phys. , 6 : 925 – 946 .
- Salcedo , D. , Onasch , T. B. , Canagaratna , M. R. , Dzepina , K. , Huffman , J. A. , Jayne , J. T. , Worsnop , D. R. , Kolb , C. E. , Weimer , S. , Drewnick , F. , Allan , J. D. , Delia , A. E. and Jimenez , J. L. 2007 . Technical Note: Use of a Beam Width Probe in an Aerosol Mass Spectrometer to Monitor Particle Collection Efficiency in the Field . Atmos. Chem. Phys. , 7 : 549 – 556 .
- Slowik , J. G. , Stainken , K. , Davidovits , P. , Williams , L. R. , Jayne , J. T. , Kolb , C. E. , Worsnop , D. R. , Rudich , Y. , DeCarlo , P. F. and Jimenez , J. L. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 2: Application to Combustion-Generated Soot Aerosols as a Function of Fuel Equivalence Ratio . Aerosol Sci. Technol. , 38 : 1206 – 1222 .
- Slowik , J. G. , Cross , E. S. , Han , J.-H. , Kolucki , J. , Davidovits , P. , Williams , L. R. , Onasch , T. B. , Jayne , J. T. , Kolb , C. E. and Worsnop , D. R. 2007 . Measurements of Morphology Changes of Fractal Soot Particles using Coating and Denuding Experiments: Implications for Optical Absorption and Atmospheric Lifetime . Aerosol Sci. Technol. , 41 : 734 – 750 .
- Stoffels , J. J. and Lagergren , C. R. 1981 . On the Real-Time Measurement of Particles in Air by Direct-Inlet Surface-Ionization Mass Spectrometry . Int. J. Mass Spec. Ion Phys , 40 : 243 – 254 .
- Takegawa , N. , Miyazaki , Y. , Kondo , Y. , Komazaki , Y. , Miyakawa , T. , Jimenez , J. L. , Jayne , J. T. , Worsnop , D. R. , Allan , J. D. and Weber , R. J. 2005 . Characterization of an Aerodyne Aerosol Mass Spectrometer (AMS): Intercomparison with Other Aerosol Instruments . Aerosol Sci. Technol. , 39 : 760 – 770 .
- Tang , I. N. 1980 . “ Deliquescence Properties and Particle Size Change of Hygroscopic Aerosols ” . In Generation of Aerosols and Facilities for Exposure Experiments , Edited by: Willeke , K. 153 – 167 . Ann Arbor, MI : Ann Arbor Science Publishers .
- Tobias , H. J. and Ziemann , P. J. 2000 . Thermal Desorption Mass Spectrometric Analysis of Organic Aerosol Formed from Reactions of 1-Tetradecene and O3 in the Presence of Carboxylic Acids . Environ. Sci. Technol. , 34 ( 11 ) : 2105 – 2115 .
- Topping , D. , Coe , H. , McFiggans , G. , Burgess , R. , Allan , J. , Alfarra , M. R. , Bower , K. , Choularton , T. W. , Decesari , S. and Facchini , M. C. 2004 . Aerosol Chemical Characteristics from Sampling Conducted on the Island of Jeju, Korea During ACE Asia . Atmos. Environ. , 38 : 2111 – 2123 .
- Turpin , B. J. and Lim , H. J. 2001 . Species Contributions to PM2.5 Mass Concentrations: Revisiting Common Assumptions for Estimating Organic Mass . Aerosol Sci. Technol. , 35 : 602 – 610 . doi: 10.1080/02786820152051454.
- Vasiliou , J. G. , Sorenson , D. and McMurry , P. H. 1999 . Sampling at Controlled Relative Humidity with a Cascade Impactor . Atmos. Environ. , 33 : 1049 – 1056 .
- Venkatachari , P. , Zhou , L. , Hopke , P. K. , Schwab , J. J. , Demerjian , K. L. , Weimer , S. , Hogrefe , O. , Felton , D. and Rattigan , O. 2006 . An Intercomparison of Measurement Methods for Carbonaceous Aerosol in the Ambient Air in New York City . Aerosol Sci. Technol. , 40 : 788 – 795 . doi:10.1080/02786820500380289
- Weber , R. J. , Orsini , D. , Daun , Y. , Lee , Y-N. , Klotz , P. J. and Brechtel , F. 2001 . A Particle-Into-Liquid Collector for Rapid Measurements of Aerosol Bulk Chemical Compositions . Aerosol Sci. Technol. , 35 : 718 – 727 .
- Weimer , S. , Drewnick , F. , Hogrefe , O. , Schwab , J. J. , Rhodes , K. , Orsini , D. , Canagaratna , M. , Worsnop , D. R. and Demerjian , K. L. 2006 . Size-Selective Nonrefractory Ambient Aerosol Measurements during the Particulate Matter Technology Assessment and Characterization Study—New York 2004 Winter Intensive in New York City . J. Geophys. Res.—Atmos. , 111 (D18305): doi:10.1029/2006JD007215
- Wexler , A. S. and Clegg , S. L. 2002 . Atmospheric Aerosol Models for Systems Including the Ions H+, NH4 +, Na+, SO4 2−, NO3 −, Cl−, Br− and H2O . J. Geophys. Res.—Atmos , 107 (D14): doi:10.1029/2001JD000451
- Zelenyuk , A. , Imre , D. and Cuadra-Rodriguez , L. A. 2006b . Evaporation of Water from Particles in the Aerodynamic Lens Inlet: An Experimental Study . Anal. Chem , 78 : 6942 – 6947 . doi: 10.1021/ac061184o
- Zelenyuk , A. , Cai , Y. and Imre , D. 2006a . From Agglomerates of Spheres to Iregularly Shaped Particles: Determination of Dynamic Shape Factors from Measurements of Mobility and Vacuum Aerodynamic Diameters . Aerosol Sci. Technol. , 40 ( 1 ) : 197 – 217 . doi:10.1080/02786820500529406
- Zelenyuk , A. , Cai , Y. , Chieffo , L. and Imre , D. 2005 . High Precision Density Measurements of Single Particles: The Density of Metastable Phases . Aerosol Sci. Technol. , 39 : 972 – 986 . doi:10.1080/02786820500380206
- Zhang , Q. , Canagaratna , M. R. , Jayne , J. T. , Worsnop , D. R. and Jimenez , J. L. 2005 . Time- and Size-Resolved Chemical Composition of Submicron Particles in Pittsburgh: Implications for Aerosol Sources and Processes . J. Geophys. Res.—Atmos , 110 (D07S09): doi:10.1029/2004JD004649
- Zhang , Q. , Jimenez , J. L. , Canagaratna , M. R. , Allan , J. D. , Coe , H. , Ulbrich , L. , Alfarra , M. R. , Takami , A. , Middlebrook , A. M. , Sun , Y. L. , Dzepina , K. , Dunlea , E. , Docherty , K. , DeCarlo , P. F. , Salcedo , D. , Onasch , T. , Jayne , J. T. , Miyoshi , T. , Shimono , A. , Hatakeyama , S. , Takegawa , N. , Kondo , Y. , Schneider , J. , Drewnick , F. , Borrmann , S. , Weimer , S. , Demerjian , K. , Williams , P. , Bower , K. , Bahreini , R. , Cottrell , L. , Griffin , R. J. , Rautiainen , J. , Sun , J. Y. , Zhang , Y. M. and Worsnop , D. R. 2007 . Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes . Geophys. Res. Lett , 34 (L13801): doi:10.1029/2007GL029979